Abstract
Proteins with domains that recognize and bind post-translational modifications (PTMs) of histones are collectively termed epigenetic readers. Numerous interactions between specific reader protein domains and histone PTMs and their regulatory outcomes have been reported, but little is known about how reader proteins may in turn be modulated by these interactions. Tripartite motif–containing protein 24 (TRIM24) is a histone reader aberrantly expressed in multiple cancers. Here, our investigation revealed functional cross-talk between histone acetylation and TRIM24 SUMOylation. Binding of TRIM24 to chromatin via its tandem PHD-bromodomain, which recognizes unmethylated lysine 4 and acetylated lysine 23 of histone H3 (H3K4me0/K23ac), led to TRIM24 SUMOylation at lysine residues 723 and 741. Inactivation of the bromodomain, either by mutation or with a small-molecule inhibitor, IACS-9571, abolished TRIM24 SUMOylation. Conversely, inhibition of histone deacetylation markedly increased TRIM24's interaction with chromatin and its SUMOylation. Of note, gene expression profiling of MCF7 cells expressing WT versus SUMO-deficient TRIM24 identified cell adhesion as the major pathway regulated by the cross-talk between chromatin acetylation and TRIM24 SUMOylation. In conclusion, our findings establish a new link between histone H3 acetylation and SUMOylation of the reader protein TRIM24, a functional connection that may bear on TRIM24's oncogenic function and may inform future studies of PTM cross-talk between histones and epigenetic regulators.
Keywords: SUMOylation, chromatin modification, histone acetylation, cell adhesion, breast cancer, epigenetics, bromodomain, histone reader, reader protein, TRIM24
Introduction
Epigenetic regulatory proteins play an essential role in chromatin-related events by imposing and/or assessing the modification status and accessibility of chromatin (1–3). Based on their functional roles in these processes, epigenetic regulators are broadly categorized as “writer” enzymes, “reader” proteins, or “eraser” enzymes (4). These regulators are responsible for depositing, recognizing, or removing chemical modifications, respectively, and largely influence chromatin-dependent functions. Studies to date offer a good understanding of how different reader proteins engage their respective histone modifications and influence chromatin-dependent functions (5, 6). However, whether engagement of chromatin by reader proteins induces signaling in the reverse direction and what consequences this may have is ill-defined. We addressed this question by focusing on TRIM24 (tripartite motif-containing protein 24), also known as transcription intermediary factor 1α (TIF1α), a plant homeodomain (PHD)6/bromodomain acetyllysine reader protein (7). TRIM24 has a characteristic N-terminal RING, B-box, coiled-coil (RBCC) motif, common to members of the TRIM family of proteins (8). TRIM24 also functions as a transcriptional co-regulator of numerous nuclear receptors, including estrogen receptor α (ERα) (9–11). TRIM24 recognizes and binds a specific combinatorial signature of H3K4me0/K23ac via its C-terminal tandem PHD-bromodomain. TRIM24 expression is aberrantly up-regulated in many cancers, including breast cancer, where overexpression correlates with poor survival of patients (7, 12–14). Using an immortalized human mammary epithelial cell culture model, we previously reported oncogenic function of TRIM24 in promoting malignant transformation in vitro and xenograft tumor formation in vivo (15, 16). Although many reports have correlated TRIM24 overexpression with multiple cancers, the basic mechanisms of how TRIM24 functions, especially with regard to regulation of TRIM24 itself, are not well understood.
Here, we report that human TRIM24 is SUMOylated and uncover an essential role for the TRIM24 tandem PHD-bromodomain in this process. SUMOylation is a PTM where a small ubiquitin-like modifier (SUMO) protein is covalently added at lysine residues, most frequently at a consensus recognition motif. SUMOylation involves a cascade of E1, E2, and E3 enzymes, which function together to covalently link SUMO proteins to target lysines of substrates. Four isoforms of SUMO proteins, SUMO-1, SUMO-2, SUMO-3, and SUMO-4, are known in humans. This is a reversible process, as SUMO-specific proteases catalyze de-SUMOylation events (17). SUMOylation is involved in multiple cellular processes, including cellular localization, protein-protein interaction, cell cycle, and transcriptional regulation (17, 18).
In the current study, we show that SUMOylation of human TRIM24 occurs at SUMO consensus sites, which are conserved and SUMOylated in murine Trim24 (19). We find that TRIM24 association with chromatin is a prerequisite for TRIM24 SUMOylation by SUMO1 and SUMO2/3 proteins, and this association and SUMOylation are dependent on the TRIM24 PHD-bromodomain. We show that chemical modulation of H3K23 acetylation levels regulates TRIM24 SUMOylation, and a small molecule inhibitor of the TRIM24 bromodomain, IACS-9571 (20, 21), disrupts TRIM24 association with chromatin and decreases TRIM24 SUMOylation. TRIM24 SUMOylation alters global gene expression, primarily regulating genes involved in cell adhesion pathways. Our studies establish that a novel PTM cross-talk exists between histone H3 acetylation and TRIM24 SUMOylation, which may impact TRIM24 oncogenic function.
