Abstract
Glycogen, the primary storage form of glucose, is a rapid and accessible form of energy that can be supplied to tissues on demand. Each glycogen granule, or “glycosome,” is considered an independent metabolic unit composed of a highly branched polysaccharide and various proteins involved in its metabolism. In this Minireview, we review the literature to follow the dynamic life of a glycogen granule in a multicompartmentalized system, i.e. the cell, and how and where glycogen granules appear and the factors governing its degradation. A better understanding of the importance of cellular compartmentalization as a regulator of glycogen metabolism is needed to unravel its role in brain energetics.
Keywords: actin, carbohydrate, carbohydrate biosynthesis, cell compartmentalization, endoplasmic reticulum (ER), glycogen, protein complex, sarcoplasmic reticulum (SR), glycogenolysis
Introduction
Glycogen granules and their metabolic regulation differ widely between tissues, cell types, and even between intracellular compartments. Although the biochemical pathways of glycogen synthesis and degradation are similar between tissues, the enzymes involved and their regulation are uniquely adapted to the metabolic demands of each cell type (Table 1).
Table 1.
Overview of differences in glycogen levels and metabolism in brain, skeletal muscle, and liver
Specialization of different tissues and cells types has led to the diversification of glycogen metabolism regulation. An overview of the main differences in glycogen content, inter- and intracellular localization, and glycogen-related regulatory enzyme expression in brain, muscle, and liver are presented.
| Attributes | Brain | Skeletal muscle | Liver |
|---|---|---|---|
| Average particle size (inner diameter, nm) | 10–30 | 10–40 | 110–290 |
| Glycogen concentration (human, μmol/g wet weight) | 3–10 | 30–100 | 100–500 |
| Estimated % of tissue weight | 0.1 | 1–2 | 6–8 |
| Estimated whole organ content (human, fed state, g) | 0.5–1.5 | 100 | 400 |
| Tissue localization | Regional variability. Gray matter > white matter | Muscle type-dependent. Type II > type I | Uniform |
| Cellular/subcellular localization | Cell-dependent, highest in astrocytes. Greater in areas with high synaptic density, primary branches and fine processes | Subsarcolemmal > myofibrillar | Hepatocytes. subcellular location modulated by metabolic conditions |
| Glycogenin isoform | GN1 | GN1 | GN2 |
| Glycogen phosphorylase isoform | GPB | GPM | PGPL |
| GPM | |||
| Glycogen synthase isoform | GS1 | GS1 | GS2 |
Each glycogen granule contains carbohydrate with a diverse complement of associated regulatory proteins, considered together as an organelle-like structure or “glycosome” (1). This Minireview is designed to summarize and integrate the existing physiology, cell biology and biochemical literature to propose a generalized model for the dynamic “life” of a glycogen granule or glycosome. Although this series of Minireviews is focused on the brain, a great deal of the understanding of the granule comes from investigations of muscle glycogen. Unless specified otherwise, the information in this Minireview originates from skeletal muscle; however, the overriding principles should apply to the brain as well.
The glycosome
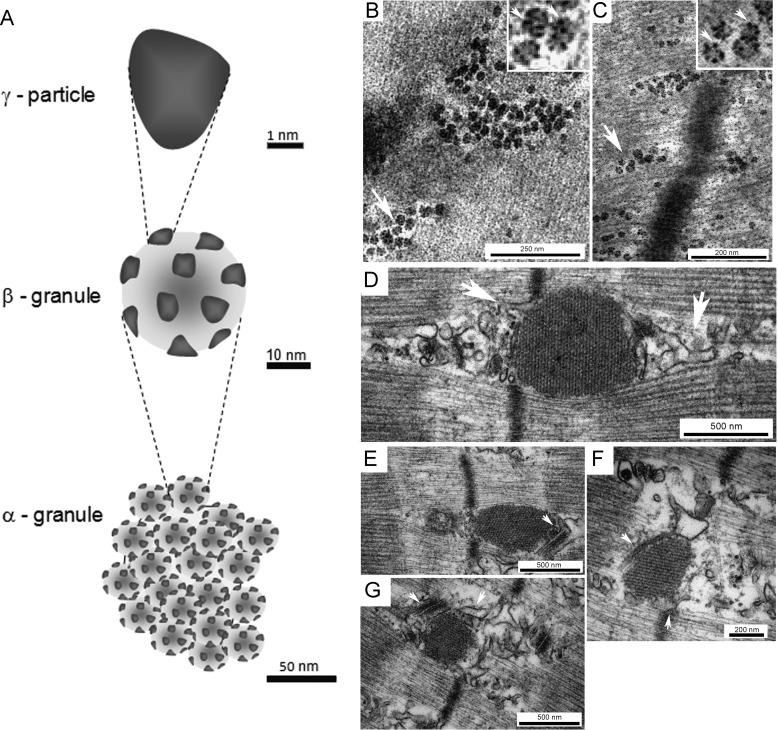
Glycogen granules are composed of protein-glycogen (2). Three types of glycogen structures have been identified by EM and termed α- and β-granules and γ-particles (1, 3) (Fig. 1A). The α-granules are mainly found in liver and are formed by several β-granules arranged in a broccoli-like fashion. The β-granules are individual glycogen granules, which include several γ-particles, 3 nm protein-rich subunits that are highly electrodense after staining with lead and uranyl acetate (4) (Fig. 1, B and C). The β-granules are considered a rapid energy source and are ∼20–30 nm in diameter and ∼106–107 in molecular weight, whereas the liver α-granules are considered a slower energy source (5) and can be as large as 300 nm in diameter and of >108 in molecular weight (1, 3). The molecular structure of a β-granule includes a central priming protein, glycogenin (GN)3 (6), covalently bound to the glucose polymer, which is formed by chains of ∼13 glucose residues bound through α-1,4-glycosidic linkages and interconnected by α-1,6-glycosidic linkages at branching points (7).
Figure 1.
Glycogen granule and the actin-rich spherical structures: EM observations. Analysis of glycogen granules by transmission EM has led to the identification of three structural entities: the γ-particle, the β- granule, and the α-granule (A). The γ-particle is highly electrodense after lead and uranyl acetate staining visualized as black clusters in the magnified arrow-marked glycogen granules (top-right corner) (B and C). The β- granule includes the carbohydrate polymer and the bound γ-particles, and the α-granule is composed of several β-granules bound via a protein backbone rich in disulfide bonds. At the start of glycogen re-synthesis after severe storage depletion, actin-rich spherical structures form (37), which in skeletal muscle are visualized by transmission EM as electrodense structures at the I-band of the sarcomeres in close proximity to the sarcoplasmic reticulum and transverse tubuli (D–G, white arrows). Scale bars, B, 250 nm; C and F, 200 nm; and D, E, and G, 500 nm.
According to the tiered model (8) for glycogen organization, β-granules are organized as concentric layers of glucose chains (tiers). The theoretical maximum size for an independent β-granule has been set to 42 nm, i.e. to consist of 12 tiers (9). Because the amount of glucose residues stored doubles in each increasing tier, a hypothetical 13th tier has been estimated to be physically impossible due to spatial constraints (7, 8, 10). In liver, several β-granules can form an α-granule; however, the process by which β-granules aggregate and the nature of the linkage between them remain elusive (11, 12). Based on the observation that GN accounts for 0.0025% of liver glycogen mass, 200-fold lower GN content than muscle glycogen (13), it was initially suggested that one GN molecule could prime the synthesis of several β-granules, forming an α-granule (6). However, the majority of the literature indicates that the β-granules in an α-granule are formed independently. EM studies of mouse liver glycogen during cycles of fasting/feeding show that when liver glycogen reaches a maximum concentration, it consists almost entirely of β-granules, which later form α-granules by binding via a protein backbone (5, 12, 14) capable of forming disulfide bonds (15). Further studies are needed to unravel the sequential processes involved in the initiation and formation of α-granules.
