Abstract
Background and aim
Although sitafloxacin (STFX)-containing regimens are effective rescue treatments for Helicobacter pylori infection, prevalence of fluoroquinolone resistance in H. pylori has increased rapidly worldwide. The change in resistance levels and gyrA mutations, a major cause of fluoroquinolone resistance, after unsuccessful STFX-containing treatment has not been investigated.
Methods
We conducted a retrospective, non-randomized study to compare the minimum inhibitory concentrations (MICs) of STFX and the location of gyrA mutations in H. pylori before and after unsuccessful eradication with STFX-containing regimens at Keio University Hospital between December 2011 and March 2015.
Results
A total of 266 patients treated with STFX-containing regimens for third-line H. pylori eradication were evaluated. Double mutations in gyrA were acquired by 20.8% of strains that exhibited seven-fold increased STFX MICs, compared to pre-treatment MICs. The STFX MICs did not increase, however, when the location of the gyrA mutations did not change after treatment. Double mutations in gyrA developed in 60.0% of the strains in which eradication failed, which exhibited a baseline mutation at position D91, and in 11.1% of strains with baseline mutations at position N87.
Conclusion
Acquisition of double mutations in gyrA evoked high-level resistance to STFX in H. pylori after unsuccessful eradication with STFX-containing regimens.
Keywords: Helicobacter pylori, gyrA, sitafloxacin, double mutation, minimum inhibitory concentration
Key summary
Summarize the established knowledge on this subject.
Sitafloxacin (STFX)-containing regimens are one of the most effective rescue treatments for Helicobacter pylori infection.
Whether exposure to STFX-containing therapies leads to further resistance through acquisition of a second gyrA mutation has not been studied.
What are the significant and/or new findings of this study?
Our study showed that STFX-containing regimens led to the accumulation of double mutations in gyrA with a certain degree of probability.
The minimum inhibitory concentrations (MICs) of double-mutated strains were significantly higher than those of single-mutated strains.
The D91-mutated strains were more likely to gain resistance to fluoroquinolones via the acquisition of a secondary mutation in gyrA than the N87-mutated strains.
Introduction
Fluoroquinolone-containing triple therapies have been a well-conducted option as a rescue treatment after failure of standard first-line and second-line eradication therapies against Helicobacter pylori infection.1 The efficacy of levofloxacin-containing triple therapy is limited, however, by a high prevalence of fluoroquinolone resistance.2 Sitafloxacin (STFX), a novel developed quinolone, exhibits potent activity against H. pylori with gyrA mutations.3 STFX-containing therapy is clearly more effective than levofloxacin-containing therapy as a third-line treatment; therefore, STFX-containing therapy is highly recommended as a third-line treatment in Japan.4–6 Mutations in gyrA play a critical role in fluoroquinolone resistance in H. pylori.7,8 The position of the gyrA mutation is usually limited to N87 or D91, both of which are in the DNA-binding region on the N-terminal domain of the gyrA protein, which includes fluoroquinolone-binding sites.8 Thus far, strains with double mutations at both N87 and D91 in gyrA are quite rare.9 We reported a greater than 96% successful eradication rate with STFX-containing therapies in patients infected with gyrA mutation-negative strains.4,5 In addition, the eradication rate was approximately 70% even in patients infected with gyrA mutation-positive strains when STFX-containing therapies were used as a third-line regimen.
Thus, to maintain susceptibility of STFX in H. pylori, it is important to know the mechanism for the development of STFX resistance. However, whether exposure to STFX-containing therapies leads to further resistance through acquisition of a second gyrA mutation has not been studied. We assessed changes in the location of gyrA mutations and the minimum inhibitory concentration (MIC) for STFX in H. pylori after unsuccessful treatment with STFX-containing regimens.
