Abstract
There are substantial disparities in the incidence and prognosis of oesophageal cancer across social population groups, including sex, race/ethnicity, geographical location and socio-economic status. Both squamous cell carcinoma and adenocarcinoma of the oesophagus are more common in men than in women, but the male predominance in adenocarcinoma is stronger and less well understood. The varying incidence and prognosis of oesophageal cancer across racial/ethnic groups show distinct patterns by histological type. Individuals residing in rural areas have a higher incidence and worse prognosis than those in urban areas in developing regions. Lower socio-economic status is associated with an increased incidence and reduced survival in oesophageal cancer. Sustained research identifying novel preventive and therapeutic strategies are needed to reduce the risk of oesophageal cancer and improve the prognosis in all social groups.
Keywords: Oesophageal neoplasm, inequalities, gender, race/ethnicity, socioeconomic status
Introduction
Oesophageal cancer is the sixth leading cause of cancer-related deaths globally, causing over 400,000 deaths in 2015.1 The prognosis of oesophageal cancer remains poor with the overall five-year survival rate below 20% in most developed countries.2 The incidence varies remarkably between countries, which is mainly attributable to differences in the prevalence of environmental risk factors and distribution of oesophageal tumour histology across populations. Oesophageal squamous cell carcinoma (OSCC) and adenocarcinoma (OAC) are the two main histological subtypes of oesophageal cancer. OSCC accounts for over 80% of all oesophageal cancer cases globally and its incidence is highest in South-Eastern and Central Asia.3,4 Many Western populations in Europe, Northern America and Oceania have witnessed a rapidly increasing incidence of OAC during the past four decades, while the incidence of OSCC has decreased in these populations during the same period.5,6 Tobacco smoking and alcohol use are the main established risk factors for OSCC in Western populations, but cannot explain the high incidence in other regions where dietary factors may play a greater role.4 Gastro-oesophageal reflux disease and obesity are the two main risk factors for OAC, whereas tobacco smoking is a moderately strong risk factor for this cancer type.5,7 Gastric colonisation with Helicobacter pylori (H. pylori), on the other hand, is associated with a decreased risk of OAC.5,7
In addition to the distinct incidence patterns between countries, oesophageal cancer is also characterised by notable disparities in incidence and prognosis across social groups within populations, i.e. sex, race and ethnicity, geographic location and socio-economic status.4,5 In this review, we evaluate and summarise the available literature examining how social group disparities influence the incidence and prognosis in oesophageal cancer.
Sex differences
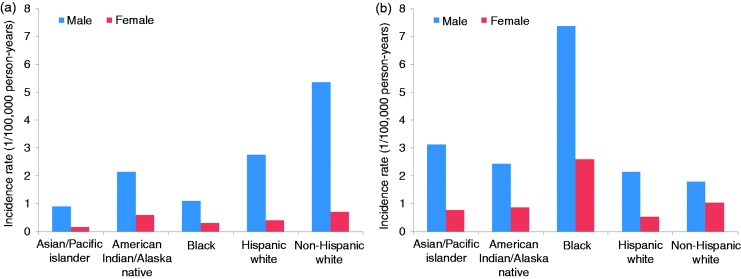
OAC has a striking male predominance in incidence (Figure 1). The male-to-female incidence ratio of OAC is as high as eight-to-one in North America, six-to-one in European and Oceanian countries, and four-to-one in Asia and Latin America and the Caribbean.3,8 The male-to-female ratio has remained fairly stable over time in most populations.8 Barrett’s oesophagus, the premalignant condition of OAC, is also characterised by substantial male predominance, suggesting that the sex disparity may begin early on in the disease pathway.9 The strong male predominance in OAC is not explained by the relatively equal population prevalence of main risk factors or their similarly sized associations with OAC across the sexes.7,10 It has been hypothesised that sex hormones may play a role, i.e. either female sex hormones protecting against OAC and/or male hormones increasing the risk, but existing evidence remains inconsistent and far from conclusive.5,10 Interestingly, a pooled analysis of three population-based case-control studies has revealed a 40% decreased risk of OAC associated with breastfeeding (odds ratio 0.58, 95% confidence interval (CI) 0.37–0.92), and the association was stronger for longer duration of breastfeeding.11 Women who breastfed for over one year had a 60% decreased risk (odds ratio 0.42, 95% CI 0.23–0.77).11 The seemingly protective effect of breastfeeding may be related to long-lasting changes in female sex hormone levels or expression of sex hormone receptors caused by lactation.
