Abstract
The effects of solar ultraviolet (UV)-B and UV-A radiation on the potential efficiency of photosystem II (PSII) in leaves of tropical plants were investigated in Panama (9°N). Shade-grown tree seedlings or detached sun leaves from the outer crown of mature trees were exposed for short periods (up to 75 min) to direct sunlight filtered through plastic or glass filters that absorbed either UV-B or UV-A+B radiation, or transmitted the complete solar spectrum. Persistent changes in potential PSII efficiency were monitored by means of the dark-adapted ratio of variable to maximum chlorophyll a fluorescence. In leaves of shade-grown tree seedlings, exposure to the complete solar spectrum resulted in a strong decrease in potential PSII efficiency, probably involving protein damage. A substantially smaller decline in the dark-adapted ratio of variable to maximum chlorophyll a fluorescence was observed when UV-B irradiation was excluded. The loss in PSII efficiency was further reduced by excluding both UV-B and UV-A light. The photoinactivation of PSII was reversible under shade conditions, but restoration of nearly full activity required at least 10 d. Repeated exposure to direct sunlight induced an increase in the pool size of xanthophyll cycle pigments and in the content of UV-absorbing vacuolar compounds. In sun leaves of mature trees, which contained high levels of UV-absorbing compounds, effects of UV-B on PSII efficiency were observed in several cases and varied with developmental age and acclimation state of the leaves. The results show that natural UV-B and UV-A radiation in the tropics may significantly contribute to photoinhibition of PSII during sun exposure in situ, particularly in shade leaves exposed to full sunlight.
Absorption of light in excess of photosynthetic utilization by green plant leaves may lead to a reduction in the potential efficiency of photosystem II (PSII), which persists in low light or darkness and is regarded as the major cause of “photoinhibition of photosynthesis” (Baker and Bowyer, 1994). It has been shown for many plant species that photoinhibition of photosynthesis does occur under natural conditions (Long et al., 1994). In studies with tropical plants, substantial reductions in the potential efficiency of PSII, indicated by a decline in the “dark-adapted” ratio of variable to maximum chlorophyll (Chl) a fluorescence (Fv/Fm) have been observed upon direct exposure to the solar beam of outer crown leaves of mature forest trees (Krause et al., 1995) and of plants situated in treefall gaps within the tropical forest (Krause and Winter, 1996; Thiele et al., 1998).
Currently, it is unknown whether and to what extent natural UV radiation (UV-B, 280–320 nm, and UV-A, 320–400 nm) is involved in photoinhibition of PSII in vascular plants in situ. To date, the effects of ambient UV-B and UV-A on PSII have only been reported for a green alga, Dunaliella salina (Herrmann et al., 1997). Although the effects of UV light, particularly those of the biologically more active UV-B light, on plants (Rozema et al., 1997; Jansen et al., 1998), and specifically on photosynthesis (Teramura and Ziska, 1996; Allen et al., 1998), have been extensively investigated, little information is available on plant responses to ambient UV radiation. With a few exceptions, published studies have involved the application of artificial supplementary UV light, often creating conditions unlikely to occur in nature. Unrealistically high ratios of UV-B/UV-A and UV-B/photosynthetically active radiation (PAR) in experiments performed in growth chambers and greenhouses have been criticized (Ziska, 1996; Rozema et al., 1997). Studies with artificial UV-B sources have shown that UV-B radiation may strongly affect PSII, whereas PSI appears to be relatively insensitive (Teramura and Ziska, 1996).
The aim of the present study was to evaluate the possible contribution of ambient UV-B and UV-A to photoinhibition of PSII in tropical plants. In the tropics, due to the small solar zenith angle and the thin stratospheric ozone (O3) layer, terrestrial plants encounter much higher levels of UV-B radiation than at higher latitudes (Caldwell et al., 1989; Madronich et al., 1995). Strong anthropogenic reduction of the O3 layer is well known in the antarctic region, and a significant decline of total O3 over middle and high latitudes of both hemispheres has been proven (Bojkov and Fioletov, 1996). Assessment of changes in O3 levels in the tropics is more problematic. Model calculations by Bojkov and Fioletov (1996) show negative trends of O3 levels in the tropical belt, which are not significant over the equator, but become significant at 25°N and 25°S. Although these trends may have been influenced by recent natural events (Bojkov and Fioletov, 1996), a future anthropogenic O3 decline over the tropics cannot be excluded, particularly if wide compliance with international agreements to phase out O3-depleting chemicals is missing. Recent evidence suggests that tropical plants, despite high constitutive resistance to UV-B, may be affected by increased UV-B levels resulting from a reduction of the O3 layer (Ziska, 1996). As discussed by Caldwell et al. (1989), a thinned O3 layer over the tropics would increase UV-B irradiance above maximum levels encountered by plants on the earth's surface in recent geological time. The conclusion drawn in a recent review (Allen et al., 1998) that the rise in UV-B radiation associated with O3 depletion does not directly threaten photosynthetic performance might have to be modified in the case of tropical plants.
To assess the possible consequences of increased UV-B light for tropical plants, it is important to elucidate the effects of present ambient UV-B levels. We studied the responses to direct sun exposure of leaves of tropical plants in various states of development and sun/shade acclimation in Panama (9°N) using plastic films and glass filters that excluded or transmitted UV-B or UV-A+B radiation. The potential efficiency of PSII was determined by measuring Chl a fluorescence. We analyzed photosynthetic pigments and assessed the amounts of protective UV-absorbing substances in leaf extracts. The opening of treefall gaps in the tropical forest was simulated by exposing shade-grown tree seedlings for short periods to direct sunlight. In addition, the effects of UV on leaves of the outer crown of mature trees acclimated to full sunlight were investigated. Preliminary results of the present study have been presented at the XI International Congress on Photosynthesis (Budapest, August 17–22, 1998).
MATERIALS AND METHODS
Experiments were carried out at the Smithsonian Tropical Research Institute (Tupper Center) in Panama City, Panama (9°N).
Plant Material
Plants of Anacardium excelsum (Bertero and Balb.) Skeels (Anacardiaceae) and Virola surinamensis (Rol.) Warb. (Myristicaceae) were grown in soil from seeds at temperatures between approximately 24°C and 31°C in a shaded greenhouse (neutral-density shading; PAR, 10–60 μmol m−2 s−1, corresponding to deep shade on the forest floor). The shading was achieved with a narrow black plastic lattice. The absence of glass walls facilitated ventilation and allowed transmittance (from the side) of unfiltered sunlight to the plants. A. excelsum plants were used for experiments after 9 months of culture, when they had developed seven to nine leaves and reached a stem height of 25 to 30 cm. V. surinamensis plants were used after 7 to 8 months (three leaves, stem height 11–15 cm). A. excelsum is a pioneer tree common in moist secondary forest, and V. surinamensis is a late successional tree widespread in tropical forests in Central and South America. Seedlings of both trees are shade tolerant.
