Abstract
The brain contains a fairly low amount of glycogen, mostly located in astrocytes, a fact that has prompted the suggestion that glycogen does not have a significant physiological role in the brain. However, glycogen metabolism in astrocytes is essential for several key physiological processes and is adversely affected in disease. For instance, diminished ability to break down glycogen impinges on learning, and epilepsy, Alzheimer's disease, and type 2 diabetes are all associated with abnormal astrocyte glycogen metabolism. Glycogen metabolism supports astrocytic K+ and neurotransmitter glutamate uptake and subsequent glutamine synthesis—three fundamental steps in excitatory signaling at most brain synapses. Thus, there is abundant evidence for a key role of glycogen in brain function. Here, we summarize the physiological brain functions that depend on glycogen, discuss glycogen metabolism in disease, and investigate how glycogen breakdown is regulated at the cellular and molecular levels.
Keywords: astrocyte, brain, calcium, cyclic AMP (cAMP), glycogen, neurological disease, disease
Introduction
Glycogen in the brain is mostly but not exclusively confined to astrocytes (1), and astrocytic glycogen metabolism is vital for a number of fundamental processes in the brain. For instance, brain glycogen is affected in hypoglycemia (2). Curiously, the glycogen level rebounds to a higher level following a single but not repeated hypoglycemic episodes in humans (2, 3). Interestingly, it has repeatedly been reported that the ability to synthesize or break down brain glycogen is important in learning and memory formation (4–7), and maladaptive learning, measured as the conditioned response to cocaine in mice, is reduced when glycogen breakdown is blocked (8). Thus, a linkage exists between breakdown of glycogen and the neural plasticity involved in both learning and addiction. In addition, glycogen breakdown is essential for key astrocytic processes, such as uptake of K+ and neurotransmitter glutamate, and the subsequent synthesis of glutamine as part of the glutamate–glutamine cycle (9–11). However, several details regarding the role and regulation of glycogen metabolism in physiology and pathology are still obscure. The importance of astrocyte glycogen is somewhat enigmatic in light of the fact that the ample supply of extracellular glucose would appear to be sufficient to serve the brain's energetic needs. Hence, two questions are as follows. (i) What is it about glycogen that gives it this prominent position in brain biochemistry and physiology? (ii) what extracellular and intracellular cues regulate these processes? As will be evident from the discussions below, we are really just beginning to understand this at the cellular and molecular levels.
Glycogen in health and disease
Historically, brain glycogen was thought to be vestigial due to the limited amount present (12). Later, glycogen was considered to constitute an emergency fuel, which was degraded only when there was a discontinuation in the cerebral glucose supply. Now we know that degradation of glycogen is crucial for sustaining a number of physiological processes. It should be noted that alterations in glycogen metabolism may be a consequence of the changes related to disease, rather than the underlying cause of disease. The altered glycogen metabolism may, of course, bring about new complications because glycogen breakdown is a key process involved in many aspects of proper brain function.
Implication of glycogen in learning, memory, and Alzheimer's disease
Degradation of glycogen is important for learning and memory formation, as well as long-term memory consolidation (4, 5, 13, 14). Disruptions of glycogen metabolism have also been linked to Alzheimer's disease, potentially due to overactivation of GSK-3 and a resulting inhibition of glycogen synthase (15). Moreover, mice lacking glycogen synthase in the brain display a significant deficiency in the acquisition of an associative learning task (4). Furthermore, intracerebral injection of β-amyloid (Aβ(1–42)) into 1-day-old chicks caused memory loss, which could not be rescued by stimulation of glycogen breakdown likely because β-amyloid impairs glycogen synthesis via activation of GSK-3 (15). Impairments in glycogen synthesis could reduce brain glycogen levels, hampering the physiological flux of glucose units through glycogen, which is important for learning and memory. In line with the importance of noradrenergic regulation of flux through the glycogen shunt (11), noradrenergic dysfunction was proposed to be an important component of Alzheimer's disease (16–18). The importance of glycogen with regard to Alzheimer's disease is underlined by a recent study that found facilitated spatial learning and increased glycogen stores in an Alzheimer's disease model fed a diet containing pyruvate (19).
Implication of glycogen in seizures and epilepsy
The involvement of glycogen in glutamatergic neurotransmission has repeatedly been demonstrated. Glycogen may be converted to lactate that can be released to the extracellular space and used by neurons or other cells as fuel (20, 21). Furthermore, glycogen degradation fuels glutamate transport in astrocytes (11, 22) or glycogen in astrocytes can be used for synthesis of glutamine, thus serving as precursor for biosynthesis of neurotransmitter glutamate (13). In line with this, it has been suggested that reduced glycogen degradation contributes to the imbalance of glutamatergic and GABAergic neurotransmission associated with epilepsy and seizures (24). Some findings indicate that epileptic animals contain alternatively structured glycogen molecules that are resistant to degradation (24), consistent with the increased levels of hippocampal glycogen detected in epileptic patients (25). Compatible with this, epileptic seizures are the symptomatic hallmark of Lafora disease characterized by abolished glycogen degradation (see Minireview by Gentry et al. (39)).
In contrast to epilepsy involving repetitive seizures, acute kainate-induced seizures led to a dose-dependent decrease in cerebral glycogen content (26). This indicates that under this condition glycogen can be degraded and contributes to the maintenance of cerebral energy homeostasis. Furthermore, based on mathematical modeling, it was recently concluded that glycogen is mobilized as an early event after induced spreading of depression in rats, an event that, like seizure activity, leads to synchronized activity in brain encompassing a drastic increase in local energy demand to restore ion homeostasis (28). This may be explained by excessive neuronal activity being associated with efflux of K+, which is subsequently removed from the extracellular space by active transport into astrocytes (23). In primary astrocyte cultures, glycogen is involved in fueling the removal of K+ from the extracellular space (11), and in line with this, it has been suggested that glucose is spared for neuronal energy metabolism when glycogen is used for fueling astrocytic K+ clearance (27). In conclusion, altered glycogen metabolism seems to be clearly involved in seizure activity and epilepsy, although the two scenarios appear to affect glycogen metabolism differently.