Glycogen fractions according to chemical properties
In addition to GN, many different proteins have been identified as part of the glycogen granule proteome (Table 2). The protein composition and/or the ratio of protein to carbohydrate within a glycogen granule and its association with cellular compartments affect its solubility in acid. As early as 1934, two fractions of glycogen were described according to their solubility in boiling water or cold TCA; the extractable fraction was named lyoglycogen, and the nonextractable fraction was named desmoglycogen (16). Desmoglycogen was reported to include glycogen granules associated with filaments and/or sarcoplasmic reticulum, and lyoglycogen included free unbound granules (17). Cells with a high content of glycogen contained mainly lyoglycogen, whereas the proportion of desmoglycogen increased as cell glycogen was depleted (18). The solubility of glycogen to acid treatment was also used to propose the existence of two distinct populations of glycogen granules, termed pro- and macro-glycogen (19–21). The acid-insoluble form, pro-glycogen, was thought to consist of smaller granules (1–8 tiers), which were the substrate for subsequent addition of carbohydrates, eventually becoming acid-soluble macro-glycogen (granule size >400,000 Da) (21, 22). Subsequently, James et al. (23) reported that alterations in the extraction conditions influenced the proportions of acid-soluble/insoluble glycogen and that these fractions did not correspond to specific granule sizes. Nevertheless, there is clear evidence in the literature of the existence in the cell of acid-labile (desmoglycogen/pro-glycogen) and acid-soluble (lyoglycogen/macro-glycogen) glycosomes, which within this Minireview will be termed acid-soluble and acid-insoluble fractions.
Table 2.
Primary proteins in the glycogen granule proteome and their interactions
List of main human glycosome-associated proteins as identified by name and UNIPROT identifier (ID) as well as primary interactions relevant to glycogen metabolism. Protein–protein interactions were derived from databases within UniProt (uniprot.org) (80), mainly The Biological General Repository for Interaction Datasets (BioGrid) (81) and the Protein Interaction Database and Analysis System (IntAct) (82). Abbreviations used are as follows: AMPK, 5′-AMP-activated protein kinase; EMP2A, laforin; GBE, branching enzyme; GDE, debranching enzyme; GN, glycogenin; GP, glycogen phosphorylase; GS, glycogen synthase; PhK, phosphorylase kinase; PP1, protein phosphatase; STDB1, starch-binding domain-containing protein 1; TRIM7, tripartite motif-containing protein.
| Protein | Role | UniProt ID | Key glycogen-related interactions |
|---|---|---|---|
| Glycogenin (GN) | Initiation | P46976 (GN1, muscle) O15488 (GN2, liver) | GS, AMPK, GBE, GP, STBD1, PP1 (PPP1R3C, PPP1CA, PPP1R5), TRIM7 |
| Tripartite motif-containing protein (TRIM7, GNIP) | Initiation, regulation | Q9C029 (TRIM7) | GN |
| Glycogen synthase (GS) | Synthesis | P13807 (GS1, muscle) P54840 (GS2, liver) | GN, AMPK, GBE, PP1 (PP1R3B, PPP1R3C, PPP1CA), STBD1, KAPCA, CSK21, MAPKAPK2, GSK3, PAST, laforin, MLP3B/3C, DYRK |
| Glycogen branching enzyme (GBE) | Synthesis | Q04446 | GP, GS, GN, STBD1, GBE, VAPA |
| Glycogen phosphorylase (GP) | Degradation | P11217 (PYGM, muscle) P11216 (PYGB, brain) P06737 (PYGL, liver) | AMPK, PKC, GBE1, PP2, MAPKAPK2, PP1 |
| Glycogen debranching enzyme (GDE, AGL) | Degradation | P35573 | AMPK, PP1, malin, AMPK, STBD1 |
| Malin (E3 ubiquitin-protein ligase NHLRC1) | Ubiquitin ligase | Q6VVB1 | Laforin, GS, PP1, GDE, AMPK |
| 5′-AMP-activated protein kinase (AMPK) | Kinase | P54646 (α2) Q9Y478 (β1) O43741 (β2) | Laforin, PP1 (PPP2CA, PPP2R1B), PHKG2, CAMK, GN |
| Laforin (EPM2A) | Carbohydrate phosphatase, ubiquitin ligase | O95278 | AMPK, PP1 (PPP1R3C, PPP1R3D), GSK3B, STBD1, GS, malin, |
| Protein phosphatase I (PP1) and targeting subunits | Phosphatase, main regulatory and catalytic subunits | PP1 | AMPK, laforin, GSK3B, GN, GS |
| Q16821 (PPP1R3A, GM) Q86X16 (PPP1R3B, GL) Q0VCR4 (PPP1R3C, PTG) O95685 (PPP1R3D, PPP1R6) P67775 (PPP2CA) | |||
| P62136 (PPP1CA) P30154 (PPP2R1B) | |||
| Phosphorylase kinase (PhK) | Kinase | P15735 (γ, liver, testis) Q16816 (γ, muscle) | AMPK, PP1 (PPP1R3B), GP |
| Starch-binding domain- containing protein 1 (STBD1) | Cargo receptor for glycogen | O95210 | GDE, GBE, PP1, malin, GN, GS, AMPK, GP, MLP3B/3C |
The birth of a new glycogen granule
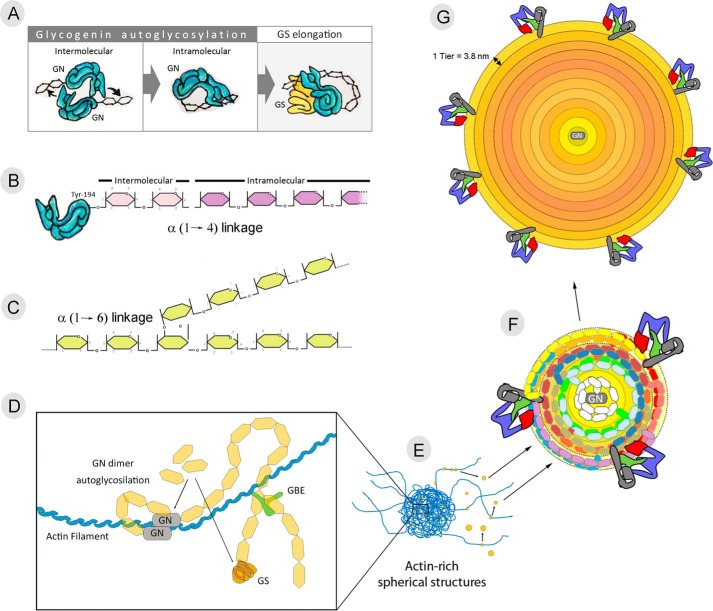
Biosynthesis of a new glycogen granule is initiated by GN dimerization and autoglycosylation (Fig. 2A). GN autoglycosylation occurs in two sequential steps: initial intermolecular glycosylation and subsequent intramolecular glucose chain lengthening (Fig. 2A) (24, 25). During intermolecular glycosylation, GN catalyzes the transfer of glucose from UDP-glucose to Tyr-194 of a separate GN molecule, forming a 1-O-tyrosyl linkage (Fig. 2B) (24, 25). GN then catalyzes the addition of 6–17 additional glucose residues onto the O-linked glucose, forming α-1,4-glucosidic linkages (25). Dimerization of the proteins is weak, potentially allowing the units to separate after the initial intermolecular glycosylation reaction (26). GN binds to a conserved amino acid sequence in the C-terminal domain of glycogen synthase (GS) (27, 28). In skeletal muscle, GS and GN are expressed in equimolar amounts, suggesting an average of one GS molecule associated with each glycogen granule in vivo (28). As the glycogen granule grows, GS unbinds from GN and binds the polysaccharide through a glycogen-binding site at its C terminus (6), adding new glucosyl residues to the outer polysaccharide chains. Once the initial chains are formed, the glycogen-branching enzyme (GBE) cuts the distal end of the newly-formed chain and attaches it to a glucose residue from the older existing chain through an α-1,6 linkage (Fig. 2C) (29). The coordinated action of GS and GBE create the spherical and branched structure of the granule, which ensures solubility and creates a hydrophilic surface necessary to reduce the osmotic pressure exerted by each granule (30, 31).