Materials and methods
Study design and study population
We conducted a retrospective, non-randomized study to compare the MICs of STFX in H. pylori before and after unsuccessful eradication with STFX-containing regimens at Keio University Hospital between December 2011 and March 2015. We analyzed the data collected in our previous clinical trials to evaluate the eradication rates of STFX-contained regimens (UMIN000001558, UMIN000006483, UMIN000013194, UMIN000013195). All participants in this study gave written informed consent for the previous studies, and were given an opportunity to opt out of this study. The protocol for this study was approved by the ethics committee of the Keio University School of Medicine (No. 20150384; January 29, 2016), and conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Susceptibility of H. pylori to antimicrobial agents and detection of gyrA mutation
The susceptibility of the isolated H. pylori to STFX was determined by the agar dilution method according to the guidelines established by the Clinical and Laboratory Standards Institute (CLSI).10 To identify the gyrA mutation status, we isolated H. pylori chromosomal and plasmid DNA using previously described methods.11 Then, we amplified by polymerase chain reaction (PCR) and sequenced the “quinolone resistance-determining region (QRDR)” of the gyrA (from codon 38 to 154) gene; gyrA (forward), 5′-TTTRGCTTATTCMATGAGCGT-3′; gyrA (reverse), 5′-GCAGACGGCTTGGTARAATA-3′. PCR was performed with 35 cycles of denaturation at 94℃ for 30 seconds (s), annealing at 52℃ for 30 s, and extension at 72℃ for one minute.4,5,8,12 PCR products for sequencing were purified with a QIAquick Gel Extraction Kit (QIAGEN). PCR templates of all strains were sequenced directly on both strands by the BigDye Terminator Cycle Sequencing method using the Applied Biosystems 3100 Genetic Analyzer (Applied Biosystems). The obtained sequences were compared with the published sequences of the H. pylori gyrA gene (GenBank accession no. L29481).13 The MIC defining the antimicrobial resistance of H. pylori was defined as 0.12 µg/ml for STFX based on our previous reports.5,14
Statistical analysis
Comparisons between the factors affecting eradication rates were conducted with the Fisher’s exact test, the Student’s t test and Wilcoxon signed-rank test, as appropriate. Statistical analyses were performed using SPSS 24 for Windows (SPSS Inc, Chicago, IL, USA). Data are expressed as means ± standard deviations. P values smaller than 0.05 were regarded as statistically significant.
Results
Patient characteristics
A total of 266 patients were treated with STFX-containing regimens for third-line H. pylori eradication, and 49 patients failed treatment. Characteristics of the 266 strains before STFX-containing treatment are shown in Table 1. There was no double mutation detected in gyrA prior to treatment. The rate of resistant strains to STFX among the strains mutated at N87 was significantly higher than that of D91 (88.1% (89/101) vs. 64.5% (40/62), p < 0.001). The eradication rate in the patients infected with the strains mutated at N87 was significantly lower than in those mutated at D91 (65.3% (66/101) vs. 82.3% (51/62), p = 0.02). Eighty, 80 and 106 strains were treated with seven-day rabeprazole, amoxicillin and sitafloxacin triple regimen (RAS), 10-day esomeprazole, amoxicillin and sitafloxacin triple regimen (EAS) and 10-day esomeprazole, metronidazole and sitafloxacin triple regimen (EMS), respectively.
Table 1.
Characteristics of Helicobacter pylori strain before the treatments.
|
gyrA mutation |
p value | |||
|---|---|---|---|---|
| N87 (n = 101) | D91 (n = 62) | None (n = 103) | ||
| Mean age (years (mean ± SD)) | 53.4 ± 12.3 | 52.4 ± 14.0 | 50.1 ± 14.5 | 0.68a |
| Gender, male/female, n | 47/54 | 18/44 | 58/45 | 0.03 b |
| H. pylori status before treatment | ||||
| MICs of STFX (µg/ml (mean ± SD)) | 0.40 ± 0.96 | 0.24 ± 0.44 | 0.03 ± 0.08 | 0.96a |
| Resistant to STFX, n (%) | 89 (88.1) | 40 (64.5) | 8 (7.8) | <0.001 b |
| Subtypes of mutation, n (%) | ||||
| N87I | 19 (18.8) | |||
| N87K | 78 (77.2) | |||
| N87T | 4 (4.0) | |||
| D91Y | 23 (37.1) | |||
| D91N | 12 (19.4) | |||
| D91G | 27 (43.5) | |||
| Successful eradication, n (%) | 66 (65.3) | 51 (82.3) | 100 (97.1) | 0.02 b |
| Failed eradication, n (%) | 35 (34.7) | 11 (17.7) | 3 (2.9) | |
| Eradication regimen, n (%) | ||||
| RAS | 25 (24.8) | 23 (37.1) | 32 (31.1) | 0.05b |
| EAS | 37 (36.6) | 11 (17.7) | 32 (31.1) | |
| EMS | 39 (38.6) | 28 (45.2) | 39 (37.8) | |
SD: standard deviation; H. pylori: Helicobacter pylori; MIC: minimum inhibitory concentration; STFX: sitafloxacin; isolates were defined as resistant to sitafloxacin, when the MICs were ≥0.12 µg/ml; RAS: seven-day rabeprazole, amoxicillin and sitafloxacin triple regimen; EAS: 10-day esomeprazole, amoxicillin and sitafloxacin triple regimen; EMS: 10-day esomeprazole, metronidazole and sitafloxacin triple regimen. Boldface types indicate significance at the p < 0.05.