Sun leaves of the outer crown of mature trees of A. excelsum were obtained from the humid, seasonally dry lowland forest in the Metropolitan Natural Park near Panama City, Panama, using a construction crane. Sun leaves of Anacardium occidentale L. (cashew) and Mangifera indica L. (mango) (Anacardiaceae) were harvested from the outer crown of trees growing close to the Smithsonian Tropical Research Institute. The sun leaves had experienced up to approximately 2,300 μmol m−2 s−1 PAR. The leaves were cut in the early morning prior to strong sunlight exposure and stored until use in the greenhouse. During storage and experiments, the petioles were immersed in water. Previous tests (Krause et al., 1995) showed that the CO2 assimilation activity of the detached leaves was comparable to that in situ.
Sun Exposure
Attached leaves of shade-grown tree seedlings and detached sun leaves of mature trees were exposed to direct sunlight for 15 to 75 min (between 10 and 16 h, local time) under 0.13-mm polyester (Mylar-D, DuPont, Wilmington, DE) or 0.08-mm Aclar (type 22A, Allied Signal, Pottsville, PA) plastic films (Searles et al., 1995). Mylar excluded most UV-B light, particularly that with higher photon energy (10% transmission at 318 nm) and a proportion of UV-A (20% transmission at 320 nm; 76% transmission at 400 nm) and transmitted 80% to 85% of light between 430- and 700-nm wavelength). Aclar transmitted 87% to 89% UV-B, 89% to 92% UV-A, and 92% to 93% PAR (measured with a UV-2100 spectrophotometer, Shimadzu, Kyoto). Alternatively, zones of 25 cm2 in the center of leaf blades were exposed under cut-off glass filters (WG 320, Schott, Mainz, Germany), which absorbed most UV-B (50% transmission at 320 nm; about 90% transmission of PAR) or GG 400 (Schott), which absorbed UV-B and UV-A (50% transmission at 400 nm; about 90% transmission of PAR).
PAR was determined with a quantum sensor (model LI 189B, LI-COR, Lincoln, NE). PAR values given were measured directly under the filters at leaf level or have been corrected for an average filter transmission of 90%. Tests indicated that differences in PAR of about 10% did not significantly alter the degree of decline in potential PSII efficiency (Fv/Fm, see below). UV-A and UV-B were measured with a radiometer (model IL 1400A, International Light, Newburyport, MA). In full sunlight of 2,000 μmol m−2 s−1 PAR, UV-A radiation was about 40 W m−2 and UV-B about 2 W m−2. As the sensors of this radiometer have a fixed wavelength sensitivity, the data provide only estimates of solar UV. A biologically weighted UV-B dose based, for example, on DNA damage has not been determined, as the UV-B effects on the photosynthetic electron transport system are much less wavelength dependent than effects on DNA (Caldwell et al., 1989). The limitations of biological action spectra have been discussed by Madronich et al. (1995). Leaf temperatures during sun exposure were measured with a thermocouple attached to the lower leaf surface, and temperatures ranged between 32°C and 40°C (air temperatures, 29°C–33°C).
Chl a Fluorescence Measurements
Fv/Fm was determined with a fluorometer (model PAM-2000, Walz, Effeltrich, Germany) by the saturation pulse method. This ratio served as an approximate measure of the potential PSII activity (Giersch and Krause, 1991; Krause and Weis, 1991). Leaf discs were dark adapted for 10 min before measurement of Fv/Fm. Previous studies on various plants have shown that during such dark periods, a plateau of Fm values is reached, indicating relaxation of fluorescence quenching (qE) related to the trans-thylakoid proton gradient and possibly of quenching caused by the state 1 to state 2 transition (qT). Thus, the decrease in Fv/Fm persisting after 10 min in the dark provides an approximate measure of photoinhibitory fluorescence change (Krause and Weis, 1991; Leitsch et al., 1994; Thiele et al., 1997). The kinetics of fluorescence rise from initial (Fo) to maximum (Fm) fluorescence yield in shade/sun leaves were recorded during a 2-s/1-s pulse of approximately 100/360 μmol m−2 s−1 actinic red light (maximum intensity at 655 nm) using the PAM-2000 software (Walz).
Photosynthetic Pigments and UV-Absorbing Compounds
For photosynthetic pigment analyses, leaf discs (1.54 cm2) were excised and frozen in liquid nitrogen either immediately after sun exposure of leaves or prior to exposure (controls) when adapted for several hours to low light (about 20 μmol m−2 s−1) in the greenhouse. Pigments were extracted with acetone and quantified by HPLC as described by Färber et al. (1997). The limit of detection of antheraxanthin and zeaxanthin was below 1 mol % of the total pool of xanthophyll cycle pigments.
UV-absorbing compounds were extracted from leaf discs of 0.51 cm2 in a final volume of 2.5 ± 0.1 mL of 80% (v/v) ethanol. Further extractions of pellets with more diluted ethanol did not yield additional UV-absorbing substances. The maximum absorbance of the extracts (after appropriate dilution) in the UV region, calculated per unit leaf area, served as a relative measure of UV-absorbing compounds. The wavelength of the absorbance maximum (λmax) varied between species studied. For better reproducibility of data, measurement at λmax, rather than at a fixed wavelength in the flank of the absorbance band, was preferred.
RESULTS
Shade-Grown Tree Seedlings
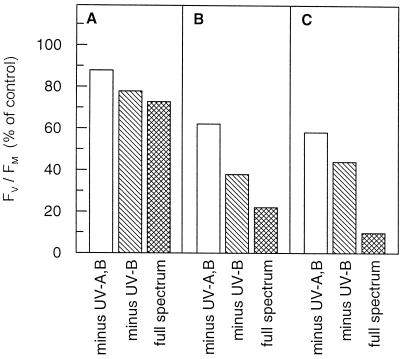
The effects of UV light on potential PSII efficiency (Fv/Fm) in leaves of shade-grown tree seedlings are shown in Figures 1–5 and Table I. Figure 1 depicts a typical time course of photoinhibition of PSII during exposure of shade-acclimated V. surinamensis seedlings to direct sunlight. A pronounced decrease in Fv/Fm occurred at a more rapid rate in the complete solar spectrum (under Aclar foil) compared with UV-B exclusion (under Mylar foil). Similar results were obtained with shade-grown A. excelsum (data not shown). Figure 2 depicts three additional experiments with shade-grown A. excelsum under different irradiation conditions. Both UV-B and UV-A light contributed to the decline in potential PSII efficiency that occurred in direct sunlight. The degree of PSII photoinhibition increased with the PAR dose received and, at a given PAR exposure, was lowest when UV-A and UV-B light were excluded. The particularly strong UV-B effect seen in Figure 2C might have been caused by fluctuations between cloud cover and extremely high light (up to 2,600 μmol m−2 s−1 PAR) during the exposure period. The clouds reduced the time in high PAR and UV-A light, but probably had less effect on UV-B irradiance due to the higher proportion of stray UV-B light (Flint and Caldwell, 1998).