Implication of glycogen in sleep
Levels of cerebral glycogen are reduced during wakefulness, and especially during sleep deprivation, but are replenished during sleep (29, 30). However, it seems that all brain areas are not equally affected by sleep and sleep deprivation (30), and brain glycogen appears to respond distinctly to sleep deprivation in different mouse strains (31). Based on the alterations in cerebral glycogen content in response to sleep, an hypothesis was formulated proposing that glycogen (in combination with adenosine) was a key regulator of sleep (32). However, current evidence does not support a direct correlation between regulation of sleep and glycogen content (33). It should be noted that the cerebral glycogen level increases only during the first 15 min of sleep and decreases slowly 20 min after awakening (34), but otherwise the cerebral glycogen content remains quite constant compatible with a persistent flux through the glycogen shunt. Recent studies have demonstrated that pharmacological inhibition of glycogen degradation by intracerebroventricular (ICV)4 injection of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB; a blocker of glycogen phosphorylase and thus glycogen breakdown, see below) did not affect either rapid eye movement or nonrapid eye movement sleep in mice (33). In contrast, ICV injection of DAB led to a decrease in spontaneous locomotor activity of almost 40%, suggesting that glycogen breakdown may be linked to locomotion because sleep/a quiet wake period is induced whenever glycogen degradation is inaccessible (33).
Astrocyte glycogen in diabetes
Diabetes is characterized by a persistent high concentration of glucose in plasma, and it is unclear whether glucose transport into the brain is affected (35). In a rat model of type 2 diabetes, glycogen metabolism was impaired resulting in a lower amount of brain glycogen (36). This is further supported by the observations that cerebral glycogen levels are reduced during hypoglycemia but then rebound to even higher levels during subsequent normo- or hyperglycemic periods (37–40). Remarkably, this increase in glycogen content was observed only after the first incidence of hypoglycemia and not after recurrent episodes, which led to the conclusion that impairments in glycogen metabolism may be involved in hypoglycemia unawareness (38).
Intracellular signals regulating glycogen breakdown
Intracellular elevations in the two canonical second messengers, 3′,5′-cyclic adenosine monophosphate (cAMP) and Ca2+, are required to elicit glycogen breakdown. The receptor-mediated regulation of glycogen breakdown has been known for decades and has been reviewed extensively (41). However, less is known about the compartmentalization of the intracellular signals and how this influences glycogen breakdown. We predict this to be an important area of future research, and thus we devote most of this section to explore this topic, rather than attempting to reproduce what has been previously discussed in the review literature.
Glycogen phosphorylase
Very briefly, astrocytes express two of the three known isoforms of GP, i.e. the brain form, bGP, and the muscle form, mGP (42, 43). As outlined in Fig. 1, cAMP and Ca2+ may elicit glycogen breakdown because phosphorylase kinase (PhK) that phosphorylates, and thus activates, GP is itself activated by Ca2+ and phosphorylation by protein kinase A (PKA) (41, 44). Activity of GP is also linked to the energetic status of the cell, because AMP activates GP allosterically, although of the two isoforms of GP in astrocytes, bGP responds more strongly to AMP than does mGP (41, 45, 46). For a further description of astrocytic glycogenolysis, see Minireview by Nadeau et al. (97).
Figure 1.
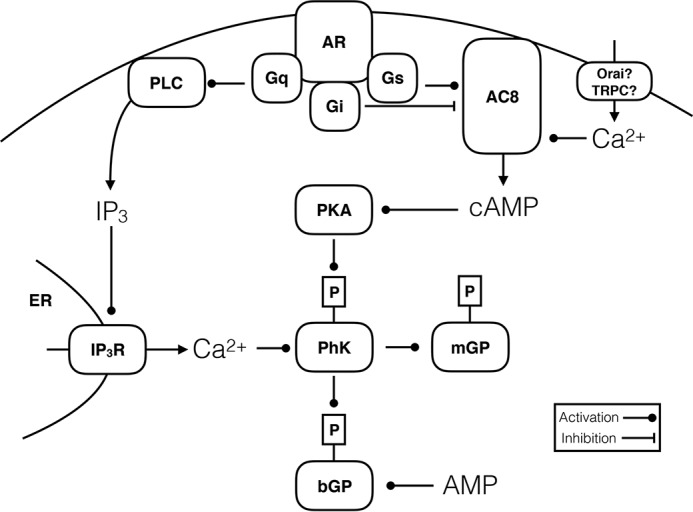
Cartoon depicting the two major signaling pathways regulating breakdown of glycogen. Glycogen phosphorylase brain (bGP) or muscle (mGP) forms are both activated by phosphorylation by a dedicated kinase, phosphorylase kinase (PhK). In addition, bGP is only fully active in the presence of ample levels of AMP. PhK, in turn, is activated by Ca2+ and phosphorylation by protein kinase A (PKA), and both signals are needed for full activation. In astrocytes, cAMP may be generated by plasma membrane-bound adenylate cyclase (AC), which in turn is regulated by the Gαs or Gαi protein-coupled adrenergic receptors (AR; see text for details). Depending on the isoform of AC expressed, Ca2+ flowing in via Orai or TRPC channels activated during store-operated Ca2+ entry may activate or inhibit the cAMP signal adding to the complexity; AC8 is activated by Ca2+ and is expressed in astrocytes. Finally, Gαq-coupled α1-adrenergic receptors may regulate glycogen breakdown via phospholipase C (PLC)-IP3 mediated release by IP3 receptors (IP3R) in the endoplasmic reticulum (ER).
Generation of receptor-coupled cAMP and Ca2+ signals in astrocytes
In astrocytes, cAMP and Ca2+ signals may arise following activation of G protein–coupled receptors, and mouse brain astrocytes express α1, α2, and β1 and possibly low but functionally important amounts of β2-adrenergic receptors (47, 48). This is essential because astrocytes are thought to be a major target of noradrenergic signaling stemming from the locus coeruleus regulating (among others) glycogen metabolism, e.g. in relation to the circadian rhythm, arousal, and emotional stress (47). Thus, norepinephrine may promote or inhibit cytosolic cAMP signaling via Gαs-coupled β-adrenergic receptors or Gαi-coupled α2-adrenergic receptors, respectively, and induce cytosolic Ca2+ signals via Gαq-coupled α1-adrenergic receptors (Fig. 1) (47). The effects of activating the different adrenergic receptors on astrocytes for glycogen breakdown have been studied extensively, although some studies show somewhat conflicting results (41). Concomitant Ca2+ and cAMP signals are needed (at least in muscle) to elicit glycogen breakdown via activation of PhK, which might explain the presence of both Gαq- and Gαs-coupled noradrenergic receptors on astrocytes. The remaining part of this section will deal with what we know and, more importantly what we need to know about these intracellular signals.