Figure 1.
Age-standardised incidence rates of oesophageal adenocarcinoma (a) and oesophageal squamous cell carcinoma (b) by sex and racial/ethnic group in the USA, 1992–2014, using the US standard population in 2000 as the reference (data source: the Surveillance, Epidemiology and End Results Program 13 registries).
OSCC is also more common in men than in women, though the male predominance is weaker compared with that for OAC (Figure 1). The global average of the male-to-female incidence ratio of OSCC is 2.7-to-one, but it varies greatly from 1.2-to-one in Northern Africa and Western Asia to 7.8-to-one in Eastern Europe.3,4 The higher incidence of OSCC in men is at least partly explained by the differential prevalence of heavy alcohol use and tobacco smoking between the sexes.4,10 Evidence regarding the role of sex hormones in OSCC is limited.10
Sex may also influence the prognosis of oesophageal cancer. Female patients have a lower disease-specific mortality compared with males, but this survival advantage seems to be restricted to patients with OSCC and not those with OAC.12,13 The reasons for this potential sex difference are unknown, but differences in heavy alcohol use and smoking among men and women might potentially influence also the prognosis. The reasons for any influence of sex itself on the survival of OAC and the underlying mechanisms are currently unknown.
Racial and ethnic disparities
Oesophageal cancer is characterised by notable disparities across racial/ethnic groups in incidence, with distinctly different patterns by histological type (Figure 1). In the USA, the incidence of OSCC in blacks, despite a steady decrease in recent years, is clearly higher than in other ethnic groups, whereas the incidence in whites is lowest.5,14,15 The incidence of OAC has increased dramatically in whites, particularly in non-Hispanic whites, since the 1970s in the USA. However, the increase seems to have slowed down from the year 2000 onwards.5,15 A recent analysis with multiple disparity measures of the racial and ethnic disparities in the USA in 1992–2013 showed decreased disparities in OSCC incidence over time and seemingly increasing absolute differences of OAC incidence across racial/ethnic groups.15 Some of the disparities by race may be explained by differences in the prevalence of exposure to major risk factors.15 In the UK, the incidence of OAC is also substantially higher in whites compared with black and Asian populations, while the incidence of OSCC is highest in blacks.16
An analysis of cancer risks among immigrants in Sweden showed higher risk of OSCC in Finnish immigrants (standardised incidence ratio (SIR) 1.32, 95% CI 1.06–1.64, for men and 1.90, 95% CI 1.50–2.38 for women) and Iranian women (SIR 3.80, 95% CI 1.23–8.87), compared to the native population in Sweden.17 The increased risk might be attributable to the higher prevalence of risk factors for OSCC, e.g. smoking, alcohol use and a diet low in fruit and vegetables, in these populations.17–20 Compared with native Swedes, increased risks of OAC have been observed in male immigrants from Denmark to Sweden (SIR 1.66, 95% CI 1.08–2.45), which might be due to a higher mean percentage of body fat in Danish men, whereas the risk of OAC was lower in male immigrants from Finland, former Yugoslavia, and Asia, possibly due to lower body mass index.17,21
Earlier studies have suggested a worse prognosis in black patients with oesophageal cancer compared with patients of other ethnic groups.22,23 However, some recent studies have suggested that this difference does not remain after adjustment for socio-economic status.24,25 An earlier study in California in the USA has shown that black patients with oesophageal cancer were less likely to undergo oesophagectomy surgery than whites, and that Asian and Hispanic patients were less likely to undergo such surgery at high-volume hospitals.26 A study of 2946 white patients and 367 black patients aged 65 years or older in the USA found that black patients had lower rates of seeing a surgeon and undergoing resectional surgery.27 The overall two-year survival rate was lower in black patients than in white patients (18% versus 25%), but the difference did not remain after adjustment for treatment characteristics.27 Thus, the ethnic survival disparity is likely to be attributable to differences in healthcare access in black patients compared to patients of other ethnic groups.