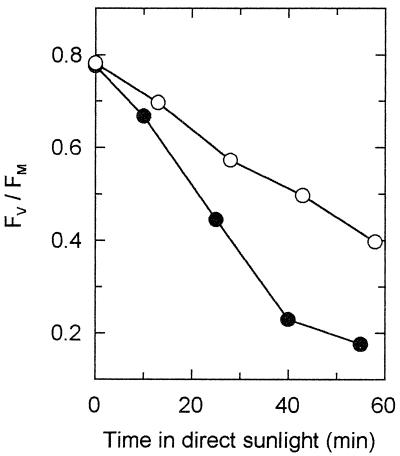
Figure 1.
Effect of ambient UV-B radiation on potential efficiency of PSII (Fv/Fm) during exposure of shade-grown V. surinamensis seedlings to direct sunlight. PAR dose was about 4.6 mol m−2 received over 55 min of exposure. A representative experiment with young leaves containing about 210 μmol Chl a+b m−2 is shown; additional duplicated experiments, both with young and mature shade-acclimated leaves of V. surinamensis and A. excelsum gave similar results. Due to varying irradiance caused by clouds, averaging of data was not possible. ●, exposure under Aclar film (+UV-B); ○, exposure under Mylar film (−UV-B).
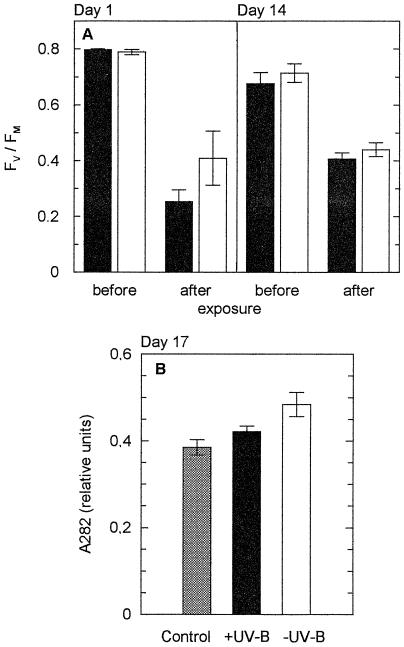
Figure 5.
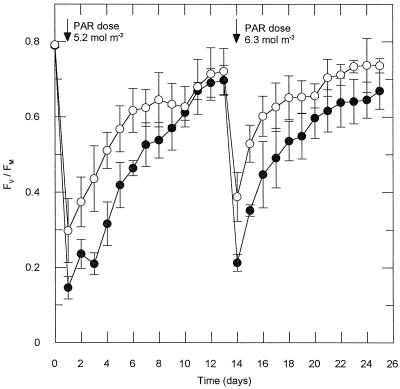
Long-term acclimation of mature leaves of shade-grown V. surinamensis seedlings to daily direct sun exposure in the presence (black bars, Aclar) and absence (white bars, Mylar) of ambient UV-B light. Plants were sun-exposed for 30 min (between 11 and 14 h, local time) on 8 consecutive d (d 1–8) and again on d 14. Between exposure periods, the plants were kept under shade conditions (maximum PAR, 110 μmol m−2 s−1). A, Fv/Fm before and after sun exposure on d 1 at a PAR dose of 3.4 mol m−2 (left panel) and d 14 at a PAR dose of 3.6 mol m−2 (right panel), recorded subsequent to 10 min of dark adaptation. Means ± se of three leaves from different plants are given. B, Relative contents of UV-absorbing substances on d 17 (absorbance measured at λmax in the UV region, 282 nm). Data are means ± se from two control plants (six leaf segments) and three sun-exposed plants. Control plants were not exposed to direct sunlight, but otherwise kept until d 17 under the same conditions as sun-exposed plants.
Table I.
Effects of 1 h of daily exposure to direct sunlight of shade-grown seedlings of A. exelsum on Fv/Fm and photosynthetic pigments
| Control | Exposure | d 1 | d 2 | d 3 | d 4 | d 7 | d 8 | |
|---|---|---|---|---|---|---|---|---|
| PAR dose (mol m−2) | 3.6 | 3.2 | 2.1 | 3.0 | 3.2 | – | ||
| Fv/Fm | (a) +UV | 0.79 ± 0.001 | 0.70 ± 0.02 | 0.45 ± 0.13 | 0.62 ± 0.05 | 0.30 ± 0.13 | 0.42 ± 0.12 | |
| −UV | 0.79 ± 0.005 | 0.73 ± 0.01 | 0.52 ± 0.08 | 0.66 ± 0.07 | 0.54 ± 0.10 | 0.58 ± 0.10 | ||
| (b) +UV | 0.44 ± 0.01 | 0.23 ± 0.08 | 0.45 ± 0.05 | 0.15 ± 0.08 | 0.22 ± 0.10 | |||
| −UV | 0.51 ± 0.02 | 0.26 ± 0.07 | 0.53 ± 0.06 | 0.37 ± 0.05 | 0.38 ± 0.08 | |||
| (V+A+Z)/Chl a+b | 22.3 ± 2.1 | +UV | 20.7 ± 1.4 | 22.3 ± 0.8 | 24.5 ± 2.1 | 23.8 ± 1.6 | 30.3 ± 2.3 | 32.2 ± 3.1 |
| (mmol mol−1) | −UV | 20.0 ± 1.4 | 22.9 ± 1.4 | 23.9 ± 1.9 | 28.0 ± 1.8 | 28.2 | 33.0 | |
| Z/(V+A+Z) | ND | +UV | 34.5 ± 3.7 | 51.5 ± 2.7 | 42.5 ± 1.1 | 41.4 ± 5.3 | 54.4 ± 7.1 | 15.3 ± 1.0 |
| (mol %) | −UV | 34.6 ± 1.5 | 50.5 ± 4.7 | 39.1 ± 1.0 | 32.6 ± 5.6 | 57.3 | 10.9 | |
| A/(V+A+Z) | ND | +UV | 8.9 ± 1.5 | 3.1 ± 1.6 | 12.3 ± 2.7 | 14.7 ± 4.5 | 10.5 ± 2.3 | 7.0 ± 1.6 |
| (mol %) | −UV | 9.2 ± 2.9 | 4.0 ± 0.9 | 13.3 ± 2.0 | 22.7 ± 2.2 | 8.4 | 2.7 | |
| L/Chl a+b | 179 ± 8 | +UV | 202 ± 6 | 220 ± 7 | 213 ± 6 | 216 ± 4 | 284 ± 8 | 284 ± 6 |
| (mmol mol−1) | −UV | 208 ± 12 | 208 ± 3 | 225 ± 11 | 217 ± 11 | 254 | 256 | |
| α-Car/β-Car | 1.19 ± 0.20 | +UV | 1.26 ± 0.24 | 1.41 ± 0.16 | 1.03 ± 0.07 | 0.97 ± 0.04 | 0.72 ± 0.06 | 0.69 ± 0.01 |
| (mol mol−1) | −UV | 1.05 ± 0.15 | 1.03 ± 0.06 | 1.18 ± 0.18 | 1.06 ± 0.16 | 0.84 | 0.81 | |
| Chl a/Chl b | 2.64 ± 0.05 | +UV | 2.68 ± 0.10 | 2.86 ± 0.06 | 2.72 ± 0.04 | 2.89 ± 0.05 | 2.78 ± 0.06 | 2.79 ± 0.07 |
| (mol/mol−1) | −UV | 2.60 ± 0.03 | 2.70 ± 0.01 | 2.77 ± 0.05 | 2.74 ± 0.03 | 2.65 | 2.65 |
Plants were kept under shade conditions and exposed daily for 1 h to sunlight in the presence and absence of UV-B radiation on 7 consecutive d (d 1–7). PAR doses during 1-h sun exposures varied with cloudiness. Fv/Fm was determined before (a) and after (b) sun exposure. Pigments were analyzed in unexposed plants (control), immediately after exposure (d 1–4, d 7) and after 1 d under shade conditions (d 8). Fluorescence and pigment data are means ± se of samples from three different plants (pigment controls, n = 6; other pigment data, n = 3, except for d 7 and 8, −UV, means of two samples; ND, not detectable). V, Violaxanthin; A, antheraxanthin; Z, zeaxanthin; L, lutein; Car, carotene.