Figure 2.
Life of a glycogen granule: birth to maturity. The start of a newly synthesized glycogen granule results from the coordinated dimerization and autoglycosylation of GN (A). GN glycosylation is believe to occur in the following two steps: intermolecular glycosylation as the transfer of 1–2 glucose units to tyrosine 194 (Tyr-194) from the other GN, and subsequent intramolecular glycosylation resulting in the elongation of the primer chain by the transfer of 7–16 glucose residues (B). Further elongation of the primer chain involves the coordinated action of glycogen synthase (GS) and glycogen-branching enzyme (GBE), adding glucose residues to the granule through α-1,4-linkage (B) and α-1,6-linkage (C), respectively. GN binds to actin filaments for the start of glycogen synthesis (D), and in situations of severe low glycogen levels, the formation of spherical actin-rich cellular structures (E) in which glycogen re-synthesis machinery gathers has been reported in several cell types. As glycogen granules mature, it may be released to the cytosol as an unbound acid-soluble glycogen granule (F), less metabolically active and ranging in size from 20 to 30 nm (G).
The question arises as to where and when new glycogen granules are initiated and how this process is regulated. Accumulating evidence in the literature indicates that the start of each glycogen granule is associated with the actin cytoskeleton, with actin binding a conserved DNIKKKL C-terminal domain of GN. Disruption of the actin cytoskeleton by cytochalasin D leads to granule dispersion (32), explaining previous observations of alignments of glycogen granules associated with cytosolic filaments (1). Interestingly, several independent studies have reported that the initial stages of glycogen re-synthesis after episodes of low glycogen levels are linked to cytosolic actin-rich spherical structures. Cid et al. (33) reported GS intranuclear localization in cultured human muscle and in 3T3-L1 cells under low glucose conditions and GS translocation to cytoplasmic spherical structures upon glucose administration. In response to glucose administration after glycogen depletion, similar structures have been reported in different cell types, among them Saccharomyces cerevisiae (34), 3T3-L1 adipocytes (35), hepatocytes (36), and rabbit and human skeletal muscle (37, 38). A combination of light and EM was used to characterize the spherical structures resulting from dynamic actin cytoskeleton rearrangement in low glycogen conditions, a process that required 1.5 h and was a prerequisite for glycogen re-synthesis to start (37). These observations are in agreement with previous studies reporting that glycogenolysis results in the release of dissociated GN and GS to the cytoplasmic fraction; in vitro, 50% re-association of these two proteins took hours (28). Altogether, the start of new glycogen granules is associated with and regulated by the actin cytoskeleton; however, further studies are needed to understand the regulation and dynamics of the molecular pathways involved.
Glycogen storage (granule size versus number)
Changes in cell glycogen content may respond to changes in the size and/or number of granules. Given that the amount of carbohydrate storage increases exponentially with each tier, one might assume that to maximize storage efficiency granules would grow to their maximal size (∼42 nm). Remarkably, most investigations of granule size in brain, liver, skeletal, and cardiac muscle have consistently reported a continuum of sizes, ranging from 10 to 44 nm in diameter, with an average granule diameter of roughly 25 nm (5, 39). Thus, most of the glycogen granules contain only about 20% of the theoretical maximum amount of carbohydrate that they could store. In agreement, Elsner et al. (40) determined granule size in cultured myotubes before and after insulin stimulation, and they showed that only 33% of the insulin-stimulated glucose uptake was used to increase average granule size (from 24.9 to 28.1 nm in diameter). Similarly, labeled glucose experiments in liver showed that granules that were synthesized early after glucose administration stopped growing, and other granules were then formed, rather than enlarging the previously formed granules to their maximal capacity (41). In the brains of mice, Oe et al. (42) have shown that glycogen granules are mainly localized in the primary branches and fine processes of cortical and hippocampal astrocytes (Table 1) and that the presence of large glycogen granules (∼15 MDa (43)) is unique to glycogen-rich areas. Altogether, the growth of glycogen granules seems to be coordinated and limited in some manner, so that glycogen granules grow only modestly in size, rarely reaching their theoretical maximal capacity.
Another potential strategy for cells to up-regulate glycogen storage capacity could be to increase GN protein expression (6). 90% of GN has been reported to be isolated with the glycogen fraction in skeletal muscle (28), which suggests that the reservoir of un-primed GN available may be a limiting factor for cell glycogen storage capacity; however, that does not turn out to be the case, because overexpression of GN in COS-1 cells and rat fibroblasts with unlimited glucose supply had no effect on total cellular glycogen content. Not surprisingly, the overexpression did lead to an increased number of smaller glycogen granules (44, 45), but with no effect on the overall storage capacity. Consistent with the above observations, training-induced increases in glycogen storage in rodents showed no significant correlation with GN protein levels or activity (46, 47). Supporting the existence of coordinated mechanisms regulating the number and size of glycogen granules, Montori-Grau et al. (48) showed that the overexpression of different protein phosphatase 1 (PP1) glycogen targeting subunits in cultured myotubes resulted in distinct alterations in both granule numbers and size. Overexpression of any of the targeting subunits resulted in more granules: overexpression of PPP1R6 led to smaller average granule size (∼14 nm), but overexpression of PPP1R3C/PTG increased the average granule size (to ∼37 nm), resulting in 1.4- and 12-fold increases in glycogen content, respectively. Even though the molecular mechanisms regulating glycogen granule size and number remain elusive, a strong inverse correlation between GS activation and glycogen levels has repeatedly been reported, suggesting that glycogen regulates its own synthesis, perhaps through GS substrate inhibition.
