Abstract
Synthesis of polyhydroxyalkanoates (PHAs) from intermediates of fatty acid β-oxidation was used as a tool to study fatty acid degradation in developing seeds of Arabidopsis. Transgenic plants expressing a peroxisomal PHA synthase under the control of a napin promoter accumulated PHA in developing seeds to a final level of 0.06 mg g−1 dry weight. In plants co-expressing a plastidial acyl-acyl carrier protein thioesterase from Cuphea lanceolata and a peroxisomal PHA synthase, approximately 18-fold more PHA accumulated in developing seeds. The proportion of 3-hydroxydecanoic acid monomer in the PHA was strongly increased, indicating a large flow of capric acid toward β-oxidation. Furthermore, expression of the peroxisomal PHA synthase in an Arabidopsis mutant deficient in the enzyme diacylglycerol acyltransferase resulted in a 10-fold increase in PHA accumulation in developing seeds. These data indicate that plants can respond to the inadequate incorporation of fatty acids into triacylglycerides by recycling the fatty acids via β-oxidation and that a considerable flow toward β-oxidation can occur even in a plant tissue primarily devoted to the accumulation of storage lipids.
The catabolism of fatty acids is mediated primarily by the peroxisomal β-oxidation pathway, generating one molecule of acetyl-CoA for every turn of the cycle (Gerhart, 1993). The acetyl-CoA generated is converted by the glyoxylate cycle to succinate, which can then enter the tricarboxylic acid cycle. In plants accumulating a large proportion of carbon reserves as triacylglycerides (TAG), such as rapeseed (Brassica napus) and Arabidopsis, the activation of the β-oxidation and glyoxylate cycles during germination ensures conversion of fatty acids to carbohydrates necessary for the growth of the seedling before establishment of photosynthesis. During photosynthetic growth, the enzymes of both the β-oxidation and glyoxylate cycles are found at very low levels. Reactivation of the β-oxidation and glyoxylate cycles has been observed either during natural senescence or during artificial senescence induced by prolonged exposure to darkness or withdrawing the carbohydrate source from suspension cell cultures or non-photosynthetic tissues (e.g. excised roots) (Dieuaide et al., 1992; Pistelli et al., 1996; Ismail et al., 1997; Lee et al., 1998). These experiments indicate that plants respond to low carbohydrate availability by an increase in the β-oxidation and glyoxylate pathways necessary for carbohydrate synthesis from fatty acids. It has been shown that intracellular concentration of hexose sugars or the flow of hexose sugars into glycolysis may provide important signals that give rise to changes in expression of the genes involved in the glyoxylate cycle (Graham et al., 1994).
Although the enzymes involved in fatty acid degradation are normally present at very low levels in photosynthetic leaves, an increase in isocitrate lyase activity, a marker enzyme for the glyoxylate cycle, has been detected in leaves of transgenic rapeseed expressing the California bay lauroyl-acyl carrier protein (ACP) thioesterase under the control of the cauliflower mosaic virus (CaMV) 35S promoter (Eccleston et al., 1996). In these transgenic plants, accumulation of lauric acid is detected only in seeds and not in leaves, even though the rate of lauric acid synthesized in chloroplasts isolated from these transgenic plants is relatively high (Eccleston et al., 1996). These results suggested that the lauric acid synthesized in leaves is rapidly degraded through the peroxisomal β-oxidation cycle, a hypothesis supported by the increase in isocitrate lyase activity (Eccleston et al., 1996). In a further extension of this work, it has been shown that high-level seed-specific expression of the same medium-chain-length thioesterase leads to activation of both β-oxidation and glyoxylate cycles in developing seeds, and that a substantial proportion of fatty acids produced in these seeds are recycled to acetyl-CoA and Suc (Eccleston and Ohlrogge, 1998). The amounts of several enzymes involved in fatty acid biosynthesis were also increased, indicating the activation of a mechanism aimed at compensating the fatty acids lost though β-oxidation (Eccleston and Ohlrogge, 1998). These studies indicate that plant cells can sense levels of either free or esterified fatty acids and adjust its metabolism to degrade them and/or enhance their synthesis.