Figure 2.
Effects of ambient UV-B and UV-A light on potential PSII efficiency during exposure of mature leaves of shade-grown A. excelsum seedlings containing about 330 μmol Chl a+b m−2 to direct sunlight. Zones of leaf blades (25 cm2) were exposed under glass filter GG 400 (minus UV-A,B), glass filter WG 320 (minus UV-B), or Aclar film (full spectrum). Three independent experiments (A–C) carried out on different days are depicted: A, PAR dose, 1.3 mol m−2 for 20 min; B, PAR dose, 1.7 mol m−2 for 15 min; C, PAR dose 2.6 mol m−2 for 30 min. Varying irradiance due to clouds did not allow averaging of data from different experiments). Data are means of two measurements given in percent of control values determined prior to exposure. Control Fv/Fm values (leaves before sun exposure, dark-adapted for 10 min) were: A, 0.793, 0.799, 0.790; B, 0.792, 0.794, 0.795; C, 0.790, 0.782, 0.789.
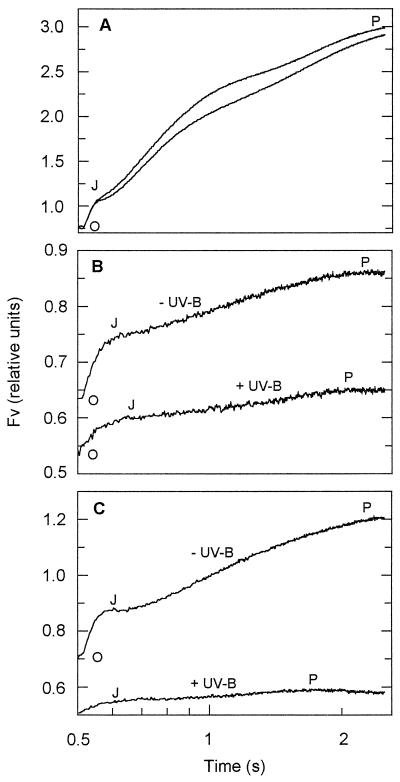
Figure 3A depicts the polyphasic kinetics of the Chl fluorescence rise in control leaves of V. surinamensis from Fo (“O”) via levels “J” and “I” to the peak (“P”) of the Kautsky curve (compare with Strasser et al., 1995). P was close to Fm under the conditions applied. In the photoinhibited leaves, the I level could not be distinguished (Fig. 3, B and C). The strong reduction in Fv/Fm that occurred upon exposure to the direct solar beam resulted predominantly (more than 90%) from a diminished J-P phase, whereas the O-J phase was less reduced (about 65%–75%). Both phases of the Fv rise were substantially more affected in the presence than in the absence of ambient UV-B light (Fig. 3B). After shading for 100 min, the onset of PSII recovery was observed only in the leaf that had been exposed in the absence of UV-B (Fig. 3C).
Figure 3.
Polyphasic kinetics of the Chl fluorescence rise from Fo (“O”) via levels “J” and “I” to the peak (“P”) of the Kautsky curve in mature leaves of shade-grown V. surinamensis seedlings containing about 370 μmol Chl a+b m−2 before and after direct sun exposure in the presence (+UV-B; Aclar film) and absence (−UV-B; Mylar film) of ambient UV-B radiation. The rise of Fv is depicted as a function of time (in logarithmic scale) in actinic light. A typical experiment is shown. A, Kinetics before exposure, recorded subsequent to 10 min of dark adaptation; B, kinetics after 30 min of sun exposure (PAR dose, about 2.6 mol m−2) and 10 min of dark adaptation; C, kinetics in the same leaves as for A and B after sun exposure followed by 90 min of recovery in low light and 10 min in the dark.
The average response of three leaves from different seedlings of V. surinamensis to 1 h of sun exposure is shown in Figure 4. The decrease in Fv/Fm was larger in the presence than in the absence of ambient UV-B, but in the long term, the decrease in potential PSII efficiency was reversible in both cases when the plants were transferred back to the shaded greenhouse. PSII recovery was extremely slow, requiring about 2 weeks to reach Fv/Fm close to the control values. During a second sun exposure of the plants after 12 d of recovery, the leaves had become slightly less UV-B sensitive. A tendency to recover more rapidly was observed after the second exposure (Fig. 4).
Figure 4.
Recovery of potential PSII efficiency (Fv/Fm) in mature leaves of shade-grown V. surinamensis seedlings after exposure to direct sunlight in the presence (●, Aclar) and absence (○, Mylar) of ambient UV-B. Plants were exposed for 1 h on d 1 and 14, respectively, to direct sunlight (arrows; approximate PAR doses given in the graph) and subsequently transferred to a shaded greenhouse (maximum PAR, 110 μmol m−2 s−1). Fv/Fm was determined after sun exposure followed by a 10-min dark period (d 1 and 14) or before sunrise (other days). Mean values ± se of three leaves from three different plants are shown.
The opening of a treefall gap in the forest was simulated by exposing shade-adapted seedlings of A. excelsum for 1 h to direct sunlight for 7 consecutive d (Table I). Exposure to high PAR doses (3.0 mol m−2 and higher, d 1, 2, and 4) resulted in a substantial decline in Fv/Fm. On d 7, this decline was seen as a tendency only, as values of Fv/Fm were already very low prior to exposure. A significant increase in PSII inhibition in the presence of UV-B light was evident on d 1 and d 4. Partial recovery (restoration of Fv/Fm values) occurred in the shade between daily exposure periods. Analyses of photosynthetic pigments revealed acclimative responses of the leaves. The pool size of xanthophyll cycle pigments (sum of V+A+Z per unit of Chl a+b) increased by approximately 60% within 8 d, but remained considerably lower than in sun leaves of mature A. excelsum trees (compare with Krause et al., 1995; Thiele et al., 1996).