Phosphorylation and granule growth
Although there have been reports of glycogen-containing phosphate groups (20), these were originally clouded with questions of contamination of the samples, etc. The identification of the glycogen phosphatase laforin and its mutation as a key factor in Lafora disease reaffirmed the relevance of glycogen phosphorylation in the regulation of glycogen metabolism. In skeletal muscle, glycogen granules contain approximately one phosphate per ∼650 glucosyl units in rabbit and one phosphate per ∼1500 glucosyl units in the mouse (49, 50), and these are found throughout the granule. Recently, Turnbull et al. (51) suggested that the hydrophilic phosphoryl groups could unfold the branch chains, exposing hydrophobic regions in a similar way as in starch. Laforin dephosphorylates glycogen, and it has been speculated that this dephosphorylation facilitates normal branching of glycogen during synthesis, allowing the granule to remain soluble (50). The origin and role of the reversible phosphorylation of glycogen remain unclear, as reviewed in the accompanying Minireview by Gentry et al. (52). Even though the role of glycogen phosphorylation remains unclear, it should be noted that two starch kinases have been identified, raising the question of whether similar glycogen kinase(s) exist. A quality control role for glycogen phosphorylation has also been suggested (53), according to which a glycogen granule accumulates phosphate throughout its lifetime, becomes less soluble, and is eventually targeted for lysosomal disposal (Fig. 3D). This idea is supported by observations in laforin-KO mice, whose glycogen has ∼4–6-fold higher phosphate content than the WT, reduced solubility, and forms characteristic Lafora bodies (49, 50). Independently of whether glycogen phosphorylation represents an enzymatic side-reaction error or is part of a regulated biochemical mechanism, there is no doubt that it has significant effects on glycogen metabolism.
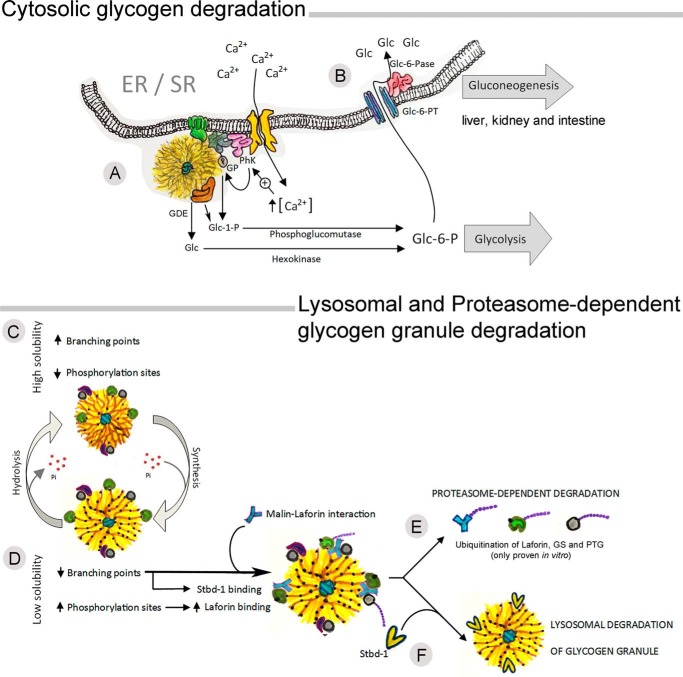
Figure 3.
Life of a glycogen granule: partial and complete degradation. Glycogen granules can be utilized by cytosolic degradation or by lysosomal degradation. Cytosolic glycogenolysis has been associated with the endoplasmic and sarcoplasmic reticulum, where the glycogenolytic complex, formed by glycogen phosphorylase (GP) and phosphorylase kinase (PhK), links glycogen utilization with calcium-ATPase (A). The coordinated action of GP and glycogen-debranching enzyme (GDE) results in the release of glucose 1-phosphate (Glc-1-P) and glucose (Glc), which are converted to glucose 6-phosphate (Glc-6-P) by phosphoglucomutase and hexokinase, respectively. Glc-6-P will either be used as substrate for glycolysis or, in gluconeogenic tissues, will enter the ER/SR through a glucose 6-phosphate transporter (Glc-6-PT) and converted to Glc by glucose-6-phosphatase (Glc-6-Pase) (B). The mechanisms by which glycogen granules are tagged for lysosomal degradation remain elusive; however, evidence indicates that phosphorylation of glycogen granules may play a role. Increased glycogen phosphorylation has been associated with increased branching points and solubility (C), whereas increases in glycogen phosphorylation are associated with lower branching degree and solubility (D). Binding and phosphorylation of laforin leads to malin recruitment, which could result in ubiquitination of glycogen-bound proteins directing them toward proteasome degradation (E). However, the starch binding domain 1 (Stbd-1) will bind to less branched glycogen granules tagging them for lysosomal degradation (F).
Utilization of a glycogen granule and the fate of glycogen-bound proteins
Glycogen granules can be utilized by two pathways: 1) cytosolic degradation by the coordinated action of glycogen phosphorylase (GP) and glycogen-debranching enzyme (GDE) (Fig. 3A), and 2) lysosomal degradation by the action of α-glucosidase (Fig. 3D) (54).
Cytosolic degradation is mediated by the rate-limiting enzyme GP, which cleaves a terminal glucose residue bound to a glycogen branch by substitution of a phosphoryl group for the α-1,4 linkage. Four residues before a branching (α-1,6-linked glucosyl residue), the GDE catalyzes the transfer of three of the remaining four glucose units to the end of another glycogen chain, where they can be degraded by GP. The exposed α-1,6 branching point is then hydrolyzed by a second catalytic domain of GCE, releasing a molecule of glucose and eliminating a branch point. Thereafter, The exposed α-1,6-branching point is hydrolyzed by a second catalytic domain of GDE, releasing a molecule of glucose and eliminating the branch point. Thus, the degradation of a glycogen granule results in the release of Glc-1-P and glucose (Fig. 3A). Glc-1-P is converted to glucose 6-phosphate (Glc-6-P) by phosphoglucomutase, and glucose is phosphorylated to Glc-6-P by hexokinase. In most tissues, the resulting Glc-6-P is used internally to feed glycolysis flux; however, in gluconeogenic tissues, such as the liver, kidney, and intestine, endogenous Glc-6-P can be transported into the ER lumen, where it is dephosphorylated to glucose and secreted by the cell to the interstitial space (Fig. 3B).
In skeletal muscle, glycogen and glycogenolysis have been associated with the sarcoplasmic reticulum (SR). A high proportion of total GP and phosphorylase kinase (PhK) activities, 39 and 53% respectively, were recovered associated with purified SR vesicles (55), forming part of the ER–SR glycogenolytic complex, linking glycogenolysis to the SR calcium (Ca2+)-ATPase. Highlighting the compartmentalized nature of the complex, lowering extravesicular Ca2+ concentration (56) or inhibiting GDE (57) in vitro decreased the phosphorylation of only SR-bound GP, with no effect on the phosphorylation state of unbound GP. The coordination of GP activation with SR Ca2+-flux in muscle allows for rapid GP activation at the onset of muscle contraction. A similar link between glycogenolysis regulation and the endoplasmic reticulum Ca2+-ATPase has been reported in primary cultures of murine cerebellar and cortical astrocytes. By stimulating store-operated Ca2+ entry or blocking glycogenolysis, Müller et al. (58) showed that ER Ca2+-uptake triggers astrocytic glycogenolysis in a cAMP-dependent manner. The existence of a compartmentalized cellular structure regulating GP activity, the glycogenolytic complex, can explain accumulating observations in the literature reporting that not all granules within a cell are regulated in an identical fashion; instead, specific intracellular pools of glycogen exist that are designated for cytosolic degradation (36). Furthermore, the brain expresses two different GP isoforms, the muscle and the brain isoforms (59), that are differently regulated by phosphorylation and AMP (60) and thus are expected to serve different physiological roles (60). Norepinephrine-induced up-regulation of glycogen degradation has been shown to be mainly mediated by the GP muscle isoform (60), whereas the glycolytic supercompensation seems to depend on brain GP activation (61). Whether the role of the two GP isoforms in the regulation of glycogen utilization can be explained by differential intracellular compartmentalizations in the astrocytes remains elusive.