Urban–rural disparities
Except for geographical variations across continents and countries, the incidence of oesophageal cancer also shows disparities within countries, particularly when comparing rural and urban areas. An analysis of data from 177 cancer registries across China in 2011 showed at least two-fold increased incidence rates of oesophageal cancer (almost only OSCC) in rural areas compared to urban areas.28 A prospective cohort study of 0.5 m participants from five urban and five rural areas in China reported a substantially higher incidence of oesophageal cancer in rural areas than in urban areas (87 versus 25 per 100,000 person-years in men; 35 versus four in women).29 The higher incidence of oesophageal cancer in rural areas in China suggests a role of risk factors linked with lower socio-economic status, such as higher prevalence of tobacco smoking and heavy alcohol use, and low intake of fruit and vegetables in rural residents of China.29,30 Higher incidence of OSCC in rural areas than in urban areas was also reported in Northern Iran, and patients in rural areas also had shorter median survival time compared with their urban counterparts.31 These disparities might also be related to the exposure to established risk factors.32 In the USA, lower incidence of OSCC was observed in whites residing in rural areas compared with those in metropolitan areas (rate ratio 0.76, 95% CI 0.64–0.88), whereas the incidence was instead higher in blacks in rural areas than those in metropolitan areas (rate ratio 1.60, 95% CI 1.24–2.04).33 Such observations might be explained by a lower prevalence of alcohol use in blacks and a higher prevalence in whites in rural areas.34 On the other hand, the incidence of OAC was slightly higher in rural areas (rate ratio 1.15, 95% CI 1.05–1.25), which might be due to the higher prevalence of obesity in rural areas.35 A nationwide Swedish case-control study found an increased risk of OSCC associated with residing in rural areas during childhood, whereas no such association was found for OAC.36
The mortality rates of oesophageal cancer in rural areas were clearly higher than in urban areas in China.28 However, an analysis of 12,482 patients with oesophageal cancer (mainly OSCC) in Taiwan found similar survival in patients who lived in areas with different urbanisation levels.37 No difference was observed in the prognosis between OAC patients in metropolitan and rural areas in the USA.33
Overall, the urban–rural disparity in oesophageal cancer in the developed world seems lower than in less developed regions, which might be partially due to differences in the distribution of the exposure to environmental risk factors.
Socio-economic disparities
Lower socio-economic status has been consistently reported to increase the risk of OSCC in studies from different parts of the world, including China and other Asian countries,30,38–40 the USA,41 and Europe.16,36,42,43 However, most studies using area-based indicators of socio-economic status did not find any association with the risk of OAC.16,44,45 A cohort study of one million Israeli men suggested no association between socio-economic status determined by the area of residence and OAC risk, whereas higher education level decreased the risk of this cancer.45 An earlier population-based case-control study from the USA found a lower risk of OAC in individuals with higher education or income.41 Another population-based case-control study in Sweden also revealed an increased risk of OAC associated with lower socio-economic status as indicated by occupational histories; the risk in unskilled workers was 3.7 (95% CI 1.7–7.7) times higher compared with age- and sex-matched professionals.36 A recent nationwide study in Sweden found that lower education attainment and lower income were associated with an increased risk of OAC.43 Overall, the risk of OAC seems to be dependent on individuals’ socio-economic status levels, whereas no such association has been established when only the area of residence is used to assess socio-economic status.