The α-carotene to β-carotene ratio, which is known to be high in shade leaves and low in sun leaves (Thayer and Björkman, 1990), decreased significantly during the 8 d of the experiment. The change in this ratio resulted from a decrease in α-carotene and an increase in β-carotene levels (data not shown). In addition, the amounts of lutein per unit Chl a+b increased by approximately 50% (Table I). Exclusion of most of the ambient UV-B under the Mylar film had no significant influence on these responses. Also, the turnover of the xanthophylls, indicated by high proportions of zeaxanthin and antheraxanthin formed during sunlight exposure, was not affected by the ambient UV-B dose. One day after the last exposure (d 8), substantial levels of zeaxanthin and antheraxanthin were still present, whereas these xanthophylls were not detectable in shade-adapted control leaves. Chl a/b ratios did not change significantly during the experiment (Table I). There were no significant alterations in the level of neoxanthin and the sum of α- and β-carotene expressed on a Chl a+b basis (data not shown).
Repetitive daily short-term exposure of V. surinamensis seedlings to the full solar spectrum diminished UV-B sensitivity of the leaves (Fig. 5A). There was a substantial UV-B effect on the 1st d of exposure, i.e. in the nonacclimated state of the plants. After 8 d of daily 30-min sun exposure followed by 5 d of recovery in the shade, no UV-B effect could be detected upon renewed solar irradiation. Slightly increased levels of foliar UV-B-absorbing substances were present at the end of the experiment (d 17; Fig. 5B), but this effect was also observed when ambient UV-B light had been excluded during sun exposure.
Sun Leaves of Mature Trees
Sun leaves from the outer crown of mature trees were much less susceptible to high-light stress than leaves of shade-grown tree seedlings. However, even in these sun-acclimated leaves, an enhancement of photoinhibition of PSII by ambient UV-B was observed in several instances (Figs. 6–8). The course of the decline in Fv/Fm upon exposure of young sun leaves of A. excelsum to direct sunlight including or excluding UV-B is demonstrated in Figure 6. The kinetics of the fluorescence rise (O-J-P) in young sun leaves of A. excelsum (induction curves not shown) were affected by sun exposure in a manner similar to that in shade-grown seedlings (compare with Fig. 3), although to a lesser degree; i.e. substantial reductions of the J-P phase, but not of the O-J phase were found. The decrease in Fv/Fm was fully reversible within 1 d (data not shown).
Figure 6.
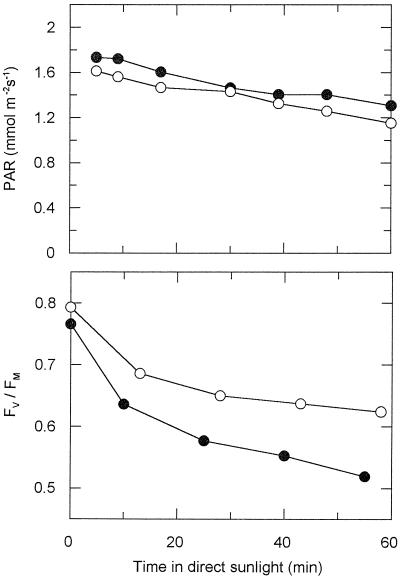
Effect of ambient UV-B radiation on potential PSII efficiency (Fv/Fm) during exposure of young sun leaves of mature A. excelsum trees to direct sunlight. A typical experiment is shown. In three independent further measurements, similar results were obtained upon 45 to 55 min of sun exposure and PAR doses between 4.2 and 5.5 mol m−2; due to varying irradiance caused by clouds, averaging of data was not possible. Upper panel, PAR under Aclar (+UV-B; ●) and Mylar (−UV-B; ○) films, respectively; lower panel, Fv/Fm as function of exposure time.
Figure 8.
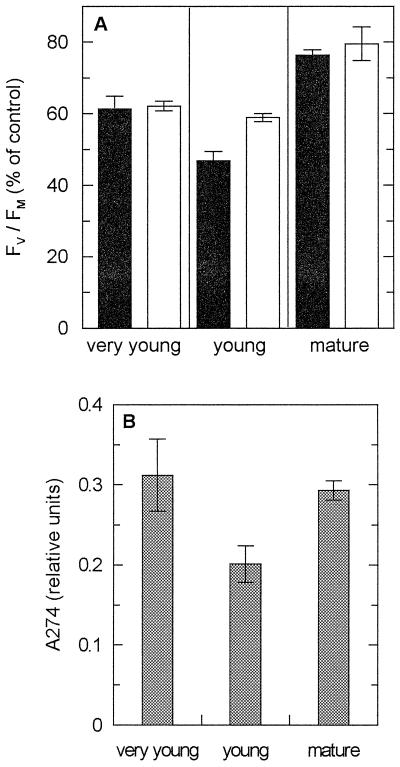
Effects of ambient UV-B radiation on potential efficiency of PSII (Fv/Fm) during direct sun exposure (A), and content of vacuolar UV-absorbing substances (B) of sun leaves of M. indica. A, Leaves in three developmental stages (very young, young, and mature) were exposed to a PAR dose of about 4.2 mol m−2 (45 min). Fv/Fm determined subsequent to sun exposure in the presence (black bars, Aclar) and absence (white bars, Mylar) of UV-B is given as percent of controls. Data are means ± se of three measurements made at different leaf regions. Control values of Fv/Fm (leaves before sun exposure; n = 6) were: 0.72 ± 0.01 (very young), 0.67 ± 0.04 (young), and 0.79 ± 0.01 (mature leaves). A representative experiment is depicted. B, Relative content of UV-absorbing substances in the three developmental stages of M. indica sun leaves (absorbance measured at λmax in the UV region, 274 nm). Data are means ± se (n = 6). Note that the leaf extracts were 10 times more dilute than for shade-grown V. surinamensis leaves (compare with Fig. 5B).
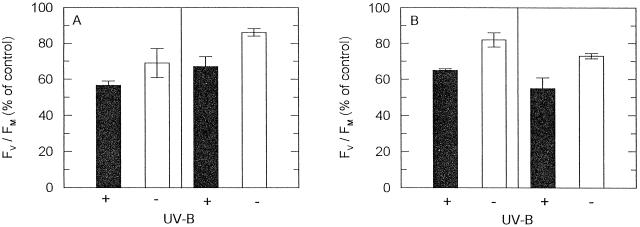
Young, light-green sun leaves of tropical trees, containing about one-half of the Chl a+b of the mature leaves, are usually more susceptible to light stress than mature leaves (Krause et al., 1995). This is shown in Figure 7A for leaves of A. occidentale. In Figure 7B (A. excelsum), a higher PAR dose, i.e. longer exposure time, was chosen for the mature than for the young leaves to obtain a strong effect on PSII in the mature leaves. Both in young and mature leaves of A. excelsum and A. occidentale, the decline in Fv/Fm was significantly more pronounced when the leaves were sun-exposed in the presence of UV-B. These results are remarkable considering the efficient UV absorption by protective substances in the sun leaves; both young and mature sun leaves of A. excelsum contained substantially higher levels of extractable UV-absorbing compounds per unit leaf area than leaves of shade-grown seedlings (data not shown). However, in other experiments with sun leaves of A. excelsum, A. occidentale, and M. indica (see below), UV-B effects on PSII were often less pronounced, or even absent, and the degree of UV-B response seemed to depend on the developmental stage and the light acclimation state of the leaves. Similarly, the response to UV-A light was variable or absent in sun leaves (data not shown).