Lysosomal glycogen is enriched in very large molecular weight granules, and its degradation affects 5% of total muscle glycogen and 10% of total liver glycogen (15, 62). The following questions arise. When and how are glycogen granules targeted for lysosomal degradation? What is the metabolic relevance of such a cellular process? In newborns, liver lysosomal glycogen is the product of glycogen autophagy, and it has been suggested to serve as an extra glucose source during and after birth (63). Several studies have reported evidence suggesting a role for laforin and malin in the regulation of glycogen and removal of glycogen-associated proteins via autophagy–lysosomal and ubiquitin–proteasome pathways, respectively (64). The autophagy–lysosomal pathway involves chaperone-mediated autophagy and unspecific invagination of a fraction of the cytoplasm into an autophagosome, which then fuses with a lysosome for content breakdown. Selective down-regulation of hepatic chaperone-mediated autophagy leads to glycogen depletion, potentially explained by the reduced degradation of glycolytic enzymes, leading to enhanced glycolysis rates (65). The ubiquitin–proteasome pathway, in which substrate proteins are targeted for 26S proteasome degradation by ubiquitination, is highly selective (66). Malin has been shown to ubiquitinate several glycogen-associated proteins in vitro, among them laforin, PTG, GDE, and GS (67–69); however, the substrates of malin in vivo remain unclear, as discussed by Gentry et al. (52). In contrast, starch binding domain 1 (Stbd-1) has been identified as a selective autophagy receptor for glycogen. Stbd-1 has a higher affinity for less branched polysaccharides (70), and it has an autophagy-related 8-family interacting motif (71).
Intracellular compartmentalization and dynamics of a glycogen granule
An interesting quandary in glycogen metabolism involves the intracellular localization of granules. As introduced above, glycogenolysis is associated with the ER/SR, whereas glycogen synthesis seems to be associated with actin filaments. Thus, are glycogen granules formed in specific locations, and do they move during their lifetime?
As an attempt to integrate the reviewed literature, we take the opportunity to propose a generalized hypothetical model for the dynamic life of a glycogen granule (Figs. 1 and 2). Critically low glycogen levels trigger actin cytoskeleton rearrangement, forming actin-rich spherical-like structures, which could facilitate GN dimerization and interaction with GS for efficient glycogen re-synthesis (Fig. 2, A and D). In mouse astrocytes, the start of glycogen resynthesis after fasting has been reported in close association with the vasculature (72). Restored glycogen levels would lead to dissolution of the actin-rich structures and explain the observation by Rybicka (1) of lines of cytoplasmic glycogen granules associated with filaments (Fig. 2E). As actin-associated glycogen granules grow, they may eventually dissociate from actin filaments, becoming free unbound granules (Fig. 2F) that could eventually associate with the ER/SR, becoming the glycogenolytic complex for cytosolic degradation (Fig. 3A). In skeletal muscle, ∼75% of glycogen granules are found between myofibrils in the intermyofibrillar space (73) where the SR is located, and the rest are associated with contractile filaments inside the myofibrils at the actin I-band of the sarcomere or underneath the plasmalemma (39, 74). Investigation into the distinct roles for the skeletal muscle subcellular glycogen pools has suggested that intramyofibrillar glycogen is tightly associated with muscle resistance to fatigue, whereas intermyofibrillar glycogen appears to be linked with muscle relaxation time and the regulation of Ca2+ uptake by the SR (73). These observations support the proposed model in which actin-associated (intramyofibrillar) granules are key for glycogen re-synthesis after depletion, and intermyofibrillar glycogen granules associated with the SR–glycogenolytic complex link glycogenolysis with SR Ca2+ uptake.
When a granule is not recruited for cytosolic degradation, accumulative glycogen phosphorylation would lead to alterations in the polysaccharide's structure (branching) (50), making it less soluble. Binding of 5′-AMP-activated protein kinase-phosphorylated laforin to the highly phosphorylated granule would increase malin binding (Fig. 3D), ubiquitination of glycogen-associated proteins, and subsequent proteasome-dependent degradation. In addition, because Stbd-1 has high affinity for less branched polysaccharides, it may tag aging granules toward lysosomal degradation (Fig. 3F) (71, 75). This idea is supported by the Lafora bodies that form and accumulate when laforin is absent, and these granules are water-insoluble, phosphate-rich, and ubiquitin-positive (76, 77).
In the proposed model, the acid-insoluble glycogen fraction would include the nascent glycogen granules associated with actin filaments and glycogen granules associated with the ER–SR-glycogenolytic complex, whereas the acid-soluble fraction may include cytosolic unbound glycogen granules. This idea is supported by studies reporting that the acid-insoluble glycogen fraction is more metabolically active (10) and has lower average external chain lengths (11). Interestingly, the amount of glycogen acid-soluble fraction has been reported to be more responsive to fasting/feeding and exercise/re-feeding cycles (23, 78). These results may seem contradictory; yet, they can be rationalized by the proposed model. In the model, the amount of metabolically active acid-insoluble glycogen granules (actin- and ER/SR-bound) could be stable, with the regained glycogen storage capacity predominantly occurring in the less metabolically active acid-soluble unbound glycogen granules. Whether glycogen synthesis and degradation co-exist in individual glycogen granules remains unsolved. It is interesting to note that newly formed granules in periportal hepatocytes have been reported to be closely associated with ER (79), and the actin-rich spherical structures in which glycogen re-synthesis localizes interact closely with SR–membrane systems and transverse tubules in skeletal muscle (Fig. 1, D–G, white arrows). A close physical proximity between the protein complexes regulating glycogen synthesis (actin-associated GN and GS and GLUT4 glucose uptake) and degradation (ER/SR-associated PhK, GP, and Ca2+-ATPase) suggests that the regulation of the two events is likely coordinated.
Closing remarks and open ends
The dynamic life of a glycogen granule is tissue-specific. A large amount of the available literature originates from skeletal muscle and liver, and thus further studies investigating the regulation of glycogen metabolism in other tissues, especially brain, are needed. In addition, the importance of intracellular compartmentalization in the regulation of glycogen metabolism makes the integration of physiology, biochemistry, and structural biology studies essential.
This is the first article in the Thematic Minireview Series “Brain glycogen metabolism.” The authors declare that they have no conflicts of interest with the contents of this article.
- GN
- glycogenin
- GS
- glycogen synthase
- GBE
- glycogen-branching enzyme
- GDE
- glycogen-debranching enzyme
- GP
- glycogen phosphorylase
- SR
- sarcoplasmic reticulum
- PTG
- protein targeting to glycogen
- AMPK
- 5′-AMP-activated protein kinase.