Putative role of nonreceptor-coupled Ca2+ and cAMP signals
Astrocytic Ca2+ signals have been researched intensively for the last few decades (49), whereas the other canonical second messenger, cAMP, has received less attention, although the protein biosensors for detecting cAMP have been available for about 2 decades (50). We know that both cAMP and Ca2+ signals may be diffusing across the cytosol or only be present in discrete micro (or rather nano) domains within the cell (51, 52). Clearly, this spatial compartmentalization of signaling pathways must influence the functional outcomes, and it has indeed been shown to do so in different preparations (51). In Müller et al. (53), we provide evidence that coordinated cross-talk between Ca2+ and cAMP induces astrocytic glycogen degradation, potentially allowing increases in nearby neuronal activity to engage this important supportive astrocytic process (49, 54). We induced store-operated Ca2+ entry (SOCE) in cultured astrocytes by depleting the intracellular stores through inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase pumps in the absence of extracellular Ca2+, and we then re-introduced extracellular Ca2+ to provoke SOCE (53). In this way, a cytosolic Ca2+ signal is generated in the absence of GPCR activation, and SOCE induced a significant breakdown of glycogen within minutes. As expected, if Ca2+ and cAMP-PKA jointly activate PhK, the breakdown was curbed by inhibition of adenylate cyclases (ACs); because SOCE was induced separately from GPCR activation, this indicates that the breakdown depends on Ca2+-induced cAMP signaling, presumably by Ca2+-activated AC isoform 8 (AC8; Fig. 1), which is present in astrocytes (48, 53). AC8 can be activated by SOCE in the absence of GPCR activation, perhaps due in part to a physical association with Orai1 channels (55–57), although the relative roles of TRPC versus Orai channels for mediating SOCE remains controversial (58, 59). Clearly, SOCE represents a nonreceptor-mediated way of initiating Ca2+/cAMP-dependent glycogen breakdown; however, in situ SOCE would only occur following an initial cytosolic Ca2+ signal depleting intracellular Ca2+ stores such as a GPCR-Gαq-IP3–mediated store depletion. Thus, the AC8-mediated cAMP signal represents a post-signaling signal arising subsequent to the initial cytosolic Ca2+ signal.
Putative role of compartmentalized intracellular signals
An interesting but little explored aspect of glycogen dynamics is whether compartmentalized Ca2+/cAMP-PKA signals can selectively affect glycogen breakdown. It has been shown in cardiac myocytes that activation of β1-adrenergic receptors produces a far-reaching cytosolic cAMP signal, whereas activation of β2-adrenergic receptors results in a signal that is restricted to the T-tubules (T-tubules are invaginations in the cell membrane that allow a fast cytosolic response to myocyte depolarization) (60)). It is therefore likely that two different pools of glycogen are degraded upon activation of β1- and β2-adrenergic receptors, respectively. We know from electron micrographs of cultured astrocytes that glycogen granules in some places line the inside of the plasma membrane but are also present in “belts” in the cytosol (61); thus, should astrocytes show the same compartmentalization of β-adrenergic signals, one might expect very different glycogenolytic responses to β1- versus β2-adrenergic receptor stimulation. Thus, it would be interesting to ascertain whether populations of astrocytes functionally express either β1- or β2-adrenergic receptors or perhaps both receptor subtypes. For instance, one could imagine that astrocytes expressing β2-adrenergic receptors might be tuned to release downstream metabolites from glycogenolysis, such as glutamine or lactate, whereas β1-adrenergic receptor-expressing astrocytes may respond to norepinephrine by breaking down glycogen for internal fuel or building blocks for anaplerosis of TCA cycle intermediates. Studying these aspects is not only interesting in terms of exploring the basic neurobiology, but also in terms of revealing putative drug targets.
Glycogen shunt activity
Metabolism of glucose via transient incorporation into glycogen, i.e. with no significant change in the amount of glycogen, is known as the glycogen shunt. Following phosphorylation of glucose to glucose 6-phosphate by the first enzyme of glycolysis, hexokinase, the glucose molecule may be incorporated into glycogen, a process that consumes UTP and is hence energy-demanding (Fig. 2). The subsequent degradation of glycogen to glucose 6-phosphate via glucose 1-phosphate does not require energy. This means that glucose metabolism via the glycogen shunt produces one ATP molecule less per molecule of glucose metabolized compared with “pure” glycolysis, i.e. one instead of two ATPs. However, despite being energetically unfavorable, glycogen shunt activity appears to operate persistently in muscle as well as in brain (11, 62–64). During increases in cerebral energy demand, the astrocytic glycogen content will, however, decrease (5, 64–68).
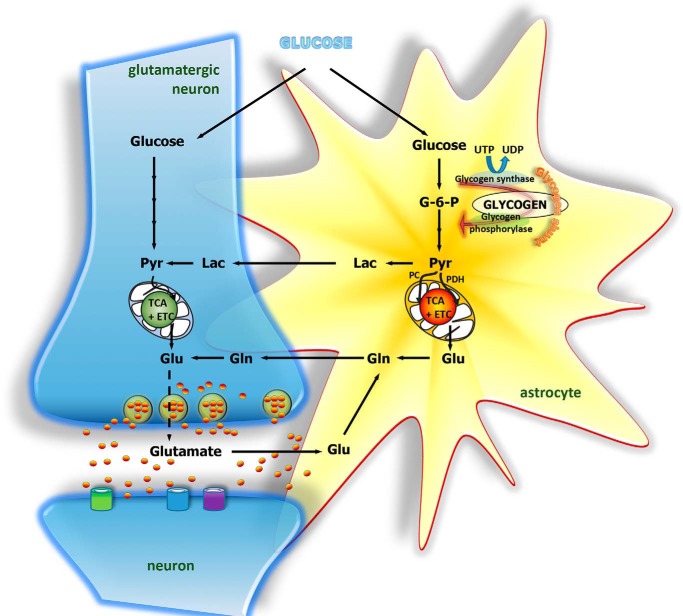
Figure 2.
Cartoon depicting glucose and glycogen metabolism in the brain as well as substrate transfer between astrocytes and neurons. In astrocytes, glucose may be metabolized via glycolysis or the glycogen shunt to pyruvate, which may be converted to lactate and transferred to neurons for oxidative metabolism to occur. Alternatively, pyruvate may enter the TCA cycle either by way of pyruvate dehydrogenase (PDH) or via pyruvate carboxylase (PC). Entrance of pyruvate via both of these pathways is required for de novo synthesis of glutamate and glutamine. Glutamine is not neuroactive and may be transferred to neurons to serve as precursor for glutamate synthesis. Following vesicular release of glutamate and interactions with receptors in the postsynaptic membrane, glutamate is cleared from the synapse mainly by transporters located in the astrocytic membrane. Glutamate can then be converted to glutamine and transferred to neurons, thereby completing the glutamate–glutamine cycle.