Student’s t test (N87 vs. D91). bFisher’s exact test (N87 vs. D91).
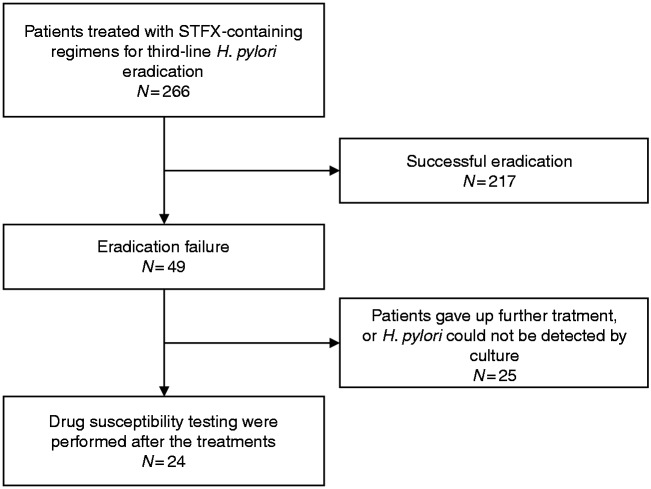
Among the 49 patients with treatment failure, 25 patients were excluded because they discontinued treatment. H. pylori from these patients could not be detected by culture after treatment. Twenty-four patients who had undergone drug susceptibility testing for STFX, and gyrA gene sequencing before and after the treatments were analyzed for changes in STFX MICs and gyrA mutations after treatment (Figure 1). Among the 24 pre-treatment strains, there were 18 strains with gyrA mutation at N87 and 5 strains at D91, respectively. One strain was not found to have a mutation in gyrA.
Figure 1.
Flow diagram of this study.
STFX: sitafloxacin.
Correlation between gyrA mutation status and minimal inhibitory concentrations of STFX
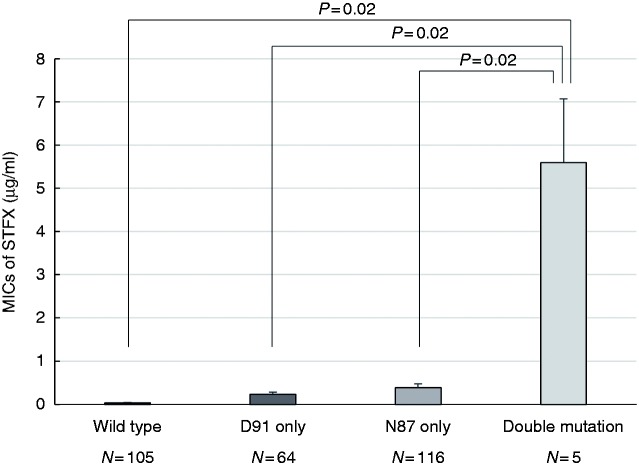
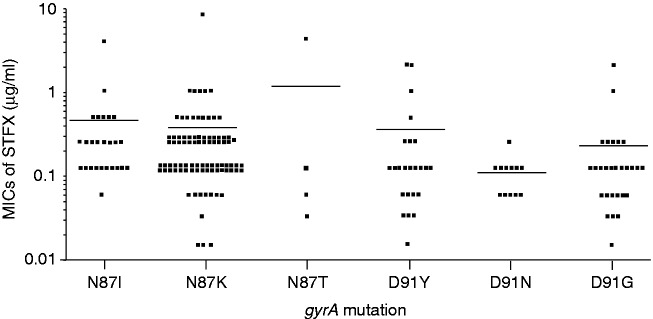
A total of 266 pre-treatment strains and 24 post-treatment failure strains were analyzed. The difference in the average MIC for each gyrA mutation position is shown in Figure 2. The average MIC of the gyrA double-mutant strains was 5.60 µg/ml (95% confidence interval (CI), 1.52–9.68 µg/ml), that of the gyrA-N87 mutant strains was 0.38 µg/ml (95% CI, 0.22–0.55 µg/ml), that of the gyrA-D91 mutant strains was 0.23 µg/ml (95% CI, 0.13–0.34 µg/ml) and that of the wild-type strains was 0.03 µg/ml (95% CI, 0.02–0.05 µg/ml). The MICs of double-mutated strains were significantly higher than those of single (N87 only or D91 only)-mutated strains (double vs. N87 only, p =0.02; double vs. D91 only, p = 0.02). The MICs of the gyrA single mutation subtypes are shown in Figure 3. No significant differences were observed among any subgroups analyzed.