References
- 1. Rybicka K. K. (1996) Glycosomes–the organelles of glycogen metabolism. Tissue Cell 28, 253–265 10.1016/S0040-8166(96)80013-9 [DOI] [PubMed] [Google Scholar]
- 2. Meyer F., Heilmeyer L. M. Jr., Haschke R. H., and Fischer E. H. (1970) Control of phosphorylase activity in a muscle glycogen particle. I. Isolation and characterization of the protein-glycogen complex. J. Biol. Chem. 245, 6642–6648 [PubMed] [Google Scholar]
- 3. Drochmans P. (1962) Morphology of glycogen. Electron microscopic study of the negative stains of particulate glycogen. J. Ultrastruct. Res. 6, 141–163 10.1016/S0022-5320(62)90050-3 [DOI] [PubMed] [Google Scholar]
- 4. Thornell L. E. (1974) The fine structure of Purkinje fiber glycogen. A comparative study of negatively stained and cytochemically stained particles. J. Ultrastruct. Res. 49, 157–166 10.1016/S0022-5320(74)80029-8 [DOI] [PubMed] [Google Scholar]
- 5. Sullivan M. A., Aroney S. T., Li S., Warren F. J., Joo J. S., Mak K. S., Stapleton D. I., Bell-Anderson K. S., and Gilbert R. G. (2014) Changes in glycogen structure over feeding cycle sheds new light on blood-glucose control. Biomacromolecules 15, 660–665 10.1021/bm401714v [DOI] [PubMed] [Google Scholar]
- 6. Smythe C., and Cohen P. (1991) The discovery of glycogenin and the priming mechanism for glycogen biogenesis. Eur. J. Biochem. 200, 625–631 10.1111/j.1432-1033.1991.tb16225.x [DOI] [PubMed] [Google Scholar]
- 7. Meléndez R., Meléndez-Hevia E., and Cascante M. (1997) How did glycogen structure evolve to satisfy the requirement for rapid mobilization of glucose? A problem of physical constraints in structure building. J. Mol. Evol. 45, 446–455 10.1007/PL00006249 [DOI] [PubMed] [Google Scholar]
- 8. Goldsmith E., Sprang S., and Fletterick R. (1982) Structure of maltoheptaose by difference Fourier methods and a model for glycogen. J. Mol. Biol. 156, 411–427 10.1016/0022-2836(82)90336-9 [DOI] [PubMed] [Google Scholar]
- 9. Shearer J., and Graham T. E. (2004) Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc. Sport Sci. Rev. 32, 120–126 10.1097/00003677-200407000-00008 [DOI] [PubMed] [Google Scholar]
- 10. Meléndez-Hevia E., Waddell T. G., and Shelton E. D. (1993) Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem. J. 295, 477–483 10.1042/bj2950477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orrell S. A., and Bueding E. (1964) A comparison of products obtained by various procedures used for the extraction of glycogen. J. Biol. Chem. 239, 4021–4026 [PubMed] [Google Scholar]
- 12. Sullivan M. A., Vilaplana F., Cave R. A., Stapleton D., Gray-Weale A. A., and Gilbert R. G. (2010) Nature of α and β particles in glycogen using molecular size distributions. Biomacromolecules 11, 1094–1100 10.1021/bm100074p [DOI] [PubMed] [Google Scholar]
- 13. Smythe C., Villar-Palasi C., and Cohen P. (1989) Structural and functional studies on rabbit liver glycogenin. Eur. J. Biochem. 183, 205–209 10.1111/j.1432-1033.1989.tb14914.x [DOI] [PubMed] [Google Scholar]
- 14. Sullivan M. A., O'Connor M. J., Umana F., Roura E., Jack K., Stapleton D. I., and Gilbert R. G. (2012) Molecular insights into glycogen α-particle formation. Biomacromolecules 13, 3805–3813 10.1021/bm3012727 [DOI] [PubMed] [Google Scholar]
- 15. Geddes R., Harvey J. D., and Wills P. R. (1977) The molecular size and shape of liver glycogen. Biochem. J. 163, 201–209 10.1042/bj1630201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilstfittcr R., and Rohdewald M. (1934) Uber den zustand des glykogens in der leben, in muskel, und in leukocyten. Hoppe Seyler's Z. Physiol. Chem. 225, 103–124 10.1515/bchm2.1934.225.2-3.103 [DOI] [Google Scholar]
- 17. Rosati G. (1967) Enzyme treatment of glycogen particles in rat liver and muscle. J. Ultrastruct. Res. 18, 444–455 10.1016/S0022-5320(67)80129-1 [DOI] [PubMed] [Google Scholar]
- 18. Roe J. H., Bailey J. M., Gray R. R., and Robinson J. N. (1961) Complete removal of glycogen from tissues by extraction with cold trichloroacetic acid solution. J. Biol. Chem. 236, 1244–1246 [PubMed] [Google Scholar]
- 19. Lomako J., Lomako W. M., and Whelan W. J. (1991) Proglycogen: a low-molecular-weight form of muscle glycogen. FEBS Lett. 279, 223–228 10.1016/0014-5793(91)80154-U [DOI] [PubMed] [Google Scholar]
- 20. Lomako J., Lomako W. M., Whelan W. J., Dombro R. S., Neary J. T., and Norenberg M. D. (1993) Glycogen synthesis in the astrocyte: from glycogenin to proglycogen to glycogen. FASEB J. 7, 1386–1393 10.1096/fasebj.7.14.8224611 [DOI] [PubMed] [Google Scholar]
- 21. Adamo K. B., and Graham T. E. (1998) Comparison of traditional measurements with macroglycogen and proglycogen analysis of muscle glycogen. J. Appl. Physiol. 84, 908–913 10.1152/jappl.1998.84.3.908 [DOI] [PubMed] [Google Scholar]
- 22. Jansson E. (1981) Acid soluble and insoluble glycogen in human skeletal muscles. Acta Physiol. Scand. 113, 337–340 10.1111/j.1748-1716.1981.tb06904.x [DOI] [PubMed] [Google Scholar]
- 23. James A. P., Barnes P. D., Palmer T. N., and Fournier P. A. (2008) Proglycogen and macroglycogen: artifacts of glycogen extraction? Metabolism 57, 535–543 10.1016/j.metabol.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 24. Hurley T. D., Walls C., Bennett J. R., Roach P. J., and Wang M. (2006) Direct detection of glycogenin reaction products during glycogen initiation. Biochem. Biophys. Res. Commun. 348, 374–378 10.1016/j.bbrc.2006.07.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smythe C., Caudwell F. B., Ferguson M., and Cohen P. (1988) Isolation and structural analysis of a peptide containing the novel tyrosyl-glucose linkage in glycogenin. EMBO J. 7, 2681–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin A., Mu J., Yang J., and Roach P. J. (1999) Self-glucosylation of glycogenin, the initiator of glycogen biosynthesis, involves an inter-subunit reaction. Arch. Biochem. Biophys. 363, 163–170 10.1006/abbi.1998.1073 [DOI] [PubMed] [Google Scholar]
- 27. Skurat A. V., Dietrich A. D., and Roach P. J. (2006) Interaction between glycogenin and glycogen synthase. Arch. Biochem. Biophys. 456, 93–97 10.1016/j.abb.2006.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smythe C., Watt P., and Cohen P. (1990) Further studies on the role of glycogenin in glycogen biosynthesis. Eur. J. Biochem. 189, 199–204 10.1111/j.1432-1033.1990.tb15477.x [DOI] [PubMed] [Google Scholar]
- 29. Thon V. J., Khalil M., and Cannon J. F. (1993) Isolation of human glycogen branching enzyme cDNAs by screening complementation in yeast. J. Biol. Chem. 268, 7509–7513 [PubMed] [Google Scholar]
- 30. Meléndez R., Meléndez-Hevia E., and Canela E. I. (1999) The fractal structure of glycogen: a clever solution to optimize cell metabolism. Biophys. J. 77, 1327–1332 10.1016/S0006-3495(99)76982-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meléndez R., Meléndez-Hevia E., Mas F., Mach J., and Cascante M., R. (1998) Physical constraints in the synthesis of glycogen that influence its structural homogeneity: a two-dimensional approach. Biophys. J. 75, 106–114 10.1016/S0006-3495(98)77498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baqué S., Guinovart J. J., and Ferrer J. C. (1997) Glycogenin, the primer of glycogen synthesis, binds to actin. FEBS Lett. 417, 355–359 10.1016/S0014-5793(97)01299-4 [DOI] [PubMed] [Google Scholar]