Results
Human TRIM24 is SUMO-modified
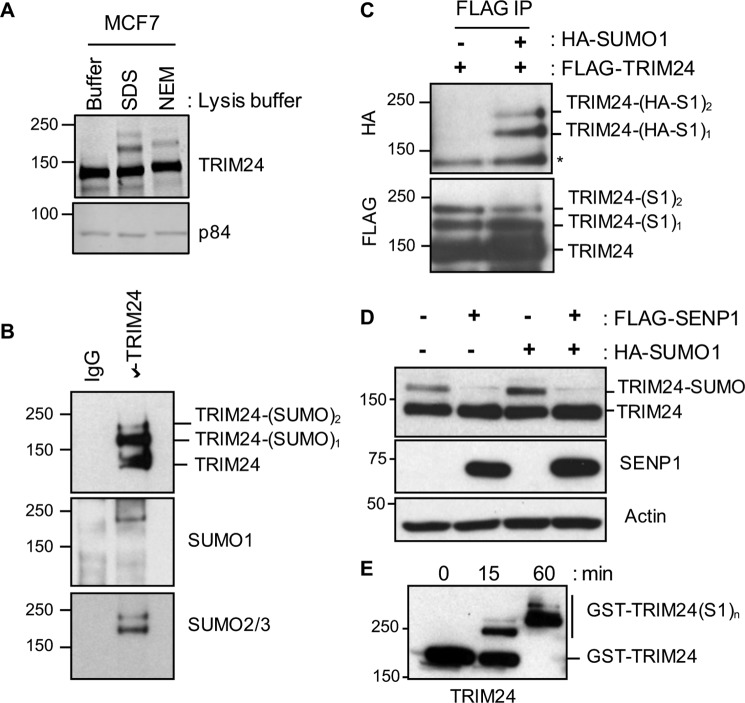
Post-translational modification by SUMO isoforms results in slowly migrating, higher-molecular weight forms of substrate proteins when separated by SDS-PAGE. Western blot analysis of endogenous TRIM24 from MCF7 cells showed the 116-kDa unmodified TRIM24 and TRIM24-positive, slower-migrating species (Fig. 1A). Typical of most SUMOylation, the slower-migrating TRIM24 bands were observed only under conditions where cell lysis was performed in the presence of N-ethylmaleimide (NEM), a general cysteine protease inhibitor, or 1% SDS, revealing two slower migrating SUMOylated species. To further evaluate the slower migrating species, we immunoprecipitated endogenous TRIM24 from HEK293T cells and analyzed with antibodies to TRIM24, SUMO1, and SUMO2/3 (Fig. 1B). In addition to the 116-kDa unmodified TRIM24 band, two distinct slower-migrating bands cross-reacted with TRIM24, SUMO1, and SUMO2/3 antibodies, consistent with TRIM24 modification by these SUMO isoforms. We then expressed ectopic FLAG-TRIM24 and HA-SUMO1 in HEK293T cells and performed immunoprecipitations using antibodies that recognize the FLAG or HA affinity tag (Fig. 1C). As with endogenous TRIM24, higher-molecular weight species of TRIM24 were enriched by FLAG immunoprecipitation. These bands cross-reacted with an antibody that recognizes an HA-moiety only in cells where HA-SUMO1 was co-expressed with FLAG-TRIM24, suggesting modification of exogenously expressed TRIM24 with HA-SUMO1.
Figure 1.
Human TRIM24 is SUMOylated. A, WCL from MCF7 cells prepared in lysis buffer with 1% SDS or NEM and subjected to TRIM24 Western blot analysis using p84 as loading control. B, TRIM24 is modified by SUMO1 and SUMO2/3. WCL from HEK293T cells were subjected to immunoprecipitation, using control IgG and TRIM24 antibody, followed by Western blot analysis with TRIM24, SUMO1, and SUMO2/3 antibodies. C, WCL from HEK293T cells transfected with FLAG-TRIM24 and HA-SUMO1 were used for FLAG immunoprecipitation (IP) followed by Western blot analysis with FLAG and HA antibodies. *, nonspecific band. D, TRIM24 is deSUMOylated by SENP1. HEK293T cells were transfected with FLAG-SENP1 and/or HA-SUMO1 plasmids followed by Western blot analysis using TRIM24 and FLAG antibodies. E, time course showing in vitro SUMOylation of bacterially expressed and purified GST-TRIM24 protein.
Protein SUMOylation is a dynamic process, subject to catalytic addition by SUMO-conjugating enzymes and de-SUMOylation by SUMO proteases (22). Overexpression of the SUMO protease FLAG-SENP1 in HEK293T cells led to complete loss of slowly migrating TRIM24 bands and a concomitant increase in the unmodified form of TRIM24, adding further support for TRIM24 modification by SUMO protein (Fig. 1D). To determine whether TRIM24 may be directly SUMOylated in vitro, we performed a SUMOylation assay using purified recombinant proteins. A 15-min reaction resulted in partial SUMOylation, and after 60 min, complete modification of GST-TRIM24 with SUMO1 was observed (Fig. 1E).
TRIM24 is SUMOylated at Lys723 and Lys741 in a PHD-bromodomain–dependent manner
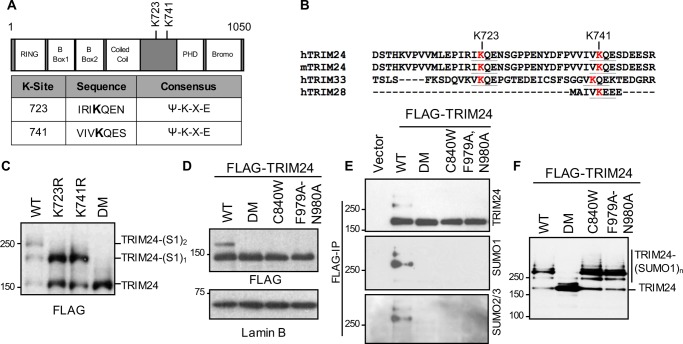
TRIM24 protein sequence analysis showed two consensus motifs as potential sites of SUMOylation (Fig. 2A), which are conserved and identical in mouse Trim24 (Fig. 2B) but less so in human TRIM/TIF1-subfamily members TRIM28 and TRIM33 that have a similar RBCC-PHD/bromodomain arrangement but distinct functions (8). To identify the sites of SUMOylation, FLAG-TRIM24 expression plasmids where lysine residues at position 723 and 741 were mutated to arginine were generated. FLAG-TRIM24 WT and mutant proteins were expressed in HEK293T cells, immunoprecipitated using FLAG antibody, and subjected to in vitro SUMOylation (Fig. 2C). Whereas FLAG-TRIM24 WT showed two slowly migrating bands, only one band was observed with the K723R and K741R mutants. The double mutant (DM) where both sites were mutated lacked slower-migrating TRIM24 bands, suggesting Lys723 and Lys741 as the only sites of SUMO modification in human TRIM24.
Figure 2.
TRIM24 is SUMOylated at Lys723–Lys741 in a PHD-bromodomain-dependent manner. A, schematic of human TRIM24 (hTRIM24) showing domain architecture and SUMO modification sites. B, sequence alignment showing conservation of hTRIM24 SUMO-modified residues, Lys723 and Lys741 (in red) in mouse Trim24 (mTrim24), hTRIM33, and hTRIM28. SUMO consensus motifs are underlined. C, HEK293T cells were transfected with FLAG-TRIM24 WT and SUMO site mutants followed by anti-FLAG immunoprecipitation. FLAG-TRIM24 immobilized on FLAG beads were washed and subjected to an in vitro SUMOylation assay followed by Western blot analysis using FLAG antibody. D, PHD finger and bromodomain mutant proteins are not SUMOylated. HEK293T cells were transfected with FLAG-TRIM24 WT, SUMOylation mutant (DM), PHD finger mutant (C840W), and bromodomain mutant (F979A/N980A) followed by Western blot analysis. E, HEK293T cells expressing control vector, FLAG-TRIM24 WT, or the indicated mutant proteins were immunoprecipitated (IP) using anti-FLAG beads followed by Western blot analysis with FLAG, SUMO1, and SUMO2/3 antibodies. F, HEK293T cells were transfected with FLAG-TRIM24 WT and mutants followed by anti-FLAG immunoprecipitation. FLAG-TRIM24 immobilized on FLAG beads were washed and subjected to in vitro SUMOylation assay followed by Western blot analysis using FLAG antibody.