In contrast to the work done in B. napus, constitutive expression of the California bay lauroyl-ACP thioesterase in Arabidopsis did not lead to a measurable activation of enzymes or genes involved in either β-oxidation or glyoxylate cycles in leaves (Hooks et al., 1999). These results indicated that in Arabidopsis, the level of β-oxidation activity present in photosynthetic leaves of wild-type plants is sufficient to cope with an increased flow of fatty acids toward β-oxidation in plants expressing a medium-chain-length thioesterase. Thus, quantification of enzymes involved in fatty acid degradation is not a reliable indicator of the carbon flow toward β-oxidation.
It has recently been demonstrated that expression of a bacterial polyhydroxyalkanoate (PHA) synthase in the peroxisomes of transgenic Arabidopsis leads to the accumulation of PHA inclusions inside the organelle (Mittendorf et al., 1998a, 1998b). In this system, PHA is synthesized from the polymerization of the 3-hydroxyacyl-CoA intermediates of β-oxidation of fatty acids. Synthesis of PHA in peroxisomes paralleled the activity of the β-oxidation cycle, being high during germination and senescence and low during photosynthetic growth (Mittendorf et al., 1998b). PHAs are polyesters normally synthesized by a wide variety of bacteria. They have attracted considerable interest because of their plastic and elastomeric properties, as well because of their biodegradability, making them an interesting source of renewable and environmentally friendly polymers (Poirier et al., 1995; Steinbüchel and Füchtenbusch, 1998). Synthesis of PHAs in agricultural crops is seen as an alternative to bacterial fermentation for the production of PHAs on a large scale and at low cost (Poirier et al., 1992; Poirier, 1999).
We were interested in examining whether the synthesis of PHA in peroxisomes can be used as a novel tool to analyze the flow of fatty acids toward β-oxidation. In this model system, changes in the quantity of PHA synthesized reflects the amount of fatty acids channeled to peroxisomal β-oxidation. Furthermore, since peroxisomal PHA is composed of saturated and unsaturated 3-hydroxyacid monomers ranging from 6 to 16 carbons, all of which are derived from the β-oxidation of endogenous fatty acids, the monomer composition of the PHA also reflects the quality (degree of saturation and length) of the fatty acids channeled toward β-oxidation. We report our analysis of the flow of fatty acids toward peroxisomal β-oxidation in developing seeds of Arabidopsis. We show that this flow can be considerably increased in developing seeds through the expression of a medium-chain-length thioesterase or by a mutation affecting diacylglycerol acyltransferase activity.
MATERIAL AND METHODS
Plant Material
Arabidopsis ecotype Columbia was used for all transformation experiments. Arabidopsis transgenic line C-PHA-3.3, expressing a PHA synthase modified for peroxisome targeting under the control of a CaMV 35S promoter, has previously been described (Mittendorf et al., 1998a, 1998b). The Arabidopsis mutant lines SK353 and SK54-3 are allelic to the mutant AS11 described by Katavic et al. (1995) and were provided by L. Kunst (University of British Columbia, Vancouver, Canada). SK353 is in the Columbia background, while SK54-3 is in the RLD background. For all experiments, plants were grown under constant fluorescent light at 19°C.