Figure 2.
Comparison of average MICs of STFX in four mutation patterns. The average MICs of STFX were higher in Helicobacter pylori strains with the gyrA double-mutant strains than in those with the gyrA single-mutant strains and the wild-type strains.
MIC: minimum inhibitory concentration; STFX: sitafloxacin.
Student’s t test was used to compare the items.
Figure 3.
The MICs of the gyrA single mutation subtypes. No significant difference were observed among any subgroups analyzed.
MIC: minimum inhibitory concentration. Bars indicate the average levels.
Changes in minimum inhibitory concentrations of STFX and gyrA mutation after treatments
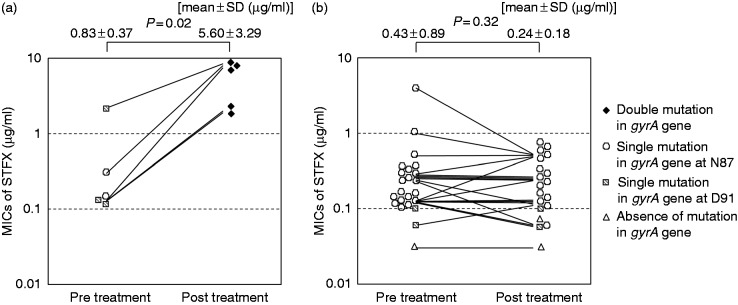
Differences in the MICs of STFX before and after treatment in the patients in whom eradication failed are shown in Figure 4. Five of 24 strains (20.8%) acquired double mutations in gyrA after treatment. The MICs of STFX for the double-mutant strains after treatment were significantly higher than before treatment (5.60 µg/ml vs. 0.83 µg/ml, p = 0.02, Wilcoxon signed-rank test, Figure 3(a)). On the other hand, the MICs of STFX for the single-mutant strains and the strain without a mutation were not significantly increased (0.24 µg/ml vs. 0.43 µg/ml, p = 0.32, Wilcoxon signed-rank test, Figure 3(b)).
Figure 4.
Changes of MICs of STFX and gyrA mutation after treatments. (a) MICs of STFX of the double-mutant strains after treatments were significantly higher than those before treatments. (b) MICs of STFX of the single or absence of mutation after treatments did not increase compared with prior values.
SD: standard deviation; MIC: minimum inhibitory concentration; STFX, sitafloxacin. Wilcoxon signed-rank test was used to compare the items.