Figure 7.
Effects of ambient UV-B radiation on potential PSII efficiency (Fv/Fm) during exposure to direct sunlight of young and mature sun leaves of A. occidentale (A) and A. excelsum trees (B). A, Young (left) and mature (right) leaves at 3.6 mol m−2 PAR for 35 min; B, young leaves (left) at 3.6 mol m−2 PAR for 35 min and mature leaves (right) at 4.5 mol m−2 PAR 75 min−1. Representative experiments are depicted. Fv/Fm recorded after sun exposure in the presence (black bars, Aclar) and absence (white bars, Mylar) of UV-B is given as percent of controls. Data are means ± se of three measurements at different regions of each leaf. Fv/Fm of controls (leaves before sun exposure; n = 6) were: A, 0.66 ± 0.03 (young leaves), 0.75 ± 0.02 (mature leaves); B, 0.78 ± 0.00 (young leaves), 0.82 ± 0.01 (mature leaves).
Sun leaves of M. indica were examined at different stages of development (Fig. 8). Young leaves (40% Chl a+b per unit leaf area of mature ones; see Table II) exhibited increased photoinhibition of PSII in the presence of ambient UV-B. Very young leaf flushes (28% Chl a+b), colored red by anthocyanins, were insensitive to UV-B, as were the mature leaves. The UV-B-sensitive young leaves possessed significantly lower amounts of UV-B-absorbing protective compounds than either the very young or the mature leaves (Fig. 8B). The Chl a/b ratios did not differ between the three developmental stages, but the pool size of xanthophyll cycle pigments on a Chl a+b basis was larger in the young and very young than in the mature leaves (Table II).
Table II.
Photosynthetic pigments in sun leaves of M. indica (mango)
| Pigments | Very Young | Young | Mature |
|---|---|---|---|
| Chl a/Chl b | 3.5 ± 0.5 | 3.7 ± 0.4 | 3.6 ± 0.5 |
| Chl a+b | 91 ± 9 | 129 ± 8 | 323 ± 19 |
| Car | 43 ± 3 | 73 ± 9 | 164 ± 4 |
| V+A+Z | 13 ± 1 | 23 ± 3 | 33 ± 4 |
| Car/Chl a+b | 497 ± 75 | 612 ± 47 | 509 ± 29 |
| (V+A+Z)/Chl a+b | 240 ± 40 | 275 ± 21 | 163 ± 19 |
Units: Chl a+b, sum of carotenoids (Car), sum of xanthophyll cycle pigments, V+A+Z (μmol m−2); Car/Chl a+b, (V+A+Z)/Chl a+b (mmol mol−1). Means ± se of three or four leaf samples are given.
DISCUSSION
Shade Leaves
Short-term sun exposure of shade-acclimated tree seedlings revealed marked effects of ambient UV-B and UV-A, in addition to effects of visible light, on PSII efficiency (Figs. 1–5). Shade-tolerant tree seedlings that grow in the understory of the tropical forest and are acclimated to deep shade encounter such conditions of sudden and repetitive exposure to full direct sunlight for short daily periods, when medium-sized treefall gaps are formed. Our results suggest that during transitions from shaded understory to gap conditions, light stress (including UV stress) causes a strong and only slowly reversible reduction in potential PSII efficiency. This may be accompanied by a reduction in photosynthetic CO2 assimilation, as indicated by preliminary gas exchange measurements (data not shown). Direct effects of UV-B light on photosynthetic carbon metabolism have not been investigated here. Therefore, we cannot exclude that the ambient UV-B caused a primary inhibition of Calvin cycle activity. Such an effect has been found when plants were exposed to high doses of supplemental UV-B light, which in the early stages caused substantial reduction in the rate of CO2 assimilation and the amount of Rubisco in the absence of a major decline in PSII photochemistry (Nogués and Baker, 1995; Allen et al., 1997, 1998).
A similarly persisting (chronic) photoinhibition of PSII as described in the present study was observed when shade-grown plants of various tropical species were repetitively exposed to direct sunlight for the whole diurnal light period (Lovelock et al., 1994). Photoinhibitory effects on PSII may also be exerted by strong sunflecks (Watling et al., 1997).
Greenhouse-grown plants were used to obtain more homogeneous leaf material than that obtained from seedlings grown under natural shade conditions. It might be argued that the seedlings cultivated in the greenhouse were more sensitive to UV-B light than plants acclimated to natural shade on the forest floor, where they are exposed to more scattered UV light. According to measurements made in Panama (Flint and Caldwell, 1998), the absolute UV-B radiation (at 305 nm) is very low in deep shade, but the UV-B to PAR ratio is higher than under the open sky, since a larger proportion of UV-B than of PAR is diffused in the atmosphere. However, as shown in Figure 5B for V. surinamensis, even in the absence of substantial UV-B irradiance, UV-absorbing substances were formed in the leaves. Further accumulation of such compounds induced by direct sun exposure did not depend on UV-B light. Moreover, in A. excelsum leaves (Table I), repetitive daily 1-h sun exposure for 3 d did not alleviate the UV-B effect on PSII (d 4, Table I). Thus, we can assume that low levels of scattered UV, as present in natural shade, would not significantly alter the results and conclusions of our study.
In several experiments we demonstrated that the tree seedlings were capable of acclimating to the simulated gap conditions (compare with Mulkey and Pearcy, 1992; Lovelock et al., 1994). A trend of faster recovery was already seen after a second sun exposure of V. surinamensis seedlings (Fig. 4). The acclimation included a substantial increase in the pool size of xanthophyll cycle pigments, i.e. in the molar ratio (V+A+Z)/Chl a+b (shown for A. excelsum seedlings in Table I), providing improved protection against stress exerted by visible light (Demmig-Adams and Adams, 1992; Horton et al., 1996). The level of xanthophyll cycle pigments reached after 7 d of 1 h of daily sun exposure was close to 60% of the level found on average in plants growing in 2- to 3-year-old natural forest gaps (Thiele et al., 1998). The maximum de-epoxidation of violaxanthin observed in response to strong solar irradiation in the shade-grown seedlings (Table I; d 2 and 7) was similar to that in the gap plants previously studied in situ. It should be noted that the de-epoxidation state was not influenced by ambient UV-B (Table I). Pfündel et al. (1992) described an inhibition of the violaxanthin de-epoxidase by artificial UV-B; but this effect was caused only by unrealistically high UV-B doses far in excess of those applied in the present study.
As a further acclimative response, the α/β-carotene ratio declined due to sun exposure (Table I), but did not reach within 8 d the average value of about 0.5 found in gap-acclimated plants (Thiele et al., 1998). Decreased α/β-carotene ratios have been demonstrated in sun leaves compared with shade leaves (Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992; Königer et al., 1995) and in young leaves compared with mature canopy sun leaves exposed to highly excessive light (Krause et al., 1995). However, a change in this ratio, as well as an increase in lutein levels (Table I) caused by periodic light stress in fully developed leaves has apparently not been reported previously.