Functional importance of glycogen shunt activity
Although glycogen shunt activity has been revealed in both muscle and brain (11, 62–64), its significance and functional importance are not clear. Abolishment of glycogen degradation in cultured astrocytes exposed to 13C-labeled glucose led to an increase in the percent of 13C labeling in lactate, both under control conditions (without increasing the cells' energy demand) and during activation of energy requiring glutamate transport by exposure to 250 μm d-aspartate (69). Such an increase in 13C-labeled lactate results when flux through the glycogen shunt is inhibited and the amount of glucose metabolized via glycolysis exceeds the amount metabolized when both glycolysis and glycogenolysis are operational, i.e. glycolytic supercompensation (11). These findings propose that the ATP generated from glycolysis and from glycogen degradation, respectively, is not equivalent, which may rely on functional and/or spatial separation of these pathways. The findings may also be in agreement with the suggestion that glycolysis and glycogenolysis in astrocytes are complementary (70). Metabolic separation of these pathways was previously demonstrated by the observation that lactate derived from glycolysis and glycogenolysis contributes to distinct pools of lactate (71). Functionally distinctive roles of glycolysis and glycogenolysis have been observed in vitro as well as in vivo. Even in the presence of glucose, elimination of glycogen degradation in cultured astrocytes was demonstrated to result in reduced accumulation of d-[3H]aspartate mediated via glutamate transporters (22). This points toward a functional role of glycogen metabolism, which cannot be substituted by glycolytic activity, a finding supported by in vivo studies demonstrating that inhibition of glycogen degradation resulted in impairment of memory consolidation in young chickens and memory deficiency in rats, effects that could not be rescued by glucose (5, 13).
Quantitative significance of glycogen shunt activity
Although it appears that a persistent flux of glucose units through glycogen is of functional importance for proper brain function, the fraction of glucose being metabolized via the glycogen shunt is unclear. Norepinephrine is known to stimulate glycogen synthesis and degradation concomitantly, i.e. accelerate glycogen shunt activity (47, 72–77), and its exposure to norepinephrine (100 μm) revealed that the glycogen shunt accounts for ∼40% of total glucose metabolism under these conditions. This might be an overestimate due to the potentiation of glycogen shunt activity in the presence of norepinephrine. Nevertheless, this may be the best approach to the in vivo situation where norepinephrine is present in brain at concentrations ranging from 1 to 15 μm, depending on the brain area (78). It should be noted that the finding that 40% of glucose is metabolized by way of glycogen is severalfold higher than earlier reports predicting glycogen synthesis to account for only 1–6% of total cerebral glucose consumption (79, 80). Taking the small glycogen reservoir into account (12), a functional role of the glycogen shunt with regard to sustaining cerebral activity and astrocytic neurotransmitter clearance implies that mobilization and the following reestablishment of glycogen are successive events mediated within seconds, as discussed by Shulman et al. (62). Such a scenario would require a high glycogen shunt activity involving only the peripheral part of the glycogen molecule, which is compatible with the finding that the cerebral glycogen content is remarkably constant under a wide range of physiological conditions (81). It should be noted that there is a dearth of data from in vivo studies evaluating the extent of glucose being metabolized via the glycogen shunt. Instead, a turnover time constant for brain glycogen has been estimated to be 5 and 24 h in conscious rats and humans, respectively (3, 79, 82, 83). This determines the total glycogen turnover time, i.e. the time needed for replacement of an amount of glycosyl units corresponding to the total brain glycogen pool at any given time. The spherical structure of glycogen and the fact that glycogen metabolism largely follows the “last-in-first-out” principle (84) would lead to the suggestion that the outer part of the molecule is much more dynamic than the inner layers. Hence, assessing a total turnover of glycogen is much more complicated than turnover of a pool of substrate exhibiting random degradation, and in addition, its relevance may be questioned.
Role of glycogen phosphorylase isoforms for glycogen shunt activity
The activity of the GP isoforms is differentially regulated, i.e. the muscle isoform is activated mainly via phosphorylation by PhK, whereas the brain isoform is more responsive to allosteric activation by AMP (46). This suggests that the two isoforms of GP serve different purposes; mGP elicits glycogen degradation secondary to receptor stimulation following neuronal activity, and bGP mediates glycogen breakdown as a consequence of energy fluctuations in the astrocytic microenvironment. We have recently suggested that the disproportionate augmentation of glycolysis observed when glycogen degradation was abolished, i.e. glycolytic supercompensation, be mediated predominantly as a result of hampered mGP activity (85). In line with this, glycolytic supercompensation in vivo was detected in response to whisker stimulation in conscious rats, i.e. as a consequence of neuronal activation (64). In contrast, the glycolytic supercompensation observed in cultured astrocytes during exposure to d-aspartate exhibits a delay of at least 30 min in onset (11). This may indicate that glycogen used to fuel glutamate transporters is degraded (at least initially) by bGP and relies on an increase in the intracellular AMP level, and only sustained exposure to d-aspartate results in phosphorylation of mGP.
Implications of glycogen in sustaining glutamatergic neurotransmission
Glycogen as energy substrate during neurotransmission
It has repeatedly been demonstrated that lactate derived from astrocyte glycogen is able to sustain neuronal activity in the absence of other energy substrates (20, 67, 68, 86–89). Although the physiological relevance of this may be questioned, glycogen degradation was also essential for maintenance of processes related to neurotransmission in the presence of a physiological glucose concentration (22, 64, 88). However, whether the energy derived from glycogen is destined for astrocytic or neuronal purposes is unclear. Several studies have demonstrated that obstructing lactate transfer between astrocytes and stimulated neurons in the absence of an exogenous energy substrate results in accelerated neuronal failure (20, 67, 68, 87–89). It is thereby suggested that astrocytes degrade glycogen to lactate, which is then oxidatively metabolized in neurons to cover the energetic demands related to neurotransmission. This is supported by the finding that abolishing glycogen degradation led to a reduction in glutamate release that was comparable with that observed when inhibiting lactate transfer between astrocytes and neurons (22). However, the notion that glycogen is mobilized upon decrements in the energy state of the local microenvironment (i.e. when the intracellular AMP concentration increases) implies that glycogen sustains energy-demanding processes within the astrocytic compartment. This is supported by the observation that impeding glycogen degradation in cultured astrocytes resulted in not only a disproportionate increase in glycolytic activity, i.e. glycolytic supercompensation, but also led to supercompensation of TCA cycle metabolism (11). Moreover, following glutamatergic neurotransmission, glutamate clearance from the synapse is one of the energy-requiring processes related to astrocytes, and astrocytic energy shortage may lead to reversal of the transporter resulting in excitotoxic levels of glutamate in the synapse (90). This, in turn, may result in neuronal failure (91, 92). It should be noted, however, that these scenarios are not mutually exclusive, and glycogen may serve as an energy substrate utilized in both astrocytes and neurons compatible with the two isoforms of GP being activated by distinct mechanisms.