The influence of socio-economic status on survival after oesophageal cancer varies across populations. Lower socio-economic status, when defined by the neighbourhood, has been associated with higher 30-day (odds ratio 1.37, 95% CI 1.03–1.85) and 90-day (1.30, 95% CI 1.04–1.63) mortality in patients who had undergone oesophagectomy in the UK.46 However, a few other studies in Western populations found no association between socio-economic status measured by the area of residence and mortality after diagnosis of oesophageal cancer or oesophagectomy.47,48 An analysis of 12,482 patients with oesophageal cancer diagnosed from 1998–2007 in Taiwan suggested higher mortality rates in patients with lower income.37 A recent study in 4097 patients younger than 50 years in the same population (Taiwan) showed that higher individual socio-economic status, as indicated by occupational category, was associated with a decreased five-year mortality (odds ratio 0.61, 95% CI 0.47–0.77, highest versus lowest categories).49 As tumour stage at diagnosis is the strongest prognostic factor for oesophageal cancer, the observed survival disadvantages in patients with low socio-economic status may be due to delayed diagnosis and treatment and less access to curative treatment.50 The lack of association between socio-economic status and survival in some previous studies may be explained by less marked socio-economic disparities in healthcare access in the study population, adopted socio-economic status indicators (neighbourhood rather than individual socio-economic status), socio-economic gradient, or chance. It is also possible that the prognosis in oesophageal cancer is similar in patients with various socio-economic status levels as long as they actually receive equal treatment and have the same opportunities for early detection to improve prognosis.
Summary
This review highlights the substantial social disparities in the incidence and prognosis in oesophageal cancer. Oesophageal cancer is characterised by a male predominance in incidence, which is especially strong for OAC, and the prognosis in OSCC might be better in women than men. The incidence and prognosis in oesophageal cancer vary across racial/ethnic groups, and the variations are partly dependent on the histological type of oesophageal cancer. Higher incidence and worse prognosis has been noted in rural areas compared with urban areas in developing regions with high incidence of OSCC. Lower socio-economic status is associated with an increased risk of both OSCC and OAC. The association between socio-economic status and prognosis in oesophageal cancer varies across populations.
Eliminating health disparities is a major overarching goal in public health. It is critical to consider the great social disparities in oesophageal cancer in order to optimise prevention and treatment. First, a continuous monitoring of the social disparities in oesophageal cancer is needed. Second, as the social disparities in the incidence of oesophageal cancer are not fully explained by known risk factors, more aetiological studies examining such disparities are needed. Third, more attention should be paid to disadvantaged social groups in terms of the risk of oesophageal cancer when planning for targeted screening programs. Fourth, more in-depth research is needed to verify how socio-economic disparities influence the prognosis of oesophageal cancer in different populations and to uncover the underlying reasons for these disparities. All individuals, independent of social groups, should have the same opportunities for timely detection of oesophageal cancer because it determines the prognosis, and all patients should have equal access to high-quality medical care and support. Thus, sustained research, prevention, and treatment efforts are needed to enable a reduction in the risk of oesophageal cancer and an improvement in the prognosis in all social groups.
Declaration of conflicting interests
None declared.
Ethics approval
Not applicable.
Funding
This work was supported by the United European Gastroenterology (UEG) Research Prize 2017 to JL; Swedish Research Council (grant number 521-2014-2536); and Swedish Cancer Society (grant number CAN 2015/460).