Among the strains that acquired double mutations, four were mutated at N87 and D91, whereas the remaining strain was mutated at N87 and A88. The locations of the amino acid substitutions of these five cases were N87I (A260T) and D91Y (G271T), N87K (C261G) and D91Y (G271T), N87Y (A259T) and D91Y (G271T), N87K (C261A) and D91Y (G271T), N87K (C261A) and A88P (G262C) (Supplementary Table 1). In strains with a mutation at position N87 prior to treatment, 11.1% (2/18) acquired a secondary mutation during treatment. Conversely, 60.0% (3/5) of strains with a mutation at position D91 prior to treatment acquired a secondary mutation. Among these, all the double-mutant strains were derived from D91Y-mutated strains. One strain (1.3%) acquired a second mutation among the strains treated with RAS, two strains (2.5%) in EAS and two strains (1.9%) in EMS, respectively. There were no significant differences in the frequency of acquisition of the second mutation between amoxicillin-containing regimens and metronidazole-containing regimens (p = 1.00). And, there were no significant differences between rabeprazole-containing regimens and esomeprazole-containing regimens (p = 1.00).
Discussion
Our results clearly revealed that the acquisition of double mutations in gyrA in H. pylori led to increased resistance to STFX. In contrast, strains in which the position of the mutation did not change during treatment did not acquire increased resistance to STFX. Although there are few reports of acquired double mutations in gyrA, Tankovic et al. reported two strains with double mutations in gyrA that were highly resistant to fluoroquinolones.15 Our study showed that STFX-containing regimens might lead to the accumulation of double mutations in gyrA with a certain degree of probability. Since both N87 and D91 are binding sites for fluoroquinolones,8 it is reasonable that the N87 and D91 double mutation would provide stronger resistance to STFX than either mutation alone. In addition, we showed that a novel double-mutant strain (N87 and A88) was resistant to a high concentration of STFX.
We previously showed that the eradication rates of N87-mutated strains were lower than those of D91-mutated strains,5 and this finding was confirmed in this study (Table 1). The present study indicates that N87-mutated strains, however, generally do not acquire a secondary mutation after treatment failure. In contrast, the D91-mutated strains were more likely to gain resistance to fluoroquinolones via the acquisition of a secondary mutation in gyrA. Comparing the D91-mutated strains, the eradication rates of D91Y-mutants (66%–70%) were lower than those of D91N and D91G mutants (80%–100%).4,5 In this study, three of four (75%) D91Y-mutated strains acquired a double mutation during failed STFX-containing therapy. Hence, careful consideration is needed before prescribing STFX-containing regimens for the eradication of D91Y-mutated H. pylori strains. If other regimens that do not contain STFX are available, such regimens should be prioritized in these cases. Furthermore, gyrA mutation status should be evaluated before prescribing STFX-containing regimens.
Stool analysis can provide a noninvasive means to obtain H. pylori DNA,16 thus the position of the gyrA mutation can be accessed more easily and cost-effectively than the methods for assessment of drug susceptibility using gastric biopsy specimens. Although it is clear that the acquisition of a second mutation in gyrA causes increased resistance to STFX in this study, the reason some single-mutant strains showed high resistance remains unclear. In H. pylori, gyrA is the only confirmed direct gene target of fluoroquinolones. Regarding mutations in other genes, previous reports showed that fluoroquinolone resistance was not associated with gyrB mutations in H. pylori.5,9,15 The genes encoding topoisomerase IV (parC/parE) mutations are representative resistant mechanisms for fluoroquinolones in several bacteria; however, the absence of genes for topoisomerase IV (parC, parE) in H. pylori has been demonstrated.17 Further studies are needed to clarify fluoroquinolone resistance mechanisms beyond gyrA.
In conclusion, some H. pylori strains treated with regimens containing STFX acquired a double mutation in gyrA, which led to increased resistance to STFX. Although single-mutant strains might be eradicated with fluoroquinolone-based regimens in combination with other drugs, double-mutant strains should be assumed to be difficult to eradicate with fluoroquinolone-containing regimens as a next rescue therapy. To prevent the development of a double mutation in H. pylori gyrA, we should ideally optimize third-line eradication strategies by assessing gyrA mutation status before treatment.