The tandem PHD-bromodomain of TRIM24 recognizes a combinatorial signature of H3K4me0/K23ac (7). We assessed whether the PHD-bromodomain, which lies C-terminal of the identified SUMOylated residues, functionally contributed to TRIM24 SUMOylation. We expressed TRIM24 PHD mutant (C840W) and bromodomain mutant (F979A/N980A) in HEK293T cells. These mutants were previously characterized as lacking histone-binding ability (7). TRIM24 PHD and bromodomain mutants, similar to SUMO site-mutant TRIM24 DM, lacked SUMOylation (Fig. 2D). To confirm our observation, FLAG-TRIM24 WT and mutant proteins were immunoprecipitated using FLAG antibody to enrich for TRIM24 SUMO-modified species (Fig. 2E). Whereas SUMO1 and SUMO2/3 cross-reactive bands were observed when TRIM24 WT was expressed, no such bands were observed with mutant proteins. These studies suggested a critical role for a functional PHD-bromodomain in SUMOylation of TRIM24. To address the possibility that PHD-bromodomain point mutants caused gross structural changes that masked the SUMOylation sites, we performed FLAG immunoprecipitations followed by an in vitro SUMOylation assay (Fig. 2F). Similar to TRIM24 WT protein, PHD and bromodomain mutants were SUMO-modified, suggesting availability of Lys723 and Lys741 for SUMO modification in vitro. Thus, whereas PHD-bromodomain mutants were able to be SUMOylated in vitro, the lack of endogenous SUMO modification in cells suggested a role for PHD-bromodomain–mediated chromatin association in SUMOylation of TRIM24.
Chromatin association is required for TRIM24 SUMOylation
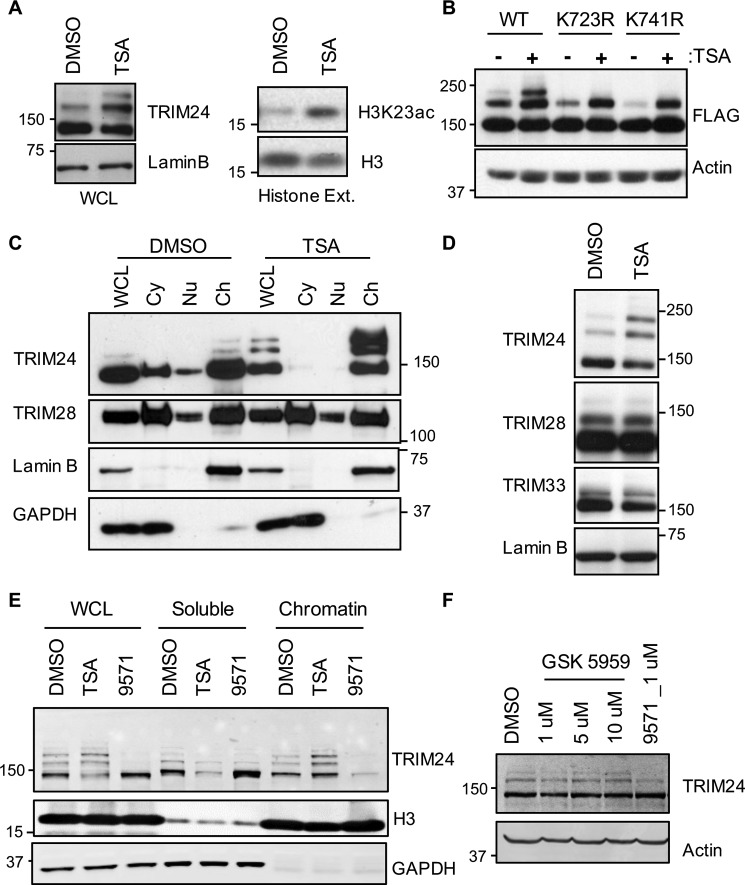
If chromatin association is important for TRIM24 SUMOylation, we reasoned that TRIM24 SUMOylation status would respond to changes in levels of histone acetylation, especially H3K23ac that is recognized by the bromodomain of TRIM24. We used a chemical approach to modulate levels of histone acetylation, including H3K23ac, via a 1-h treatment of HEK293T cells with trichostatin A (TSA), a class I and II histone deacetylase (HDAC) inhibitor (23). TSA stabilized H3K23ac levels and increased SUMOylation of TRIM24 (Fig. 3A). Immunoblot analysis of whole-cell lysates from HEK293T cells expressing FLAG-TRIM24 WT and single SUMO site mutants of TRIM24 showed that TSA-mediated up-regulation of TRIM24 SUMOylation occurred at both Lys723 and Lys741 (Fig. 3B). We assessed subcellular localization of TRIM24 to determine whether TSA-mediated up-regulation of H3K23ac and TRIM24 SUMOylation coincided with increased chromatin association of TRIM24. Consistent with results in HEK293T cells, TSA treatment led to enhanced SUMOylation of TRIM24 in MCF7 cells, and cellular fractionation experiments showed that TSA treatment mobilized TRIM24 and altered localization from cytosolic and nuclear fractions to the chromatin fraction (Fig. 3C). Interestingly, TRIM28, a closely related TIF1 family member that does not interact with H3K4me0/K23ac, did not show a similar response to TSA treatment, suggesting specificity and different mechanisms of chromatin localization within the TIF1 family. Immunoblot analysis of TSA-treated HEK293T cells further established that TSA-mediated up-regulation of SUMOylation is specific to TRIM24 but not TIF1 family members TRIM28 and TRIM33 (Fig. 3D). We wondered whether TRIM24 itself is acetylated and if that regulated TRIM24 SUMOylation. Our studies using TSA-treated HEK293T whole-cell lysates subjected to immunoprecipitation with anti-acetyllysine antibody to enrich acetylated protein did not identify TRIM24 as an acetylated protein. The reverse approach to immunoprecipitate TRIM24 and probe with anti-acetyllysine antibody also failed to show TRIM24 acetylation (data not shown). Our observation is consistent with a global acetylome study, where high-resolution MS was used to identify 3,600 acetylation sites on 1,750 proteins in three different cell lines. TRIM24 was not reported as an acetylated protein; however, acetylation sites were identified on closely related TRIM28 and TRIM33 (24).