Glycogen as a precursor for the neurotransmitter glutamate
In addition to its role as an energy substrate, glycogen serves as a precursor for glutamate and glutamine synthesis (7, 13). In order for glycogen to provide the entire carbon skeleton of glutamate/glutamine, the entrance of pyruvate into the TCA cycle must occur by means of pyruvate carboxylase as well as via pyruvate dehydrogenase (Fig. 2). Because of the importance of the glutamate–glutamine cycle for replenishment of neurotransmitter pools (69, 93–95), inhibition of glycogen degradation may lead to decrements in neuronal glutamate synthesis. In agreement with this, it was demonstrated that glutamate–glutamine cycle activity (Fig. 2) is impaired in an obese rat model displaying hampered glycogen metabolism in combination with a reduction in cerebral glycogen content (36). As filling of glutamatergic vesicles depends upon the cytosolic glutamate concentration (96), this may ultimately lead to impairments in neuronal glutamate release upon depolarization. Hence, it may be speculated that a lower vesicular glutamate release from neurons upon inhibition of glycogen degradation arises as a consequence of deficits in astrocytic glutamine synthesis and not (only) because of energy deficiency.
Concluding remarks
Astrocyte glycogen plays a vital role in a number of brain functions and is aberrant in not only neurological diseases but also type 2 diabetes. An increased understanding of the regulation and functional roles of astrocyte glycogen in health and disease will likely uncover novel drug targets for the potential benefit of patients.
This work was supported by the Hørslev Foundation and the Faculty of Health and Medical Sciences at University of Copenhagen (to L. K. B.) and Lundbeck Foundation Grant R165-2013-15334 (to A. B. W.). This is the third article in the Thematic Minireview series “Brain glycogen metabolism.” The authors declare that they have no conflicts of interest with the contents of this article.
- ICV
- intracerebroventricular
- PK
- pyruvate kinase
- TCA
- tricarboxylic acid cycle
- DAB
- 4-dideoxy-1,4-imino-d-arabinitol
- SOCE
- store-operated Ca2+ entry
- GPCR
- G protein–coupled receptor
- AC
- adenylate cyclase
- PhK
- phosphorylase kinase
- GP
- glycogen phosphorylase
- bGP
- brain GP
- mGP
- muscle GP
- IP3
- inositol 1,4,5-triphosphate
- TRPC
- transient receptor potential channel.
References
- 1. Öz G., DiNuzzo M., Kumar A., Moheet A., and Seaquist E. R. (2015) Revisiting glycogen content in the human brain. Neurochem. Res. 40, 2473–2481 10.1007/s11064-015-1664-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Öz G., DiNuzzo M., Kumar A., Moheet A., Khowaja A., Kubisiak K., Eberly L. E., and Seaquist E. R. (2017) Cerebral glycogen in humans following acute and recurrent hypoglycemia: implications on a role in hypoglycemia unawareness. J. Cereb. Blood Flow Metab. 37, 2883–2893 10.1177/0271678X16678240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oz G., Kumar A., Rao J. P., Kodl C. T., Chow L., Eberly L. E., and Seaquist E. R. (2009) Human brain glycogen metabolism during and after hypoglycemia. Diabetes 58, 1978–1985 10.2337/db09-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duran J., Saez I., Gruart A., Guinovart J. J., and Delgado-García J. M. (2013) Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J. Cereb. Blood Flow Metab. 33, 550–556 10.1038/jcbfm.2012.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki A., Stern S. A., Bozdagi O., Huntley G. W., Walker R. H., Magistretti P. J., and Alberini C. M. (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 10.1016/j.cell.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibbs M. E., Lloyd H. G., Santa T., and Hertz L. (2007) Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J. Neurosci. Res. 85, 3326–3333 10.1002/jnr.21307 [DOI] [PubMed] [Google Scholar]
- 7. Hertz L., O'Dowd B. S., Ng K. T., and Gibbs M. E. (2003) Reciprocal changes in forebrain contents of glycogen and of glutamate/glutamine during early memory consolidation in the day-old chick. Brain Res. 994, 226–233 10.1016/j.brainres.2003.09.044 [DOI] [PubMed] [Google Scholar]
- 8. Boury-Jamot B., Carrard A., Martin J. L., Halfon O., Magistretti P. J., and Boutrel B. (2016) Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry 21, 1070–1076 10.1038/mp.2015.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J., Song D., Xue Z., Gu L., Hertz L., and Peng L. (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem. Res. 38, 472–485 10.1007/s11064-012-0938-3 [DOI] [PubMed] [Google Scholar]
- 10. Sickmann H. M., Walls A. B., Bouman S. D., Schousboe A., and Waagepetersen H. S. (2009) Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J. Neurochem. 109, Suppl. 1, 80–86 10.1111/j.1471-4159.2009.05915.x [DOI] [PubMed] [Google Scholar]
- 11. Walls A. B., Heimbürger C. M., Bouman S. D., Schousboe A., and Waagepetersen H. S. (2009) Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neuroscience 158, 284–292 10.1016/j.neuroscience.2008.09.058 [DOI] [PubMed] [Google Scholar]
- 12. Clarke D. D., and Sokoloff L. (1999) in Basic Neurochemistry (Siegel G. J., ed) 6th Ed., pp. 637–670, Lippincott-Raven Publishers, Philadelphia, PA: DOI not found. PMID not found. [Google Scholar]
- 13. Gibbs M. E., Anderson D. G., and Hertz L. (2006) Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54, 214–222 10.1002/glia.20377 [DOI] [PubMed] [Google Scholar]
- 14. Newman L. A., Korol D. L., and Gold P. E. (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 6, e28427 10.1371/journal.pone.0028427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibbs M. E. (2015) Role of Glycogenolysis in memory and learning: regulation by noradrenaline, serotonin and ATP. Front. Integr. Neurosci. 9, 70 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hertz L. (1989) Is Alzheimer's disease an anterograde degeneration, originating in the brainstem, and disrupting metabolic and functional interactions between neurons and glial cells? Brain Res. Brain Res. Rev. 14, 335–353 10.1016/0165-0173(89)90017-9 [DOI] [PubMed] [Google Scholar]