- 33. Cid E., Cifuentes D., Baqué S., Ferrer J. C., and Guinovart J. J. (2005) Determinants of the nucleocytoplasmic shuttling of muscle glycogen synthase. FEBS J. 272, 3197–3213 10.1111/j.1742-4658.2005.04738.x [DOI] [PubMed] [Google Scholar]
- 34. Wilson W. A., Boyer M. P., Davis K. D., Burke M., and Roach P. J. (2010) The subcellular localization of yeast glycogen synthase is dependent upon glycogen content. Can. J. Microbiol. 56, 408–420 10.1139/W10-027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ou H., Yan L., Osmanovic S., Greenberg C. C., and Brady M. J. (2005) Spatial reorganization of glycogen synthase upon activation in 3T3-L1 adipocytes. Endocrinology 146, 494–502 10.1210/en.2004-1022 [DOI] [PubMed] [Google Scholar]
- 36. Fernández-Novell J. M., Bellido D., Vilaró S., and Guinovart J. J. (1997) Glucose induces the translocation of glycogen synthase to the cell cortex in rat hepatocytes. Biochem. J. 321, 227–231 10.1042/bj3210227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prats C., Cadefau J. A., Cussó R., Qvortrup K., Nielsen J. N., Wojtaszewski J. F., Wojtaszewki J. F., Hardie D. G., Stewart G., Hansen B. F., and Ploug T. (2005) Phosphorylation-dependent translocation of glycogen synthase to a novel structure during glycogen resynthesis. J. Biol. Chem. 280, 23165–23172 10.1074/jbc.M502713200 [DOI] [PubMed] [Google Scholar]
- 38. Prats C., Helge J. W., Nordby P., Qvortrup K., Ploug T., Dela F., and Wojtaszewski J. F. (2009) Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. J. Biol. Chem. 284, 15692–15700 10.1074/jbc.M900845200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marchand I., Chorneyko K., Tarnopolsky M., Hamilton S., Shearer J., Potvin J., and Graham T. E. (2002) Quantification of subcellular glycogen in resting human muscle: granule size, number, and location. J. Appl. Physiol. 93, 1598–1607 10.1152/japplphysiol.00585.2001 [DOI] [PubMed] [Google Scholar]
- 40. Elsner P., Quistorff B., Hansen G. H., and Grunnet N. (2002) Partly ordered synthesis and degradation of glycogen in cultured rat myotubes. J. Biol. Chem. 277, 4831–4838 10.1074/jbc.M108226200 [DOI] [PubMed] [Google Scholar]
- 41. Devos P., and Hers H. G. (1979) A molecular order in the synthesis and degradation of glycogen in the liver. Eur. J. Biochem. 99, 161–167 10.1111/j.1432-1033.1979.tb13242.x [DOI] [PubMed] [Google Scholar]
- 42. Oe Y., Baba O., Ashida H., Nakamura K. C., and Hirase H. (2016) Glycogen distribution in the microwave-fixed mouse brain reveals heterogeneous astrocytic patterns. Glia 64, 1532–1545 10.1002/glia.23020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura-Tsuruta S., Yasuda M., Nakamura T., Shinoda E., Furuyashiki T., Kakutani R., Takata H., Kato Y., and Ashida H. (2012) Comparative analysis of carbohydrate-binding specificities of two anti-glycogen monoclonal antibodies using ELISA and surface plasmon resonance. Carbohydr. Res. 350, 49–54 10.1016/j.carres.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 44. Skurat A. V., Lim S. S., and Roach P. J. (1997) Glycogenin biogenesis in rat-1 fibroblasts expressing rabbit muscle glycogenin. Eur. J. Biochem. 245, 147–155 10.1111/j.1432-1033.1997.t01-1-00147.x [DOI] [PubMed] [Google Scholar]
- 45. Skurat A. V., Peng H. L., Chang H. Y., Cannon J. F., and Roach P. J. (1996) Rate determining steps in the biosynthesis of glycogen in COS cells. Arch. Biochem. Biophys. 328, 283–288 10.1006/abbi.1996.0174 [DOI] [PubMed] [Google Scholar]
- 46. Cao Y., Skurat A. V., DePaoli-Roach A. A., and Roach P. J. (1993) Initiation of glycogen synthesis: Control of glycogenin by glycogen phosphorylase. J. Biol. Chem. 268, 21717–21721 [PubMed] [Google Scholar]
- 47. Hansen B. F., Derave W., Jensen P., and Richter E. A. (2000) No limiting role for glycogenin in determining maximal attainable glycogen levels in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 278, E398–E404 10.1152/ajpendo.2000.278.3.E398 [DOI] [PubMed] [Google Scholar]
- 48. Montori-Grau M., Guitart M., García-Martínez C., Orozco A., and Gómez-Foix A. (2011) Differential pattern of glycogen accumulation after protein phosphatase 1 glycogen-targeting subunit PPP1R6 overexpression, compared to PPP1R3C and PPP1R3A, in skeletal muscle cells. BMC Biochem. 12, 57 10.1186/1471-2091-12-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tagliabracci V. S., Girard J. M., Segvich D., Meyer C., Turnbull J., Zhao X., Minassian B. A., Depaoli-Roach A. A., and Roach P. J. (2008) Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J. Biol. Chem. 283, 33816–33825 10.1074/jbc.M807428200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tagliabracci V. S., Turnbull J., Wang W., Girard J.-M., Zhao X., Skurat A. V., Delgado-Escueta A. V., Minassian B. A., Depaoli-Roach A. A., and Roach P. J. (2007) Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 19262–19266 10.1073/pnas.0707952104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turnbull J., Tiberia E., Pereira S., Zhao X., Pencea N., Wheeler A. L., Yu W. Q., Ivovic A., Naranian T., Israelian N., Draginov A., Piliguian M., Frankland P. W., Wang P., Ackerley C. A., et al. (2013) Deficiency of a glycogen synthase-associated protein, Epm2aip1, causes decreased glycogen synthesis and hepatic insulin resistance. J. Biol. Chem. 288, 34627–34637 10.1074/jbc.M113.483198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gentry M. S., Guinovart J. J., Minassian B. A., Roach P. J., and Serratosa J. M. (February 26, 2018) Glycogen, Lafora disease offers a unique window into neuronal. J. Biol. Chem. 293, 7117–7125 10.1074/jbc.R117.803064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lomako J., Lomako W. M., Kirkman B. R., and Whelan W. J. (1994) The role of phosphate in muscle glycogen. Biofactors 4, 167–171 [PubMed] [Google Scholar]
- 54. Becker J. A., Vlach J., Raben N., Nagaraju K., Adams E. M., Hermans M. M., Reuser A. J., Brooks S. S., Tifft C. J., Hirschhorn R., Huie M. L., Nicolino M., and Plotz P. H. (1998) The African origin of the common mutation in African American patients with glycogen-storage disease type II. Am. J. Hum. Genet. 62, 991–994 10.1086/301788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wanson J. C., and Drochmans P. (1972) Role of the sarcoplasmic reticulum in glycogen metabolism. Binding of phosphorylase, phosphorylase kinase, and primer complexes to the sarcovesicles of rabbit skeletal muscle. J. Cell Biol. 54, 206–224 10.1083/jcb.54.2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Entman M. L., Keslensky S. S., Chu A., and Van Winkle W. B. (1980) The sarcoplasmic reticulum-glycogenolytic complex in mammalian fast twitch skeletal muscle. Proposed in vitro counterpart of the contraction-activated glycogenolytic pool. J. Biol. Chem. 255, 6245–6252 [PubMed] [Google Scholar]