Figure 3.
Chromatin association is required for TRIM24 SUMOylation. A, HDAC inhibitor treatment promotes TRIM24 SUMOylation. HEK293T cells were treated with TSA (2 μm) for 60 min, followed by histone extraction (right) and WCL (left) preparation and subjected to Western blot analysis using the indicated antibodies. B, HEK293T cells were transfected with the indicated FLAG-TRIM24 WT and mutant protein-expressing plasmids. 24 h post-transfection, cells were treated with TSA for 60 min, and WCL were subjected to Western blot analysis with FLAG and actin antibodies. C, HDAC inhibition promotes chromatin association of TRIM24. DMSO- and TSA-treated MCF7 cells were fractionated to prepare cytosol (Cy), nuclear (Nu), and chromatin (Ch) fractions. Western blot analysis was performed using the indicated antibodies. Lamin B and GAPDH serve as positive controls for chromatin and cytosol fractions, respectively. TRIM28 serves as a loading control for comparison of individual fractions between DMSO and TSA treatment. D, HEK293T cells treated with TSA and WCL were subjected to Western blot analysis with the indicated antibodies. E, IACS-9571 dissociates TRIM24 from chromatin and down-regulates its SUMOylation. HEK293T cells treated with DMSO, TSA, or 9571 for 60 min were used to generate WCL, soluble (cytosol and free nuclear), and chromatin-bound fractions. Western blot analysis was performed using the indicated antibodies. F, MCF7 cells were treated with the indicated concentrations of BRPF1 inhibitor, GSK 5959, or IACS-9571 for 24 h. WCL were subjected to Western blot analysis with the indicated antibodies.
HDAC inhibition established a correlation between H3K23ac and TRIM24 SUMOylation; however, HDACs, in addition to regulating H3K23ac, modulate numerous acetylation events in a cell. To focus on SUMOylation as a result of TRIM24 bromodomain interaction with acetylated lysines, we used IACS-9571, a cell-permeable, small-molecule inhibitor of TRIM24 and BRPF1 bromodomains that can displace endogenous, full-length TRIM24 from chromatin in cells (21). HEK293T cells treated with TSA showed increased SUMOylation of TRIM24, whereas treatment with IACS-9571 resulted in inhibition of TRIM24 SUMOylation (Fig. 3E, WCL samples). In the same experiment, to determine the effects of TSA and IACS-9571 treatments on association of TRIM24 with chromatin, we fractionated DMSO–, TSA–, or IACS-9571–treated cells to generate soluble (containing cytosolic and nuclear material) and chromatin-bound fractions. Comparison of the three treatments showed that TSA and IACS-9571 had opposing effects on TRIM24 localization. Compared with DMSO, TSA treatment resulted in depletion and IACS-9571 treatment enriched TRIM24 in the soluble fraction. The opposite was observed in the chromatin fraction, where TSA treatment showed enrichment of TRIM24, and IACS-9571 treatment resulted in depletion of TRIM24. IACS-9571 is a selective, dual inhibitor of the TRIM24 and BRPF1 bromodomains with a specific Kd of 1.3 and 2.1 nm, respectively (21). We tested whether IACS-9571 mediated inhibition of TRIM24 SUMOylation through the BRPF1 bromodomain. Treatment of MCF7 cells with an additional potent and specific BRPF1 bromodomain inhibitor, GSK 5959 (25), had no effect on TRIM24 SUMOylation (Fig. 3F), offering further support for specificity of TRIM24 bromodomain engagement in downstream SUMOylation of TRIM24. Our studies so far establish that bromodomain-dependent chromatin association promotes TRIM24 SUMOylation.
TRIM24 SUMOylation regulates cell adhesion
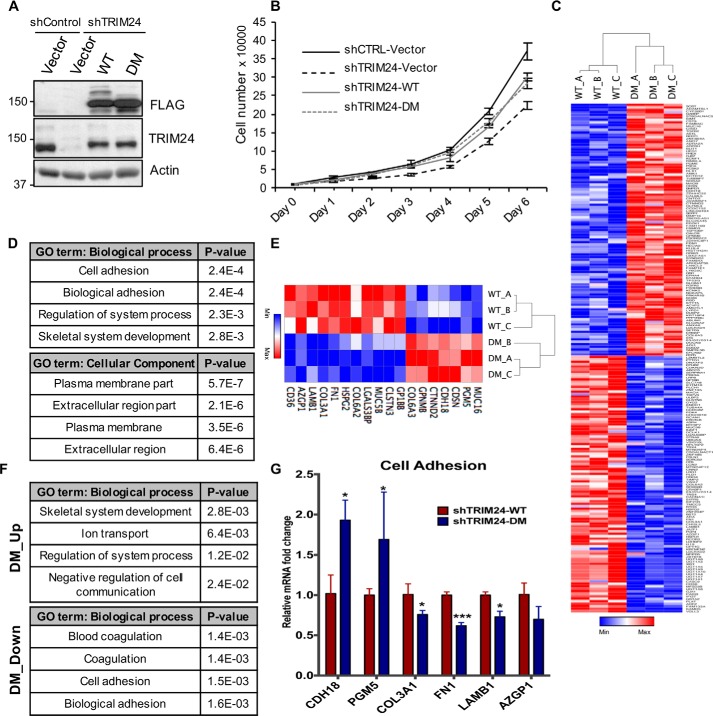
To examine the functional impact of SUMOylation of TRIM24, especially in breast cancers where TRIM24 is frequently overexpressed (26), we used breast cancer-derived MCF7 cells with which we previously showed a positive correlation between TRIM24 expression and proliferation (7, 27). We developed a stable gene rescue system, where TRIM24 was first depleted by shRNA, followed by ectopic expression of shRNA-resistant TRIM24 (FLAG-TRIM24 WT, FLAG-TRIM24 DM, or vector control) in shCtrl or shTRIM24 MCF7 cells. We confirmed TRIM24 knockdown and subsequent rescue with WT and mutant TRIM24 by immunoblot analysis (Fig. 4A). TRIM24 depletion was previously reported to negatively affect MCF7 cell proliferation (7, 27). Here, we saw that restoration of TRIM24 expression in shTRIM24 cells by ectopic TRIM24 WT partially rescued loss of cellular proliferation; however, SUMOylation was dispensable, as both TRIM24 WT and DM showed similar rescue of proliferation (Fig. 4B).
Figure 4.