As shown for V. surinamensis seedlings, acclimation to repetitive sun exposure led to reduced sensitivity to ambient UV-B (Fig. 5A). There is evidence that vacuolar flavonoids and related compounds provide efficient UV protection (e.g. Reuber et al., 1993, 1996). The increase in the content of extractable foliar UV-absorbing substances (Fig. 5B) was relatively small. To our knowledge, information on the distribution of these compounds within the leaves studied is not currently available in the literature. An accumulation of UV-protecting substances in the adaxial epidermis could explain the acclimation to UV-B stress seen in Figure 5A. In numerous studies, flavonoid synthesis and other protective effects have been found to be induced by UV-B light (e.g. Flint et al., 1985; Caldwell et al., 1989; Teramura and Ziska, 1996). However, the acclimative responses shown in Figure 5B and Table I were not specifically induced by the shorter-wavelength UV-B light (excluded by Mylar film); rather, UV-A or blue-light receptors (Teramura and Ziska, 1996; Jenkins, 1997) might be effective sensors. The changes in carotenoid content and composition (Table I) possibly are responses to excessive PAR only.
Sun Leaves
Even though the sun leaves of mature trees were better protected against UV light stress than the shade-grown tree seedlings, in several experiments we observed that ambient UV-B markedly affected PSII in both young (Figs. 6–8) and fully developed (Fig. 7) sun leaves. The variability and sometimes absence of the UV-B (and UV-A) effects in the same species indicate that leaves can become fully protected against ambient UV levels. Our data (Fig. 8) support the results of many studies using supplemental UV-B light (Allen et al., 1998; Jansen et al., 1998) that UV-B absorbing vacuolar compounds play an important protective role. Possibly, UV sensitivity varies with individual light acclimation of the leaves depending on their position in the tree crown. Moreover, the developmental stage seems to influence UV-B sensitivity of PSII in sun leaves. In the very young red and the mature dark-green leaves of M. indica, the tolerance of PSII to ambient UV-B was associated with high levels of extractable UV-absorbing substances. In the very young red leaves, anthocyanins may contribute to UV protection, as has been suggested earlier for rapidly flushing young leaves of tropical trees (Lee and Lowry, 1980). In the subsequent light-green stage of the young leaves, UV-B sensitivity was associated with a significantly reduced content of UV-absorbing compounds. As indicated by the high (V+A+Z)/Chl a+b ratio (Table II), these leaves were well acclimated to stress exerted by visible light. The high Chl a/b ratio of the three differently developed leaf types (Table II) is consistent with their role as sun leaves.
Possible Mechanisms
The very slow recovery of potential PSII efficiency in leaves of the shade-grown tree seedlings (Fig. 4, Table I) suggests that the exposure to direct sunlight, including UV light, has caused protein damage. It is known that shade leaves have a low capacity to restore PSII activity via protein degradation and resynthesis (Aro et al., 1993). Studies that applied artificial UV-B (Friso et al., 1994; Jansen et al., 1998) suggest that both the D1 and D2 proteins of the PSII reaction center might need replacing.
In the sun leaves and in leaves acclimated to forest gap conditions, a major component of PSII photoinhibition has been shown to be related to the formation of zeaxanthin (and possibly of antheraxanthin) under high light via the xanthophyll cycle (Thiele et al., 1996, 1998). This was particularly pronounced in young sun leaves, which exhibited a higher pool size and turnover of xanthophyll cycle pigments and less D1 protein degradation compared with mature leaves (Krause et al., 1995; Thiele et al., 1997). If the UV component of the solar spectrum does affect the D1 and D2 proteins in sun leaves, restoration of PSII activity would be possible in low light during several hours of a slow recovery phase that presumably involves protein turnover (Leitsch et al., 1994; Thiele et al., 1996, 1998). Thus, even when UV exposure increases photoinhibition of PSII, we do not expect permanent or long-lasting damage to PSII in sun leaves caused by ambient UV-B. Also, a loss of Calvin cycle activity (Allen et al., 1998), if it does occur, would be quickly restored. In sun leaves of young trees exposed to full sunlight during the course of a day, maximum rates of CO2 assimilation were similar in the morning and after a midday depression in the afternoon (Krause et al., 1995).
Sun exposure of shade-grown tree seedlings resulted in a predominant reduction of the J-P phase of fluorescence induction (Fig. 3). When recorded in low or moderate light, as in the present study, the J-P phase is regarded as the fluorescence induction of fully functional PSIIα units, whereas he O-J phase has been interpreted to represent emission by PSIIβ units that are incapable of transferring electrons from QA to QB (from the primary to the secondary quinone-type electron acceptor of PSII) and possibly are in the process of being repaired (Melis, 1991). The data indicate that the functional PSIIα units are predominately affected by sunlight and converted to fluorescence quenchers (compare with Krause et al., 1990; van Wijk et al., 1993). To a lesser extent, PSIIβ units were also affected in the shade leaves, particularly in the presence of UV-B (Fig. 3B). As the PSIIβ units are viewed as a reserve pool for restoring fully active PSIIα (Melis, 1991), the effect of UV-B light on the β-centers may explain the delay in recovery observed after exposure to the complete solar spectrum (Fig. 3C). There was no substantial decrease of the O-J phase of fluorescence induction in the young sun leaves of A. excelsum (data not shown), which suggests that the QB-nonreducing β-units were not markedly affected in either the presence or absence of UV-B light.
CONCLUSION
The present data demonstrate that in the leaves of tropical trees, the ambient UV-B and UV-A radiation may contribute to the reversible decline in potential PSII efficiency observed upon exposure to full, direct sunlight. Sensitivity of PSII to natural UV-B light depended on the acclimation status and developmental stages of the leaves and tended to decline with increased levels of vacuolar UV-absorbing compounds. In shade-grown tree seedlings periodically exposed to full sunlight, protein damage was indicated by the strong effects of ambient PAR and UV light on Fv/Fm and fluorescence induction kinetics, and by the extremely slow recovery. Leaves of the crown of mature trees that are acclimated to full sunlight may achieve full protection against adverse effects of UV on PSII. But at certain stages of development, or depending on the prior light environment (incomplete acclimation), ambient UV light may cause temporary losses in PSII efficiency. Although tropical plants are capable of effectively protecting their leaves against UV stress, an anthropogenic rise in ambient UV-B radiation in the tropics may markedly affect their PSII efficiency.
ACKNOWLEDGMENTS
We thank Silke Scholl and Aurelio Virgo for valuable assistance, and Darren Crayn, Joseph Holtum, Robert Ingle, and Simon Pierce for reading the manuscript.
Footnotes
This study was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB189), by the Andrew W. Mellon Foundation through the Smithsonian Institution, by the Hort-Stiftung (Düsseldorf, Germany), and by the Smithsonian Tropical Research Institute, Panama.