- 17. Marien M. R., Colpaert F. C., and Rosenquist A. C. (2004) Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res. Brain Res. Rev. 45, 38–78 10.1016/j.brainresrev.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 18. Weinshenker D. (2008) Functional consequences of locus coeruleus degeneration in Alzheimer's disease. Curr. Alzheimer Res. 5, 342–345 10.2174/156720508784533286 [DOI] [PubMed] [Google Scholar]
- 19. Koivisto H., Leinonen H., Puurula M., Hafez H. S., Barrera G. A., Stridh M. H., Waagepetersen H. S., Tiainen M., Soininen P., Zilberter Y., and Tanila H. (2016) Chronic pyruvate supplementation increases exploratory activity and brain energy reserves in young and middle-aged mice. Front. Aging Neurosci. 8, 41 10.3389/fnagi.2016.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wender R., Brown A. M., Fern R., Swanson R. A., Farrell K., and Ransom B. R. (2000) Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 20, 6804–6810 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown A. M., Baltan Tekkök S., and Ransom B. R. (2004) Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int. 45, 529–536 10.1016/j.neuint.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 22. Sickmann H. M., Walls A. B., Schousboe A., Bouman S. D., and Waagepetersen H. S. (2009) Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J. Neurochem. 109, Suppl. 1, 80–86 10.1111/j.1471-4159.2009.05915.x [DOI] [PubMed] [Google Scholar]
- 23. Nedergaard M., and Hansen A. J. (1993) Characterization of cortical depolarizations evoked in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 13, 568–574 10.1038/jcbfm.1993.74 [DOI] [PubMed] [Google Scholar]
- 24. DiNuzzo M., Mangia S., Maraviglia B., and Giove F. (2015) Does abnormal glycogen structure contribute to increased susceptibility to seizures in epilepsy? Metab. Brain Dis. 30, 307–316 10.1007/s11011-014-9524-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dalsgaard M. K., Madsen F. F., Secher N. H., Laursen H., and Quistorff B. (2007) High glycogen levels in the hippocampus of patients with epilepsy. J. Cereb. Blood Flow Metab. 27, 1137–1141 10.1038/sj.jcbfm.9600426 [DOI] [PubMed] [Google Scholar]
- 26. Walls A. B., Eyjolfsson E. M., Schousboe A., Sonnewald U., and Waagepetersen H. S. (2014) A subconvulsive dose of kainate selectively compromises astrocytic metabolism in the mouse brain in vivo. J. Cereb. Blood Flow Metab. 34, 1340–1346 10.1038/jcbfm.2014.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dinuzzo M., Mangia S., Maraviglia B., and Giove F. (2012) The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem. Res. 37, 2432–2438 10.1007/s11064-012-0802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feuerstein D., Backes H., Gramer M., Takagaki M., Gabel P., Kumagai T., and Graf R. (2016) Regulation of cerebral metabolism during cortical spreading depression. J. Cereb. Blood Flow Metab. 36, 1965–1977 10.1177/0271678X15612779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kong J., Shepel P. N., Holden C. P., Mackiewicz M., Pack A. I., and Geiger J. D. (2002) Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J. Neurosci. 22, 5581–5587 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gip P., Hagiwara G., Ruby N. F., and Heller H. C. (2002) Sleep deprivation decreases glycogen in the cerebellum but not in the cortex of young rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R54–R59 10.1152/ajpregu.00735.2001 [DOI] [PubMed] [Google Scholar]
- 31. Franken P., Gip P., Hagiwara G., Ruby N. F., and Heller H. C. (2003) Changes in brain glycogen after sleep deprivation vary with genotype. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R413–R419 10.1152/ajpregu.00668.2002 [DOI] [PubMed] [Google Scholar]
- 32. Benington J. H., and Heller H. C. (1995) Restoration of brain energy metabolism as the function of sleep. Prog. Neurobiol. 45, 347–360 10.1016/0301-0082(94)00057-O [DOI] [PubMed] [Google Scholar]
- 33. Petit J. M., Burlet-Godinot S., Magistretti P. J., and Allaman I. (2015) Glycogen metabolism and the homeostatic regulation of sleep. Metab. Brain Dis. 30, 263–279 10.1007/s11011-014-9629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karnovsky M. L., Reich P., Anchors J. M., and Burrows B. L. (1983) Changes in brain glycogen during slow-wave sleep in the rat. J. Neurochem. 41, 1498–1501 10.1111/j.1471-4159.1983.tb00853.x [DOI] [PubMed] [Google Scholar]
- 35. Sickmann H. M., and Waagepetersen H. S. (2015) Effects of diabetes on brain metabolism–is brain glycogen a significant player? Metab. Brain Dis. 30, 335–343 10.1007/s11011-014-9546-z [DOI] [PubMed] [Google Scholar]
- 36. Sickmann H. M., Waagepetersen H. S., Schousboe A., Benie A. J., and Bouman S. D. (2010) Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J. Cereb. Blood Flow Metab. 30, 1527–1537 10.1038/jcbfm.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi I. Y., and Gruetter R. (2003) In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem. Int. 43, 317–322 10.1016/S0197-0186(03)00018-4 [DOI] [PubMed] [Google Scholar]
- 38. Öz G., Tesfaye N., Kumar A., Deelchand D. K., Eberly L. E., and Seaquist E. R. (2012) Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J. Cereb. Blood Flow Metab. 32, 256–263 10.1038/jcbfm.2011.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gentry M. S., Guinovart J. J., Minassian B. A., Roach P. J., and Serratosa J. (February 26, 2018) Lafora disease offers a unique window into neuronal glycogen metabolism. J. Biol. Chem. 293, 7117–7125 10.1074/jbc.R117.803064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duarte J. M. N., Morgenthaler F. D., and Gruetter R. (2017) Glycogen supercompensation in the rat brain after acute hypoglycemia is independent of glucose levels during recovery. Neurochem. Res. 42, 1629–1635 10.1007/s11064-017-2178-z [DOI] [PubMed] [Google Scholar]
- 41. Obel L. F., Müller M. S., Walls A. B., Sickmann H. M., Bak L. K., Waagepetersen H. S., and Schousboe A. (2012) Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenerget. 4, 3 10.3389/fnene.2012.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfeiffer-Guglielmi B., Fleckenstein B., Jung G., and Hamprecht B. (2003) Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J. Neurochem. 85, 73–81 10.1046/j.1471-4159.2003.01644.x [DOI] [PubMed] [Google Scholar]
- 43. Pfeiffer-Guglielmi B., Bröer S., Bröer A., and Hamprecht B. (2000) Isozyme pattern of glycogen phosphorylase in the rat nervous system and rat astroglia-rich primary cultures: electrophoretic and polymerase chain reaction studies. Neurochem. Res. 25, 1485–1491 10.1023/A:1007676109206 [DOI] [PubMed] [Google Scholar]