TRIM24 SUMOylation regulates cell adhesion genes. A, Western blotting showing TRIM24 knockdown in MCF7 shTRIM24 cells and re-expression of FLAG-TRIM24 WT or SUMO mutant (DM) in shTRIM24 cells. B, ectopic expression of TRIM24 WT or DM in shTRIM24 cells partially rescues the proliferation defect. Shown is quantification of cell numbers from three replicates. Error bars, S.D. C, heat map showing significantly down-regulated (blue) or up-regulated (red) genes between TRIM24 WT and DM-expressing cells. D, gene ontology analysis performed using the DAVID online tool shows enrichment of cell adhesion– and ECM–related pathways. E, heat map showing differentially expressed cell adhesion–related genes between TRIM24 WT and DM-expressing cells. F, gene ontology analysis performed for DM_Up (top) and DM_Down (bottom) genes using the DAVID online tool. G, RT-PCR validation of randomly selected cell adhesion-related genes shown in E.
TRIM24 functions as a co-regulator of gene expression, serving in both repression and activation of transcription (9–11). To determine TRIM24 SUMOylation-dependent gene expression profiles, we performed RNA-Seq analysis using shTRIM24 cells expressing TRIM24 WT or DM protein. Genes with at least 1.3-fold difference in expression at 10% false discovery rate were selected as differentially expressed between WT and DM rescue cell lines. We identified 239 differentially expressed genes (Table S1), with nearly half up-regulated or down-regulated in SUMO mutant-expressing MCF7 cells (DM) compared with WT TRIM24-expressing cells (Fig. 4C), suggesting that SUMOylation of TRIM24 may either activate or repress genes co-regulated by TRIM24. To annotate and assess the biological roles of the specific subset of TRIM24-regulated genes dependent on the SUMOylation state of TRIM24, we used Database for Annotation, Visualization and Integrated Discovery (DAVID) to determine enrichment of biological process and cellular component gene ontology terms (28, 29). This analysis showed an enrichment of cell adhesion, plasma membrane, and extracellular region components among all genes differentially expressed, whether up- or down-regulated, in TRIM24 WT versus DM-expressing MCF7 cells (Fig. 4D and Table S2). A heat map representation of cell adhesion-related genes assessed by DAVID shows that most of these genes are down-regulated in DM-expressing cells (Fig. 4E). When differentially expressed genes were segregated into increased, or up-regulated (DM_Up), versus repressed, or down-regulated (DM_Down), genes down-regulated by loss of specific SUMOylation in DM-expressing cells showed enrichment of cell adhesion and extracellular region components (Fig. 4F). RT-PCR analysis was performed for select cell adhesion-related genes to validate RNA-Seq expression profiles (Fig. 4G).
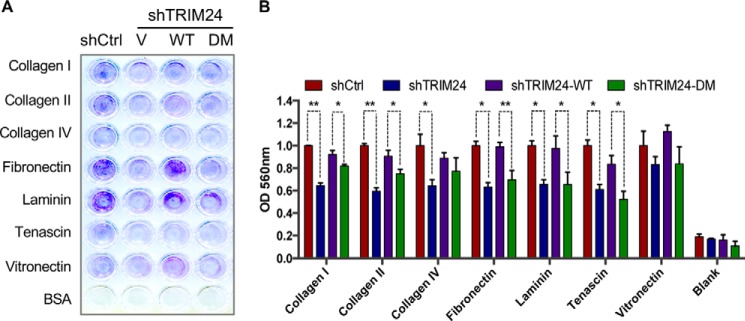
Given cell adhesion as the top enriched pathway, we performed assays to determine cell adhesion properties of TRIM24 WT and DM protein-expressing MCF7 cells; shCtrl and shTRIM24 cells were also used to determine the impact of TRIM24 knockdown on cell adhesion. Our analysis shows that shTRIM24 cells exhibit reduced binding to all extracellular matrix (ECM) proteins tested except vitronectin (Fig. 5A). The reduced binding phenotype of shTRIM24 cells was rescued by ectopic expression of TRIM24 WT, suggesting a role for TRIM24 in promoting cell adhesion to ECM proteins. Compared with TRIM24 WT-expressing cells, TRIM24 DM-expressing cells showed reduced cell adhesion to several ECM proteins (collagen I, collagen II, fibronectin, laminin, and tenascin), supporting a role for SUMO modification of TRIM24 in regulation of pathways associated with cell adhesion.
Figure 5.
TRIM24 SUMOylation regulates adhesion of MCF7 cells to ECM proteins. A, cell adhesion assay. MCF7 shCtrl, shTRIM24, shTRIM24-WT, and shTRIM24-DM cells were allowed to attach to wells coated with the indicated ECM proteins and BSA control. Following the wash step, adherent cells were stained. B, following extraction of stained cells in extraction buffer, optical density was determined and plotted as a measure of cell adhesion. Quantification from biological replicates (n = 2 for shCtrl and shTRIM24 and n = 3 for shTRIM24-WT and shTRIM24-DM). *, p < 0.05; **, p < 0.005. Error bars, S.D.
Discussion
Histone PTM cross-talk, where distinct histone modifications promote or inhibit modification of histones, occurs as either cis- or trans-nucleosomal (30, 31); however, reports of chromatin modifications that effect or alter modifications of nonhistone proteins are limited. An important study showed that signaling from chromatin to kinetochore proteins occurs via histone H2B ubiquitination that promotes SET1-dependent methylation of Dam1 in yeast (32). Chromatin PTM status has also been linked to ATM signaling in response to DNA damage, where association of KAT5 with histone H3K9me at DNA damage sites stimulates KAT5-mediated acetylation of ATM (33). Thus, these two reports show that histone modifications direct the catalytic activity of “writer” enzymes toward nonhistone protein substrates.
Our studies uncovered a previously unknown, PTM cross-talk between chromatin acetylation and SUMOylation of a bromodomain-containing reader protein. Here, we show that chromatin association of TRIM24 leads to TRIM24 SUMOylation at Lys723 and Lys741. Chromatin association of TRIM24 probably increases the proximity of this substrate for components of SUMO conjugation pathway, which are reported to be chromatin-associated (34, 35). Our studies using TSA to modulate H3K23ac levels and IACS-9571 to inhibit TRIM24 bromodomain association with chromatin show that recognition of histone acetylation by the TRIM24 bromodomain is required for TRIM24 SUMOylation. The TRIM24 bromodomain in tandem with its neighboring PHD domain functions as a combinatorial structural domain and binds with relatively high affinity to H3K23ac-containing peptides where H3K4 is unmethylated (Kd = 0.09 μm) (7). These quantified affinity determinations and our studies reported here suggest that acetylation of H3K23 forms a high-affinity platform for recruitment of TRIM24 to chromatin to promote its SUMOylation.