- 57. Entman M. L., Bornet E. P., Van Winkle W. B., Goldstein M. A., and Schwartz A. (1977) Association of glycogenolysis with cardiac sarcoplasmic reticulum: II. Effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: evidence for a phosphorylase kinase membrane complex. J. Mol. Cell. Cardiol. 9, 515–528 10.1016/S0022-2828(77)80367-2 [DOI] [PubMed] [Google Scholar]
- 58. Müller M. S., Fox R., Schousboe A., Waagepetersen H. S., and Bak L. K. (2014) Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia 62, 526–534 10.1002/glia.22623 [DOI] [PubMed] [Google Scholar]
- 59. Schmid H., Pfeiffer-Guglielmi B., Dolderer B., Thiess U., Verleysdonk S., and Hamprecht B. (2009) Expression of the brain and muscle isoforms of glycogen phosphorylase in rat heart. Neurochem. Res. 34, 581–586 10.1007/s11064-008-9825-3 [DOI] [PubMed] [Google Scholar]
- 60. Müller M. S., Pedersen S. E., Walls A. B., Waagepetersen H. S., and Bak L. K. (2015) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63, 154–162 10.1002/glia.22741 [DOI] [PubMed] [Google Scholar]
- 61. Jakobsen E., Bak L. K., Walls A. B., Reuschlein A.-K., Schousboe A., and Waagepetersen H. S. (2017) Glycogen shunt activity and glycolytic supercompensation in astrocytes may be distinctly mediated via the muscle form of glycogen phosphorylase. Neurochem. Res. 42, 2490–2494 10.1007/s11064-017-2267-z [DOI] [PubMed] [Google Scholar]
- 62. Geddes R., Jeyarathan P., and Taylor J. A. (1992) Molecular and metabolic aspects of lysosomal glycogen. Carbohydr. Res. 227, 339–349 10.1016/0008-6215(92)85083-C [DOI] [PubMed] [Google Scholar]
- 63. Kotoulas O. B., Kalamidas S. A., and Kondomerkos D. J. (2004) Glycogen autophagy. Microsc. Res. Tech. 64, 10–20 10.1002/jemt.20046 [DOI] [PubMed] [Google Scholar]
- 64. Roach P. J., Depaoli-Roach A. A., Hurley T. D., and Tagliabracci V. S. (2012) Glycogen and its metabolism: some new developments and old themes. Biochem. J. 441, 763–787 10.1042/BJ20111416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schneider J. L., Suh Y., and Cuervo A. M. (2014) Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 20, 417–432 10.1016/j.cmet.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goldberg A. L. (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426, 895–899 10.1038/nature02263 [DOI] [PubMed] [Google Scholar]
- 67. Cheng A., Zhang M., Gentry M. S., Worby C. A., Dixon J. E., and Saltiel A. R. (2007) A role for AGL ubiquitination in the glycogen storage disorders of Lafora and Cori's disease. Genes Dev. 21, 2399–2409 10.1101/gad.1553207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Worby C. A., Gentry M. S., and Dixon J. E. (2008) Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J. Biol. Chem. 283, 4069–4076 10.1074/jbc.M708712200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vilchez D., Ros S., Cifuentes D., Pujadas L., Vallès J., García-Fojeda B., Criado-García O., Fernández-Sánchez E., Medraño-Fernández I., Domínguez J., García-Rocha M., Soriano E., Rodríguez de Córdoba S., and Guinovart J. J. (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 10, 1407–1413 10.1038/nn1998 [DOI] [PubMed] [Google Scholar]
- 70. Jiang S., Heller B., Tagliabracci V. S., Zhai L., Irimia J. M., DePaoli-Roach A. A., Wells C. D., Skurat A. V., and Roach P. J. (2010) Starch binding domain-containing protein 1/genethonin 1 is a novel participant in glycogen metabolism. J. Biol. Chem. 285, 34960–34971 10.1074/jbc.M110.150839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang S., Wells C. D., and Roach P. J. (2011) Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: Identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1. Biochem. Biophys. Res. Commun. 413, 420–425 10.1016/j.bbrc.2011.08.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takado Y., Knott G., Humbel B. M., Escrig S., Masoodi M., Meibom A., and Comment A. (2015) Imaging liver and brain glycogen metabolism at the nanometer scale. Nanomedicine 11, 239–245 10.1016/j.nano.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 73. Nielsen J., Schrøder H. D., Rix C. G., and Ortenblad N. (2009) Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J. Physiol. 587, 3679–3690 10.1113/jphysiol.2009.174862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nielsen J., Holmberg H.-C., Schrøder H. D., Saltin B., and Ortenblad N. (2011) Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J. Physiol. 589, 2871–2885 10.1113/jphysiol.2010.204487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Demetriadou A., Morales-Sanfrutos J., Nearchou M., Baba O., Kyriacou K., Tate E. W., Drousiotou A., and Petrou P. P. (2017) Mouse Stbd1 is N-myristoylated and affects ER-mitochondria association and mitochondrial morphology. J. Cell Sci. 130, 903–915 10.1242/jcs.195263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Serratosa J. M., Minassian B. A., and Ganesh S. (2012) Progressive myoclonus epilepsy of Lafora in Jasper's Basic Mechanisms of the Epilepsies (Noebels J. L., Avoli M., Rogawski M. A., Olsen R. W., and Delgado-Escueta A. V., eds) 4th Ed., NCBI, Bethesda, MD: [PubMed] [Google Scholar]
- 77. Ganesh S., Puri R., Singh S., Mittal S., and Dubey D. (2006) Recent advances in the molecular basis of Lafora's progressive myoclonus epilepsy. J. Hum. Genet. 51, 1–8 10.1007/s10038-005-0321-1 [DOI] [PubMed] [Google Scholar]
- 78. Barnes P. D., Singh A., and Fournier P. A. (2009) Homogenization-dependent responses of acid-soluble and acid-insoluble glycogen to exercise and refeeding in human muscles. Metabolism 58, 1832–1839 10.1016/j.metabol.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 79. Cardell R. R., and Cardell E. L. (1990) Heterogeneity of glycogen distribution in hepatocytes. J. Electron Microsc. Tech. 14, 126–139 10.1002/jemt.1060140206 [DOI] [PubMed] [Google Scholar]
- 80. Apweiler R., Bairoch A., Wu C. H., Barker W. C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., Martin M. J., Natale D. A., O'Donovan C., Redaschi N., and Yeh L.-S. (2004) UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 32, D115–D119 10.1093/nar/gkh131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chatr-Aryamontri A, Oughtred R., Boucher L., Rust J., Chang C., Kolas N. K., O'Donnell L., Oster S., Theesfeld C., Sellam A., Stark C., Breitkreutz B.-J., Dolinski K., and Tyers M. (2017) The BioGRID interaction database: 2017 update. Nucleic Acids Res. 45, D369–D379 10.1093/nar/gkw1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Orchard S., Ammari M., Aranda B., Breuza L., Briganti L., Broackes-Carter F., Campbell N. H., Chavali G., Chen C., del-Toro N., Duesbury M., Dumousseau M., Galeota E., Hinz U., Iannuccelli M., et al. (2014) The MIntAct project–IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 42, D358–D363 10.1093/nar/gkt1115 [DOI] [PMC free article] [PubMed] [Google Scholar]