- 44. Brushia R. J., and Walsh D. A. (1999) Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 4, D618–D641 10.2741/Brushia [DOI] [PubMed] [Google Scholar]
- 45. Crerar M. M., Karlsson O., Fletterick R. J., and Hwang P. K. (1995) Chimeric muscle and brain glycogen phosphorylases define protein domains governing isozyme-specific responses to allosteric activation. J. Biol. Chem. 270, 13748–13756 10.1074/jbc.270.23.13748 [DOI] [PubMed] [Google Scholar]
- 46. Müller M. S., Pedersen S. E., Walls A. B., Waagepetersen H. S., and Bak L. K. (2015) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63, 154–162 10.1002/glia.22741 [DOI] [PubMed] [Google Scholar]
- 47. Hertz L., Lovatt D., Goldman S. A., and Nedergaard M. (2010) Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem. Int. 57, 411–420 10.1016/j.neuint.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holtman I. R., Noback M., Bijlsma M., Duong K. N., van der Geest M. A., Ketelaars P. T., Brouwer N., Vainchtein I. D., Eggen B. J., and Boddeke H. W. (2015) Glia open access database (GOAD): a comprehensive gene expression encyclopedia of glia cells in health and disease. Glia 63, 1495–1506 10.1002/glia.22810 [DOI] [PubMed] [Google Scholar]
- 49. Bazargani N., and Attwell D. (2016) Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- 50. Willoughby D., and Cooper D. M. (2008) Live-cell imaging of cAMP dynamics. Nat. Methods 5, 29–36 10.1038/nmeth1135 [DOI] [PubMed] [Google Scholar]
- 51. Ellisdon A. M., and Halls M. L. (2016) Compartmentalization of GPCR signalling controls unique cellular responses. Biochem. Soc. Trans. 44, 562–567 10.1042/BST20150236 [DOI] [PubMed] [Google Scholar]
- 52. Berridge M. J. (2006) Calcium microdomains: organization and function. Cell Calcium 40, 405–412 10.1016/j.ceca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 53. Müller M. S., Fox R., Schousboe A., Waagepetersen H. S., and Bak L. K. (2014) Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia. 62, 526–534 10.1002/glia.22623 [DOI] [PubMed] [Google Scholar]
- 54. Volterra A., and Meldolesi J. (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640 10.1038/nrn1722 [DOI] [PubMed] [Google Scholar]
- 55. Willoughby D., Everett K. L., Halls M. L., Pacheco J., Skroblin P., Vaca L., Klussmann E., and Cooper D. M. (2012) Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci. Signal. 5, ra29 DOI not found. [DOI] [PubMed] [Google Scholar]
- 56. Martin A. C., Willoughby D., Ciruela A., Ayling L. J., Pagano M., Wachten S., Tengholm A., and Cooper D. M. (2009) Capacitative Ca2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol. Pharmacol. 75, 830–842 10.1124/mol.108.051748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Willoughby D., Ong H. L., De Souza L. B., Wachten S., Ambudkar I. S., and Cooper D. M. (2014) TRPC1 contributes to the Ca2+-dependent regulation of adenylate cyclases. Biochem. J. 464, 73–84 10.1042/BJ20140766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Verkhratsky A., and Parpura V. (2014) Store-operated calcium entry in neuroglia. Neurosci. Bull. 30, 125–133 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kwon J., An H., Sa M., Won J., Shin J. I., and Lee C. J. (2017) Orai1 and Orai3 in combination with Stim1 mediate the majority of store-operated calcium entry in astrocytes. Exp. Neurobiol. 26, 42–54 10.5607/en.2017.26.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nikolaev V. O., Bünemann M., Schmitteckert E., Lohse M. J., and Engelhardt S. (2006) Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined β2-adrenergic receptor-mediated signaling. Circ. Res. 99, 1084–1091 10.1161/01.RES.0000250046.69918.d5 [DOI] [PubMed] [Google Scholar]
- 61. Obel L. F. (2011) Energy and Amino Acid Metabolism in Astrocytes: Roles of Glycogen and Ca2+. Ph.D. thesis, University of Copenhagen [Google Scholar]
- 62. Shulman R. G., Hyder F., and Rothman D. L. (2001) Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc. Natl. Acad. Sci. U.S.A. 98, 6417–6422 10.1073/pnas.101129298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shulman R. G., and Rothman D. L. (2001) The “glycogen shunt” in exercising muscle: A role for glycogen in muscle energetics and fatigue. Proc. Natl. Acad. Sci. U.S.A. 98, 457–461 10.1073/pnas.98.2.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dienel G. A., Ball K. K., and Cruz N. F. (2007) A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J. Neurochem. 102, 466–478 10.1111/j.1471-4159.2007.04595.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Swanson R. A., Morton M. M., Sagar S. M., and Sharp F. R. (1992) Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience 51, 451–461 10.1016/0306-4522(92)90329-Z [DOI] [PubMed] [Google Scholar]
- 66. Cruz N. F., and Dienel G. A. (2002) High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J. Cereb. Blood Flow Metab. 22, 1476–1489 10.1097/01.WCB.0000034362.37277.C0 [DOI] [PubMed] [Google Scholar]
- 67. Brown A. M., Tekkök S. B., and Ransom B. R. (2003) Glycogen regulation and functional role in mouse white matter. J. Physiol. 549, 501–512 10.1113/jphysiol.2003.042416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suh S. W., Bergher J. P., Anderson C. M., Treadway J. L., Fosgerau K., and Swanson R. A. (2007) Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide). J. Pharmacol. Exp. Ther. 321, 45–50 10.1124/jpet.106.115550 [DOI] [PubMed] [Google Scholar]
- 69. Schousboe A., Walls A. B., Bak L. K., and Waagepetersen H. S. (2015) Astroglia and brain metabolism: focus on energy and neurotransmitter amino acid homeostasis. Morgan & Claypool Life Sciences, Milton Keynes, UK. 2, 1–64 10.4199/C00130ED1V01Y201506NGL007 [DOI] [Google Scholar]
- 70. Pellerin L., Bouzier-Sore A. K., Aubert A., Serres S., Merle M., Costalat R., and Magistretti P. J. (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55, 1251–1262 10.1002/glia.20528 [DOI] [PubMed] [Google Scholar]
- 71. Sickmann H. M., Schousboe A., Fosgerau K., and Waagepetersen H. S. (2005) Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem. Res. 30, 1295–1304 10.1007/s11064-005-8801-4 [DOI] [PubMed] [Google Scholar]