Gene expression profiling of WT and SUMO-mutated TRIM24–expressing cells identified differentially expressed genes with nearly equal numbers up- or down-regulated. Thus, SUMOylation of TRIM24, which depends on chromatin engagement, impacts co-regulatory functions in activation as well as repression of gene expression. DAVID analysis of differentially expressed genes suggested a high-probability role for TRIM24 SUMOylation in regulation of cell adhesion. We tested this hypothesis and found that MCF7 cells, which express TRIM24 but lack SUMOylation of TRIM24 (TRIM24-DM), exhibited significant changes in expression of genes associated with adhesion, membrane integrity, and migration. Downstream of changes in gene expression, cells expressing TRIM24-DM physically lacked adhesion to a panel of extracellular matrix proteins, including fibronectin, laminin, and tenascin. Thus, TRIM24 and its SUMOylation may play a role in cancers by regulating both expression and interactions with specific cell adhesion proteins, implicated in cell proliferation, migration, and metastasis (36, 37).
Our studies reveal a novel PTM cross-talk showing that histone acetylation modulates SUMOylation of a reader protein and its downstream functions in regulation of specific genes implicated in cancers. How TRIM24 SUMOylation yields activation or repression of target gene expression requires further studies with antibodies specific to SUMO-modified TRIM24, which like other SUMO-specific antibodies are not currently available. Disruption of chromatin-dependent SUMOylation of TRIM24 offers a pharmacodynamic biomarker for the evaluation of TRIM24 bromodomain inhibitors in cells and their effectiveness in human disease. Specifically, a deeper understanding of PTM cross-talk between histones and epigenetic regulators may open new avenues for drug development and augment the value of epigenetic regulators in cancer drug discovery.
Experimental procedures
Cell culture
MCF7 and HEK293T cells were obtained from ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 1% l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. For cell proliferation, cells were seeded in ok;224-well plates at 10,000 cells/well in DMEM and counted for 6 days using a Coulter counter (Beckman Coulter). Transfections were performed using Effectene (Qiagen), following the manufacturer's recommendations. Cells were treated with 2 μm TSA (Sigma, T8552) or 1 μm IACS-9571 for 1 h.
Plasmids
HA-SUMO1 (plasmid 21154) and FLAG-SENP1 (plasmid 17357) were purchased from Addgene. pCMX-FLAG-TRIM24 and PHD finger, bromodomain mutant vector were reported previously (7). SUMO site mutants were generated using the QuikChange XL site-directed mutagenesis kit (Stratagene) using primers listed in Table S3.
Protein analysis
To prepare whole-cell lysates, cells were lysed as indicated in either radioimmunoprecipitation assay (RIPA) buffer (20 mm Tris, pH 8.0, 150 mm NaCl, 1 mm EDTA, 1% NP-40, 1, Triton X-100, 0.5% deoxycholic acid) or NETN buffer (150 mm NaCl, 1 mm EDTA, 50 mm Tris, pH 7.5, 0.1% Nonidet P-40) supplemented with protease inhibitor mixture (Calbiochem). To stabilize TRIM24 SUMOylation, lysis buffer containing 20 mm NEM or 1% SDS was used as indicated in the figure legends. Subcellular fractionation was performed as reported previously with the addition of 20 mm NEM in buffers (38). Immunoblotting was performed using standard techniques using antibodies listed in Table S4.
Immunoprecipitation
Endogenous TRIM24 SUMOylation
3 mg of protein extract from HEK293T cells harvested in RIPA buffer containing NEM was incubated overnight at 4 °C with 4 μg of rabbit IgG or TRIM24 antibody. 50 μl of protein A-Sepharose beads (GE Healthcare) equilibrated in RIPA buffer were incubated with the extracts for 1 h at 4 °C to precipitate immune complexes. Beads were washed three times with RIPA buffer, boiled in 1× SDS protein loading dye, and processed for immunoblotting.
FLAG-TRIM24 SUMOylation
2 mg of protein extract from HA-SUMO1 and FLAG-TRIM24 co-transfected HEK293T cells or cells expressing FLAG-TRIM24 WT or mutant proteins harvested in NETN buffer containing NEM was incubated overnight at 4 °C with 30 μl of anti-FLAG M2 agarose beads (Sigma, A2220) equilibrated in NETN buffer. Protein-bound beads were washed three times with NETN buffer and processed for immunoblotting.
Immunoprecipitation of FLAG-TRIM24 for in vitro SUMOylation assay
0.5 mg of protein extract was processed as above with the last two washes in reaction buffer (25 mm Tris, pH 7.5, and 25 mm NaCl) followed by an in vitro SUMOylation assay.
Histone extraction
Cells harvested in Triton Extraction Buffer (PBS containing 0.5% Triton X-100, 2 mm phenylmethylsulfonyl fluoride, and 5 mm sodium butyrate) were lysed on ice for 10 min and centrifuged at 2,500 × g for 10 min. The pellet was resuspended in 0.2 n HCl, and acid extraction was performed at 4 °C for 1 h. Samples were centrifuged at 6,500 × g for 10 min at 4 °C to collect the supernatant, which was subsequently mixed with 10 volumes of ice-cold acetone and incubated overnight at −20 °C. Samples were centrifuged at 6,500 × g for 10 min to collect the pellet, which was resuspended in water followed by immunoblotting.
SUMOylation assay
GST-TRIM24 protein was purified from bacteria. SUMO-E1, Ubc9, and SUMO1 proteins were purchased from Boston Biochem. The SUMOylation reaction contained the following in a 20-μl volume: 250 ng of GST-TRIM24 or FLAG-TRIM24 protein immobilized on 20 μl of beads, 250 ng of SUMO-E1, 500 ng of Ubc9, 1.5 μg of SUMO1, 2.5 μg of BSA, 2 mm ATP, 5 mm MgCl2, 1 mm DTT, 50 mm Tris, pH 7.5, 0.05% Tween 20. After incubation at 37 °C for the indicated times, reactions were stopped with protein loading dye and subjected to immunoblotting.