LITERATURE CITED
- Allen DJ, McKee IF, Farage PK, Baker NR. Analysis of limitations to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997;20:633–640. [Google Scholar]
- Allen DJ, Nogués S, Baker NR. Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot. 1998;49:1775–1788. [Google Scholar]
- Aro E-M, McCaffery S, Anderson JM. Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol. 1993;103:835–843. doi: 10.1104/pp.103.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR, Bowyer JR, editors. Photoinhibition of Photosynthesis. From Molecular Mechanisms to the Field. Oxford: Bios Scientific Publishers; 1994. [Google Scholar]
- Bojkov RD, Fioletov VE. Total ozone variations in the tropical belt: an application for quality of ground based measurements. Meteorol Atmos Phys. 1996;58:223–240. [Google Scholar]
- Caldwell MM, Teramura AH, Tevini M. The changing solar ultraviolet climate and the ecological consequences for higher plants. Trends Ecol Evol. 1989;4:363–367. doi: 10.1016/0169-5347(89)90100-6. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P. Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants. Plant Physiol. 1997;115:1609–1618. doi: 10.1104/pp.115.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SD, Caldwell MM. Solar UV-B and visible radiation in tropical forest gaps: measurements partitioning direct and diffuse radiation. Global Change Biol. 1998;4:863–870. [Google Scholar]
- Flint SD, Jordan PW, Caldwell MM. Plant protective response to enhanced UV-B radiation under field conditions: leaf optical properties and photosynthesis. Photochem Photobiol. 1985;41:95–99. [Google Scholar]
- Friso G, Barbato R, Giacometti GM, Barber J. Degradation of the D1 protein due to UV-B irradiation of the reaction centre of photosystem II. FEBS Lett. 1994;339:217–221. doi: 10.1016/0014-5793(94)80419-2. [DOI] [PubMed] [Google Scholar]
- Giersch C, Krause GH. A simple model relating photoinhibitory fluorescence quenching in chloroplasts to a population of altered photosystem II reaction centers. Photosynth Res. 1991;30:115–121. doi: 10.1007/BF00042009. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Häder D-P, Ghetti F. Inhibition of photosynthesis by solar radiation in Dunaliella salina: relative efficiencies of UV-B, UV-A and PAR. Plant Cell Environ. 1997;20:359–365. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jenkins GI. UV and blue light signal transduction in Arabidopsis. Plant Cell Environ. 1997;20:773–778. doi: 10.1046/j.1365-3040.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- Königer M, Harris GC, Virgo A, Winter K. Xanthophyll-cycle pigments and photosynthetic capacity in tropical forest species: a comparative field study on canopy, gap and understory plants. Oecologia. 1995;104:280–290. doi: 10.1007/BF00328362. [DOI] [PubMed] [Google Scholar]
- Krause GH, Somersalo S, Zumbusch E, Weyers B, Laasch H. On the mechanism of photoinhibition in chloroplasts: relationship between changes in fluorescence and activity of photosystem II. J Plant Physiol. 1990;136:472–479. [Google Scholar]
- Krause GH, Virgo A, Winter K. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta. 1995;197:583–591. [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Krause GH, Winter K. Photoinhibition of photosynthesis in plants growing in natural tropical forest gaps: a chlorophyll fluorescence study. Bot Acta. 1996;109:456–462. [Google Scholar]
- Lee DW, Lowry JB. Young-leaf anthocyanin and solar ultraviolet. Biotropica. 1980;12:75–76. [Google Scholar]
- Leitsch J, Schnettger B, Critchley C, Krause GH. Two mechanism of recovery from photoinhibition in vivo: reactivation of photosystem II related and unrelated to D1-protein turnover. Planta. 1994;194:15–21. [Google Scholar]
- Long SP, Humphries S, Falkowski PG. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. [Google Scholar]
- Lovelock CE, Jebb M, Osmond CB. Photoinhibition and recovery in tropical plant species: response to disturbance. Oecologia. 1994;97:297–307. doi: 10.1007/BF00317318. [DOI] [PubMed] [Google Scholar]
- Madronich S, McKenzie RL, Caldwell MM, Björn LO. Changes in ultraviolet radiation reaching the earth's surface. Ambio. 1995;24:143–152. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Mulkey SS, Pearcy RW. Interactions between acclimation and photoinhibition of photosynthesis of a tropical understorey herb, Alocasia macrorrhiza, during simulated canopy gap formation. Funct Ecol. 1992;6:719–729. [Google Scholar]
- Nogués S, Baker NR. Evaluation of the role of damage to photosystem II in the inhibition of CO2 assimilation in pea leaves on exposure to UV-B radiation. Plant Cell Environ. 1995;18:781–787. [Google Scholar]
- Pfündel EE, Pan R-S, Dilley RA. Inhibition of violaxanthin deepoxidation by ultraviolet-B radiation in isolated chloroplasts and intact leaves. Plant Physiol. 1992;98:1372–1380. doi: 10.1104/pp.98.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber S, Bornman JF, Weissenböck G. Phenylpropanoid compounds in primary leaf tissue of rye (Secale cereale): light response of their metabolism and the possible role in UV-B protection. Physiol Plant. 1996;97:160–168. [Google Scholar]
- Reuber S, Leitsch J, Krause GH, Weissenböck G. Metabolic reduction of phenylpropanoid compounds in primary leaves of rye (Secale cereale L.) leads to increased UV-B sensitivity of photosynthesis. Z Naturforsch. 1993;48c:749–756. [Google Scholar]
- Rozema J, van de Staaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- Searles PS, Caldwell MM, Winter K. The response of five tropical dicotyledon species to solar ultraviolet-B radiation. Am J Bot. 1995;82:445–453. [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol. 1995;61:32–42. [Google Scholar]
- Teramura AH, Ziska LH. Baker NR ed, Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. Ultraviolet-B radiation and photosynthesis; pp. 435–450. [Google Scholar]
- Thayer SS, Björkman O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res. 1990;23:331–343. doi: 10.1007/BF00034864. [DOI] [PubMed] [Google Scholar]
- Thiele A, Krause GH, Winter K. In situ study of photoinhibition of photosynthesis and xanthophyll cycle activity in plants growing in natural gaps of the tropical forest. Aust J Plant Physiol. 1998;25:189–195. [Google Scholar]
- Thiele A, Schirwitz K, Winter K, Krause GH. Increased xanthophyll cycle activity and reduced D1 protein inactivation related to photoinhibition in two plant systems acclimated to excess light. Plant Science. 1996;115:237–250. [Google Scholar]
- Thiele A, Winter K, Krause GH. Low inactivation of D1 protein of photosystem II in young canopy leaves of Anacardium excelsum under high-light stress. J Plant Physiol. 1997;151:286–292. [Google Scholar]
- van Wijk KJ, Schnettger B, Graf M, Krause GH. Photoinhibition and recovery in relation to heterogeneity of photosystem II. Biochim Biophys Acta. 1993;1142:59–68. [Google Scholar]
- Watling JR, Robinson SA, Woodrow IE, Osmond CB. Responses of rainforest understorey plants to excess light during sunflecks. Aust J Plant Physiol. 1997;24:17–25. [Google Scholar]
- Ziska LH. The potential sensitivity of tropical plants to increased ultraviolet-B radiation. J Plant Physiol. 1996;148:35–41. [Google Scholar]