Informed consent
Not applicable.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017; 3: 524–548. [DOI] [PMC free article] [PubMed]
- 2. Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet. Epub ahead of print 22 June 2017. DOI: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed]
- 3.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015; 64: 381–387. [DOI] [PubMed] [Google Scholar]
- 4.Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. Epub ahead of print 17 August 2017. DOI: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman HG, Xie SH and Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology. Epub ahead of print 2 August 2017. DOI: 10.1053/j.gastro.2017.07.046. [DOI] [PubMed]
- 6.Xie SH, Mattsson F, Lagergren J. Incidence trends in oesophageal cancer by histological type: An updated analysis in Sweden. Cancer Epidemiol 2017; 47: 114–117. [DOI] [PubMed] [Google Scholar]
- 7.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin 2013; 63: 232–248. [DOI] [PubMed] [Google Scholar]
- 8.Xie SH, Lagergren J. A global assessment of the male predominance in esophageal adenocarcinoma. Oncotarget 2016; 7: 38876–38883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol 2005; 162: 1050–1061. [DOI] [PubMed] [Google Scholar]
- 10.Xie SH, Lagergren J. The male predominance in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2016; 14: 338–347. [DOI] [PubMed] [Google Scholar]
- 11.Cronin-Fenton DP, Murray LJ, Whiteman DC, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: A pooled analysis. Eur J Cancer 2010; 46: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie SH, Wahlin K, Lagergren J. Cause of death in patients diagnosed with esophageal cancer in Sweden: A population-based study. Oncotarget 2017; 8: 51800–51809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012; 30: 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez L, Magno P, Ortiz AP, et al. Esophageal cancer incidence rates by histological type and overall: Puerto Rico versus the United States Surveillance, Epidemiology, and End Results population, 1992–2005. Cancer Epidemiol 2013; 37: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie SH, Rabbani S, Petrick J, et al. Racial and ethnic disparities in the incidence of esophageal cancer in the United States, 1992–2013. Am J Epidemiol 2017; 186: 1341–1351. [DOI] [PMC free article] [PubMed]
- 16.Cooper SC, Day R, Brooks C, et al. The influence of deprivation and ethnicity on the incidence of esophageal cancer in England. Cancer Causes Control 2009; 20: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 17.Mousavi SM, Hemminki K. Cancer incidence, trends, and survival among immigrants to Sweden: A population-based study. Eur J Cancer Prev 2015; 24: S1–S63. [DOI] [PubMed] [Google Scholar]
- 18.Gadd M, Sundquist J, Johansson SE, et al. Do immigrants have an increased prevalence of unhealthy behaviours and risk factors for coronary heart disease? Eur J Cardiovasc Prev Rehabil 2005; 12: 535–541. [DOI] [PubMed] [Google Scholar]
- 19.Leao TS, Johansson LM, Sundquist K. Hospitalization due to alcohol and drug abuse in first- and second-generation immigrants: A follow-up study in Sweden. Subst Use Misuse 2006; 41: 283–296. [DOI] [PubMed] [Google Scholar]
- 20.Westman J, Martelin T, Harkanen T, et al. Migration and self-rated health: A comparison between Finns living in Sweden and Finns living in Finland. Scand J Public Health 2008; 36: 698–705. [DOI] [PubMed] [Google Scholar]
- 21.Lahmann PH, Lissner L, Gullberg B, et al. Differences in body fat and central adiposity between Swedes and European immigrants: The Malmo Diet and Cancer Study. Obes Res 2000; 8: 620–631. [DOI] [PubMed] [Google Scholar]
- 22.Taioli E, Wolf AS, Camacho-Rivera M, et al. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol 2016; 113: 659–664. [DOI] [PubMed] [Google Scholar]
- 23.Kim A, Ashman P, Ward-Peterson M, et al. Racial disparities in cancer-related survival in patients with squamous cell carcinoma of the esophagus in the US between 1973 and 2013. PLoS One 2017; 12: e0183782–e0183782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erhunmwunsee L, Gulack BC, Rushing C, et al. Socioeconomic status, not race, is associated with reduced survival in esophagectomy patients. Ann Thorac Surg 2017; 104: 234–244. [DOI] [PubMed] [Google Scholar]