Supplementary Material
Declaration of conflicting interests
During the last two years, H.S. has received scholarship funds for research from AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Tsumura Co., and received service honoraria from Astellas Pharma Inc, AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd., Daiichi-Sankyo Co., Takeda Pharmaceutical Co. Ltd., Mylan EPD Co., and Zeria Pharmaceutical Co. Ltd. T.K. has received scholarship funds for research from Astellas Pharm Inc, AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Eisai Pharmaceutical Co. Ltd., Zeria Pharmaceutical Co. Ltd., Tanabe Mitsubishi Pharmaceutical Co. Ltd., JIMRO Co. Ltd., and Kyorin Pharmaceutical Co. Ltd., and received service honoraria from Astellas Pharm Inc, Eisai Pharmaceutical Co. Ltd., JIMRO Co. Ltd., and Tanabe Mitsubishi Pharmaceutical Co. Ltd. The other authors have nothing to declare.
Ethics approval
The protocol for this study was approved by the ethics committee of the Keio University School of Medicine (No. 20150384; January 29, 2016), and conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in prior approval by the institution’s human research committee.
Funding
This work was supported by a Grant-in-Aid for Young Scientists (B) (26860527, to J.M.), Grant-in-Aid for Scientific Research C (25460301, T.M.) and a Grant-in-Aid for Scientific Research B (16H05291, to H.S.), from the Japan Society for the Promotion of Science (JSPS), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to H.S.), the Princess Takamatsu Cancer Research grants (to H.S.), a grant from Takeda Science Foundation (to J.M.) and Keio Gijuku Academic Development Funds (to J.M., to T.M. and to H.S.).
Informed consent
All participants in this study gave written informed consent for the previous studies, and were given an opportunity to opt out of this study.
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 2.Thung I, Aramin H, Vavinskaya V, et al. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016; 43: 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Nishizawa T, Muraoka H, et al. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob Agents Chemother 2009; 53: 1720–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori H, Suzuki H, Matsuzaki J, et al. Efficacy of 10-day sitafloxacin-containing third-line rescue therapies for Helicobacter pylori strains containing the gyrA mutation. Helicobacter 2016; 21: 286–294. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzaki J, Suzuki H, Nishizawa T, et al. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob Agents Chemother 2012; 56: 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami K, Furuta T, Ando T, et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol 2013; 48: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 7.Moore RA, Beckthold B, Wong S, et al. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob Agents Chemother 1995; 39: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzaki J, Suzuki H, Tsugawa H, et al. Homology model of the DNA gyrase enzyme of Helicobacter pylori, a target of quinolone-based eradication therapy. J Gastroenterol Hepatol 2010; 25(Suppl 1): S7–S10. [DOI] [PubMed] [Google Scholar]
- 9.Miyachi H, Miki I, Aoyama N, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 2006; 11: 243–249. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute, 2012.
- 11.Ge Z, Taylor DE. H. pylori DNA transformation by natural competence and electroporation. Methods Mol Med 1997; 8: 145–152. [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa T, Suzuki H, Kurabayashi K, et al. Gatifloxacin resistance and mutations in gyra after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob Agents Chemother 2006; 50: 1538–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimura S, Kato S, Iinuma K, et al. In vitro activity of fluoroquinolone and the gyrA gene mutation in Helicobacter pylori strains isolated from children. J Med Microbiol 2004; 53(Pt 10): 1019–1022. [DOI] [PubMed] [Google Scholar]
- 14.Mori H, Suzuki H, Matsuzaki J, et al. Antibiotic resistance and gyrA mutation affect the efficacy of 10-day sitafloxacin-metronidazole-esomeprazole therapy for Helicobacter pylori in penicillin allergic patients. United European Gastroenterol J. Epub ahead of print 19 January 2017. DOI: 10.1177/2050640616688995. [DOI] [PMC free article] [PubMed]
- 15.Tankovic J, Lascols C, Sculo Q, et al. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob Agents Chemother 2003; 47: 3942–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan DE, Omorogbe J, Hussey M, et al. Molecular detection of Helicobacter pylori antibiotic resistance in stool vs biopsy samples. World J Gastroenterol 2016; 22: 9214–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambur OH, Davidsen T, Frye SA, et al. Genome dynamics in major bacterial pathogens. FEMS Microbiol Rev 2009; 33: 453–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.