MCF7 stable cells
MCF7 cells stably expressing nontarget or TRIM24 short hairpin RNA (shControl or shTRIM24, respectively) were described previously (27) and were cultured in complete DMEM containing 1 μg/ml puromycin. The TRIM24 shRNA for stable cell line creation was AAGCAGGTGGAACAGGATATTAAAGTTGC. TRIM24 full-length coding sequences were cloned in the empty vector pEntry4-FLAG as described previously (16). TRIM24 coding sequences and FLAG-only sequences were transferred to the vector pLX304 using a Gateway cloning system (Invitrogen). SUMO site DMs and shRNA-resistant site mutants were generated using the QuikChange XL site-directed mutagenesis kit. See Table S3 for primer sequences. Lentiviruses containing culture supernatants were prepared as described previously (16). Stable cell lines, ectopically expressing WT TRIM24, SUMO site double mutant, or FLAG only were maintained in regular culture medium containing 200 μg/ml G418.
Quantitative real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen), and quantitative RT-PCR was performed as described previously (16) using primers listed in Table S3.
Cell adhesion assay
Cell adhesion assays were performed using an ECM array kit from Millipore (catalog no. ECM540) as per the manufacturer's instructions. Briefly, a single cell suspension of cells in DMEM was added on to the ECM array plate and incubated for 2 h at 37 °C. After incubation, cells were washed with PBS and stained with the provided cell stain solution for 5 min. After washing the wells with deionized water, stain was extracted using the extraction buffer, and absorbance (560 nm) was determined.
Gene expression profiling
Total RNA was isolated using mirVana miRNA isolation kit (Life Technologies, AM1560) following the manufacturer's protocol. The RNA-Seq data reads were mapped to the HG19 assembly of the human genome with the Bowtie2 aligner. The data were quality-controlled with the FASTQC and RNA-Se-QC (39) packages. The expression was quantified with the easyRNASeq (40) package. Data were suitably filtered with DESeq package before running the differential expression analysis. Differential expression was identified with the edgeR and DESeq (41) package that assumes negative binomial distribution on RNA-Seq counts. The p values were adjusted for multiple-hypothesis correction using Benjamini–Hochberg correction (false discovery rate). Data were suitably filtered before running differential expression analysis. Genes having -fold changes of 1.3 and a significant p value after multiple-hypothesis correction were considered differentially expressed.
Author contributions
S. A. conceptualization; S. A., K. N. T., and P. K. S. formal analysis; S. A. and K. N. T. validation; S. A. and K. N. T. investigation; S. A. and K. N. T. methodology; S. A. writing-original draft; K. N. T., P. K. S., S. Y. R. D., J. N. A., and M. C. B. writing-review and editing; P. K. S. data curation; S. Y. R. D., J. N. A., and M. C. B. resources; J. N. A. and M. C. B. supervision; M. C. B. funding acquisition.
Supplementary Material
Acknowledgments
We thank Dr. A. Jain for helpful discussions, Drs. T. Bawa-Khalfe and E. Yeh for protocols, Drs. P. Jones and W. Palmer for IACS-9571 and GSK 5959, and Dr. J. Chandra for TSA.
This work was supported in part by CPRIT RP110471 and an Innovation Award from the Center for Cancer Epigenetics (to M. C. B.) and University of Texas M. D. Anderson Cancer Center NCI, National Institutes of Health, Support Grant CA016672. The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
This article contains Tables S1–S4.
RNA-Seq data can be accessed at the NCBI gene expression omnibus (GEO) under accession number GSE77140.
- PHD
- plant homeodomain
- RBCC
- RING, B-box, coiled-coil
- SUMO
- small ubiquitin-like modifier
- PTM
- post-translational modification
- NEM
- N-ethylmaleimide
- TSA
- trichostatin A
- DM
- double mutant
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- ECM
- extracellular matrix
- RIPA
- radioimmunoprecipitation assay
- HA
- hemagglutinin
- HDAC
- histone deacetylase
- DMEM
- Dulbecco's modified Eagle's medium
- WCL
- whole-cell lysate(s)
- GST
- glutathione S-transferase
- H3K4
- H3K9, and H3K23, histone H3 Lys4, Lys9, and Lys23, respectively.
References
- 1. Conaway J. W. (2012) Introduction to theme “Chromatin, epigenetics, and transcription”. Annu. Rev. Biochem. 81, 61–64 10.1146/annurev-biochem-090711-093103 [DOI] [PubMed] [Google Scholar]
- 2. Jaenisch R., and Bird A. (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 3. Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 4. Jenuwein T., and Allis C. D. (2001) Translating the histone code. Science 293, 1074–1080 10.1126/science.1063127 [DOI] [PubMed] [Google Scholar]
- 5. Musselman C. A., Lalonde M. E., Côté J., and Kutateladze T. G. (2012) Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 19, 1218–1227 10.1038/nsmb.2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yun M., Wu J., Workman J. L., and Li B. (2011) Readers of histone modifications. Cell Res. 21, 564–578 10.1038/cr.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai W. W., Wang Z., Yiu T. T., Akdemir K. C., Xia W., Winter S., Tsai C. Y., Shi X., Schwarzer D., Plunkett W., Aronow B., Gozani O., Fischle W., Hung M. C., Patel D. J., and Barton M. C. (2010) TRIM24 links a non-canonical histone signature to breast cancer. Nature 468, 927–932 10.1038/nature09542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatakeyama S. (2011) TRIM proteins and cancer. Nat. Rev. Cancer 11, 792–804 10.1038/nrc3139 [DOI] [PubMed] [Google Scholar]
- 9. Thénot S., Henriquet C., Rochefort H., and Cavaillès V. (1997) Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1. J. Biol. Chem. 272, 12062–12068 10.1074/jbc.272.18.12062 [DOI] [PubMed] [Google Scholar]
- 10. Le Douarin B., vom Baur E., Zechel C., Heery D., Heine M., Vivat V., Gronemeyer H., Losson R., and Chambon P. (1996) Ligand-dependent interaction of nuclear receptors with potential transcriptional intermediary factors (mediators). Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 569–578 10.1098/rstb.1996.0056 [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi M., Okumura F., Tsukiyama T., Watanabe M., Miyajima N., Tanaka J., Imamura M., and Hatakeyama S. (2009) TRIM24 mediates ligand-dependent activation of androgen receptor and is repressed by a bromodomain-containing protein, BRD7, in prostate cancer cells. Biochim. Biophys. Acta 1793, 1828–1836 10.1016/j.bbamcr.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 12. Liu X., Huang Y., Yang D., Li X., Liang J., Lin L., Zhang M., Zhong K., Liang B., and Li J. (2014) Overexpression of TRIM24 is associated with the onset and progress of human hepatocellular carcinoma. PLoS One 9, e85462 10.1371/journal.pone.0085462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui Z., Cao W., Li J., Song X., Mao L., and Chen W. (2013) TRIM24 overexpression is common in locally advanced head and neck squamous cell carcinoma and correlates with aggressive malignant phenotypes. PLoS One 8, e63887 10.1371/journal.pone.0063887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miao Z. F., Wang Z. N., Zhao T. T., Xu Y. Y., Wu J. H., Liu X. Y., Xu H., You Y., and Xu H. M. (2015) TRIM24 is upregulated in human gastric cancer and promotes gastric cancer cell growth and chemoresistance. Virchows Arch. 466, 525–532 10.1007/s00428-015-1737-4 [DOI] [PubMed] [Google Scholar]