- 25.Tran PN, Taylor TH, Klempner SJ, et al. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: A population-based analysis. J Carcinog 2017; 16: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA 2006; 296: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 27.Ashktorab H, Kupfer SS, Brim H, et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017; 153: 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer 2016; 7: 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan R, Zhu M, Yu C, et al. Cancer incidence and mortality: A cohort study in China, 2008–2013. Int J Cancer 2017; 141: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 30.Xibib S, Meilan H, Moller H, et al. Risk factors for oesophageal cancer in Linzhou, China: A case-control study. Asian Pac J Cancer Prev 2003; 4: 119–124. [PubMed] [Google Scholar]
- 31.Amani F, Ahari SS, Akhghari L. Epidemiology of esophageal cancer in ardabil province during 2003–2011. Asian Pac J Cancer Prev 2013; 14: 4177–4180. [DOI] [PubMed] [Google Scholar]
- 32.Golalipour G, Semnani S, Safaie B, et al. Predictors of survival in oesophageal cancer patients in a high-risk area in Northern Iran: The role of health services utilisation. Eur J Cancer Care (Engl) 2017; 26: e12549–e12549. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Goodman M, Saba N, et al. Incidence and prognosis of gastroesophageal cancer in rural, urban, and metropolitan areas of the United States. Cancer 2013; 119: 4020–4027. [DOI] [PubMed] [Google Scholar]
- 34.Dixon MA, Chartier KG. Alcohol use patterns among urban and rural residents: Demographic and social influences. Alcohol Res 2016; 38: 69–77. [PMC free article] [PubMed] [Google Scholar]
- 35.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005–2008). J Rural Health 2012; 28: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansson C, Johansson AL, Nyren O, et al. Socioeconomic factors and risk of esophageal adenocarcinoma: A nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev 2005; 14: 1754–1761. [DOI] [PubMed] [Google Scholar]
- 37.Chen MF, Yang YH, Lai CH, et al. Outcome of patients with esophageal cancer: A nationwide analysis. Ann Surg Oncol 2013; 20: 3023–3030. [DOI] [PubMed] [Google Scholar]
- 38.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005; 113: 456–463. [DOI] [PubMed] [Google Scholar]
- 39.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: Results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009; 38: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dar NA, Shah IA, Bhat GA, et al. Socioeconomic status and esophageal squamous cell carcinoma risk in Kashmir, India. Cancer Sci 2013; 104: 1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1997; 89: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 42.Sharpe KH, McMahon AD, McClements P, et al. Socioeconomic inequalities in incidence of lung and upper aero-digestive tract cancer by age, tumour subtype and sex: A population-based study in Scotland (2000-2007). Cancer Epidemiol 2012; 36: e164–e170. [DOI] [PubMed] [Google Scholar]
- 43.Lagergren J, Andersson G, Talback M, et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer 2016; 122: 207–212. [DOI] [PubMed] [Google Scholar]
- 44.Brewster DH, Fraser LA, McKinney PA, et al. Socioeconomic status and risk of adenocarcinoma of the oesophagus and cancer of the gastric cardia in Scotland. Br J Cancer 2000; 83: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi Z, Kark JD, Shamiss A, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer 2013; 119: 4086–4093. [DOI] [PubMed] [Google Scholar]
- 46.Leigh Y, Seagroatt V, Goldacre M, et al. Impact of socio-economic deprivation on death rates after surgery for upper gastrointestinal tract cancer. Br J Cancer 2006; 95: 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan MA, Lewis WG, Chan DS, et al. Influence of socio-economic deprivation on outcomes for patients diagnosed with oesophageal cancer. Scand J Gastroenterol 2007; 42: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 48.Stavrou EP, McElroy HJ, Baker DF, et al. Adenocarcinoma of the oesophagus: Incidence and survival rates in New South Wales, 1972–2005. Med J Aust 2009; 191: 310–314. [DOI] [PubMed] [Google Scholar]
- 49.Wu CC, Chang CM, Hsu TW, et al. The effect of individual and neighborhood socioeconomic status on esophageal cancer survival in working-age patients in Taiwan. Medicine (Baltimore) 2016; 95: e4140–e4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bus P, Aarts MJ, Lemmens VE, et al. The effect of socioeconomic status on staging and treatment decisions in esophageal cancer. J Clin Gastroenterol 2012; 46: 833–839. [DOI] [PubMed] [Google Scholar]