- 72. Subbarao K. V., and Hertz L. (1990) Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 536, 220–226 10.1016/0006-8993(90)90028-A [DOI] [PubMed] [Google Scholar]
- 73. Subbarao K. V., and Hertz L. (1990) Noradrenaline induced stimulation of oxidative metabolism in astrocytes but not in neurons in primary cultures. Brain Res. 527, 346–349 10.1016/0006-8993(90)91157-C [DOI] [PubMed] [Google Scholar]
- 74. Sorg O., and Magistretti P. J. (1991) Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 563, 227–233 10.1016/0006-8993(91)91538-C [DOI] [PubMed] [Google Scholar]
- 75. Waagepetersen H. S., Westergaard N., and Schousboe A. (2000) The effects of isofagomine, a potent glycogen phosphorylase inhibitor, on glycogen metabolism in cultured mouse cortical astrocytes. Neurochem. Int. 36, 435–440 10.1016/S0197-0186(99)00146-1 [DOI] [PubMed] [Google Scholar]
- 76. Hertz L., Peng L., and Dienel G. A. (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab. 27, 219–249 10.1038/sj.jcbfm.9600343 [DOI] [PubMed] [Google Scholar]
- 77. Hutchinson D. S., Summers R. J., and Gibbs M. E. (2008) Energy metabolism and memory processing: role of glucose transport and glycogen in responses to adrenoceptor activation in the chicken. Brain Res. Bull. 76, 224–234 10.1016/j.brainresbull.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 78. Mefford I. N., Foutz A., Noyce N., Jurik S. M., Handen C., Dement W. C., and Barchas J. D. (1982) Distribution of norepinephrine, epinephrine, dopamine, serotonin, 3,4-dihydroxyphenylacetic acid, homovanillic acid and 5-hydroxyindole-3-acetic acid in dog brain. Brain Res. 236, 339–349 10.1016/0006-8993(82)90719-3 [DOI] [PubMed] [Google Scholar]
- 79. Watanabe H., and Passonneau J. V. (1973) Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J. Neurochem. 20, 1543–1554 10.1111/j.1471-4159.1973.tb00272.x [DOI] [PubMed] [Google Scholar]
- 80. Choi I. Y., Tkác I., Ugurbil K., and Gruetter R. (1999) Noninvasive measurements of [1-13C]glycogen concentrations and metabolism in rat brain in vivo. J. Neurochem. 73, 1300–1308 DOI not found. [DOI] [PubMed] [Google Scholar]
- 81. Watanabe H., and Passonneau J. V. (1975) Cyclic adenosine monophosphate in cerebral cortex. Alterations following trauma. Arch. Neurol. 32, 181–184 10.1001/archneur.1975.00490450061008 [DOI] [PubMed] [Google Scholar]
- 82. Oz G., Seaquist E. R., Kumar A., Criego A. B., Benedict L. E., Rao J. P., Henry P. G., Van De Moortele P. F., and Gruetter R. (2007) Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am. J. Physiol. Endocrinol. Metab. 292, E946–E951 10.1152/ajpendo.00424.2006 [DOI] [PubMed] [Google Scholar]
- 83. Morgenthaler F. D., Lanz B. R., Petit J. M., Frenkel H., Magistretti P. J., and Gruetter R. (2009) Alteration of brain glycogen turnover in the conscious rat after 5 h of prolonged wakefulness. Neurochem. Int. 55, 45–51 10.1016/j.neuint.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Elsner P., Quistorff B., Hansen G. H., and Grunnet N. (2002) Partly ordered synthesis and degradation of glycogen in cultured rat myotubes. J. Biol. Chem. 277, 4831–4838 10.1074/jbc.M108226200 [DOI] [PubMed] [Google Scholar]
- 85. Jakobsen E., Bak L. K., Walls A. B., Reuschlein A. K., Schousboe A., and Waagepetersen H. S. (2017) The enigmatic phenomenon of glycolytic supercompensation in astrocytes may be distinctly mediated via the muscle form of glycogen phosphorylase. Neurochem. Res. 42, 2940–2494 [DOI] [PubMed] [Google Scholar]
- 86. Swanson R. A., and Choi D. W. (1993) Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J. Cereb. Blood Flow Metab. 13, 162–169 10.1038/jcbfm.1993.19 [DOI] [PubMed] [Google Scholar]
- 87. Ransom B. R., and Fern R. (1997) Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation? Glia 21, 134–141 10.1002/(SICI)1098-1136(199709)21:1%3C134::AID-GLIA15%3E3.0.CO%3B2-T [DOI] [PubMed] [Google Scholar]
- 88. Brown A. M., Sickmann H. M., Fosgerau K., Lund T. M., Schousboe A., Waagepetersen H. S., and Ransom B. R. (2005) Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 79, 74–80 10.1002/jnr.20335 [DOI] [PubMed] [Google Scholar]
- 89. Walls A. B., Sickmann H. M., Brown A., Bouman S. D., Ransom B., Schousboe A., and Waagepetersen H. S. (2008) Characterization of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) as an inhibitor of brain glycogen shunt activity. J. Neurochem. 105, 1462–1470 10.1111/j.1471-4159.2008.05250.x [DOI] [PubMed] [Google Scholar]
- 90. Rossi D. J., Oshima T., and Attwell D. (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321 10.1038/35002090 [DOI] [PubMed] [Google Scholar]
- 91. Bonde C., Sarup A., Schousboe A., Gegelashvili G., Zimmer J., and Noraberg J. (2003) Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor dl-threo-β-benzyloxyaspartate (dl-TBOA) during physiological and ischemia-like conditions. Neurochem. Int. 43, 371–380 10.1016/S0197-0186(03)00024-X [DOI] [PubMed] [Google Scholar]
- 92. Waagepetersen H. S., Qu H., Sonnewald U., Shimamoto K., and Schousboe A. (2005) Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons. Neurochem. Int. 47, 92–102 10.1016/j.neuint.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 93. Lebon V., Petersen K. F., Cline G. W., Shen J., Mason G. F., Dufour S., Behar K. L., Shulman G. I., and Rothman D. L. (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci. 22, 1523–1531 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rothman D. L., Behar K. L., Hyder F., and Shulman R. G. (2003) In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu. Rev. Physiol. 65, 401–427 10.1146/annurev.physiol.65.092101.142131 [DOI] [PubMed] [Google Scholar]
- 95. Tani H., Dulla C. G., Huguenard J. R., and Reimer R. J. (2010) Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J. Neurosci. 30, 1288–1300 10.1523/JNEUROSCI.0106-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wilson N. R., Kang J., Hueske E. V., Leung T., Varoqui H., Murnick J. G., Erickson J. D., and Liu G. (2005) Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J. Neurosci. 25, 6221–6234 10.1523/JNEUROSCI.3003-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nadeau O. W., Fontes J. D., and Carlson G. M. (February 26, 2018) The regulation of glycogenolysis in the brain. J. Biol. Chem. 293, 7099–7107 10.1074/jbc.R117.803023 [DOI] [PMC free article] [PubMed] [Google Scholar]