- 15. Thakkar K. N., Stratton S. A., and Craig Barton M. (2015) Tissue-specific metabolism and TRIM24. Aging 7, 736–737 10.18632/aging.100822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pathiraja T. N., Thakkar K. N., Jiang S., Stratton S., Liu Z., Gagea M., Shi X., Shah P. K., Phan L., Lee M. H., Andersen J., Stampfer M., and Barton M. C. (2015) TRIM24 links glucose metabolism with transformation of human mammary epithelial cells. Oncogene 34, 2836–2845 10.1038/onc.2014.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geiss-Friedlander R., and Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 10.1038/nrm2293 [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson K. A., and Henley J. M. (2010) Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 428, 133–145 10.1042/BJ20100158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seeler J. S., Marchio A., Losson R., Desterro J. M., Hay R. T., Chambon P., and Dejean A. (2001) Common properties of nuclear body protein SP100 and TIF1α chromatin factor: role of SUMO modification. Mol. Cell. Biol. 21, 3314–3324 10.1128/MCB.21.10.3314-3324.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmer W. S., Poncet-Montange G., Liu G., Petrocchi A., Reyna N., Subramanian G., Theroff J., Yau A., Kost-Alimova M., Bardenhagen J. P., Leo E., Shepard H. E., Tieu T. N., Shi X., Zhan Y., et al. (2016) Structure-guided design of IACS-9571, a selective high-affinity dual TRIM24-BRPF1 bromodomain inhibitor. J. Med. Chem. 59, 1440–1454 10.1021/acs.jmedchem.5b00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhan Y., Kost-Alimova M., Shi X., Leo E., Bardenhagen J. P., Shepard H. E., Appikonda S., Vangamudi B., Zhao S., Tieu T. N., Jiang S., Heffernan T. P., Marszalek J. R., Toniatti C., Draetta G., et al. (2015) Development of novel cellular histone-binding and chromatin-displacement assays for bromodomain drug discovery. Epigenetics Chromatin 8, 37 10.1186/s13072-015-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hickey C. M., Wilson N. R., and Hochstrasser M. (2012) Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13, 755–766 10.1038/nrm3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida M., Kijima M., Akita M., and Beppu T. (1990) Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174–17179 [PubMed] [Google Scholar]
- 24. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., and Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 10.1126/science.1175371 [DOI] [PubMed] [Google Scholar]
- 25. Demont E. H., Bamborough P., Chung C. W., Craggs P. D., Fallon D., Gordon L. J., Grandi P., Hobbs C. I., Hussain J., Jones E. J., Le Gall A., Michon A. M., Mitchell D. J., Prinjha R. K., Roberts A. D., Sheppard R. J., and Watson R. J. (2014) 1,3-Dimethyl benzimidazolones are potent, selective inhibitors of the BRPF1 bromodomain. ACS Med. Chem. Lett. 5, 1190–1195 10.1021/ml5002932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Appikonda S., Thakkar K. N., and Barton M. C. (2016) Regulation of gene expression in human cancers by TRIM24. Drug Discov. Today Technol. 19, 57–63 10.1016/j.ddtec.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 27. Allton K., Jain A. K., Herz H. M., Tsai W. W., Jung S. Y., Qin J., Bergmann A., Johnson R. L., and Barton M. C. (2009) Trim24 targets endogenous p53 for degradation. Proc. Natl. Acad. Sci. U.S.A. 106, 11612–11616 10.1073/pnas.0813177106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang da W., Sherman B. T., and Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 29. Huang da W., Sherman B. T., and Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Izzo A., and Schneider R. (2010) Chatting histone modifications in mammals. Brief Funct. Genomics 9, 429–443 10.1093/bfgp/elq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee J. S., Smith E., and Shilatifard A. (2010) The language of histone crosstalk. Cell 142, 682–685 10.1016/j.cell.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Latham J. A., Chosed R. J., Wang S., and Dent S. Y. (2011) Chromatin signaling to kinetochores: transregulation of Dam1 methylation by histone H2B ubiquitination. Cell 146, 709–719 10.1016/j.cell.2011.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaidi A., and Jackson S. P. (2013) KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 498, 70–74 10.1038/nature12201 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Neyret-Kahn H., Benhamed M., Ye T., Le Gras S., Cossec J. C., Lapaquette P., Bischof O., Ouspenskaia M., Dasso M., Seeler J., Davidson I., and Dejean A. (2013) Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res. 23, 1563–1579 10.1101/gr.154872.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niskanen E. A., Malinen M., Sutinen P., Toropainen S., Paakinaho V., Vihervaara A., Joutsen J., Kaikkonen M. U., Sistonen L., and Palvimo J. J. (2015) Global SUMOylation on active chromatin is an acute heat stress response restricting transcription. Genome Biol. 16, 153 10.1186/s13059-015-0717-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okegawa T., Pong R. C., Li Y., and Hsieh J. T. (2004) The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 51, 445–457 [PubMed] [Google Scholar]
- 37. Lester B. R., and McCarthy J. B. (1992) Tumor cell adhesion to the extracellular matrix and signal transduction mechanisms implicated in tumor cell motility, invasion and metastasis. Cancer Metastasis Rev. 11, 31–44 10.1007/BF00047601 [DOI] [PubMed] [Google Scholar]
- 38. Wysocka J., Reilly P. T., and Herr W. (2001) Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol. Cell. Biol. 21, 3820–3829 10.1128/MCB.21.11.3820-3829.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeLuca D. S., Levin J. Z., Sivachenko A., Fennell T., Nazaire M. D., Williams C., Reich M., Winckler W., and Getz G. (2012) RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 10.1093/bioinformatics/bts196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delhomme N., Padioleau I., Furlong E. E., and Steinmetz L. M. (2012) easyRNASeq: a bioconductor package for processing RNA-Seq data. Bioinformatics 28, 2532–2533 10.1093/bioinformatics/bts477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anders S., and Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.