DNA Constructs and Plant Transformation
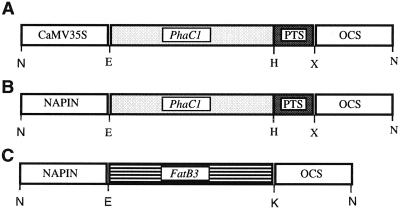
The PhaC1 synthase gene from Pseudomonas aeruginosa was modified at the carboxy terminus by the addition of the last 34 amino acids of the B. napus isocitrate lyase gene, as previously described (Mittendorf et al., 1998a, 1998b). The modified PhaC1 synthase was cloned in the pART7 vector (Gleave, 1992), putting the gene under the control of the CaMV 35S promoter, to give the construct C-PHA (Fig. 1A). The CaMV 35S promoter was removed from the pART7 vector by a SacI-EcoRI digestion and replaced by a 1.1-kb fragment harboring the napin promoter from B. napus (Stålberg et al., 1993) to give the plasmid pART7-napin. The modified PhaC1 synthase was then cloned in the pART7-napin vector to give the N-PHA construct (Fig. 1B). The Cuphea lanceolata FatB3 gene, encoding a medium-chain-length acyl-ACP thioesterase (Martini et al., 1999) was cloned as a EcoRI-KpnI fragment into the pART7-napin vector to give the N-FatB3 construct (Fig. 1C). All pART7-derived constructs were cloned into the pART27 binary vector as NotI fragments and transferred into Agrobacterium tumefaciens pGV3101 (Koncz and Schell, 1986) by electroporation. Arabidopsis was transformed by vacuum infiltration, as described by Betchold et al. (1993). Transformants were isolated by plating seeds on medium containing Murashige and Skoog salts, 1% (w/v) Suc, 0.7% (w/v) agar, and 50 μg/mL kanamycin.
Figure 1.
Diagrams of three DNA constructs used to express the PhaC1 synthase and FatB3 thioesterase genes in transgenic plants. Only the portion of the constructs containing the genes and regulatory elements (CaMV 35S and napin promoters, octopine synthase 3′-untranslated region [OCS]) are shown. A, C-PHA; B, N-PHA; C, N-FatB3. PTS, Peroxisomal targeting sequence derived from the last 34 amino acids of the isocitrate lyase from B. napus; N, NotI; E, EcoRI; H, HindIII; X, XbaI; K, KpnI.
Protein Analysis
Leaf tissue or developing siliques (10- to 14-d-old) were homogenized in a protein extraction buffer containing 0.25 mm EDTA, 100 mm Tris-HCl pH 8.0, 5 mm dithiothreitol, and 1 mm phenylmethylsulphonyl fluoride. The extracts were centrifuged for 30 s at 4°C and the supernatants were denatured according to the method of Laemmli (1970). Proteins were separated on a 12% (w/v) SDS-PAGE and blotted onto nitrocellulose membrane using an electrophoretic cell (Trans-Blot, Bio-Rad Laboratories, Hercules, CA). Free binding sites were saturated by incubation in blocking buffer (0.4 m NaCl, 2 mm KCl, 20 mm Tris-HCl [pH 7.4], 0.1% [v/v] Tween, and 5% [w/v] milk powder) for 1 h. The membranes were incubated for 2 h in blocking buffer with the PhaC1 antibody (provided by A. Steinbüchel, University of Münster, Germany). The antigen-antibody complexes were visualized with horseradish peroxidase-coupled goat anti-rabbit antibodies using the enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
PHA and Lipid Analysis
Extraction of PHA from plant material and analysis by gas chromatography-mass spectrometry (GC-MS) was done essentially as previously described (Mittendorf et al., 1998b). Approximately 2 to 4 g of seeds was ground in a mortar. The powder was extracted with methanol in a Soxhlet apparatus for 24 h, followed by PHA extraction with chloroform for 24 h. The PHA-containing chloroform was concentrated using a Rotovapor (Büch, Flawil, Switzerland) and filtered over glass wool. PHA was precipitated by the addition of 10 volumes of cold methanol and subsequently washed by two cycles of chloroform solubilization and methanol precipitation. PHA was transesterified by mixing 1 volume of PHA dissolved in chloroform with 1 volume of methanol containing 3% sulfuric acid and reacting the mixture at 100°C for 4 h. Methyl esters of 3-hydroxyacids were extracted by adding 1 volume of an aqueous solution of 0.9% (w/v) NaCl, and the chloroform phase was collected for analysis by GC-MS using a gas chromatograph (model 5890 and model HP-5MS column, Hewlett-Packard) coupled to a mass spectrometer (model 5972, Hewlett-Packard).
In some experiments a simplified procedure was used in which homogenized seeds were extensively extracted with methanol at 65°C, the residual material was trans-esterified by acid methanolysis, and the PHA content was analyzed by GC-MS utilizing the ion-selective mode. Identification of monomers present in plant PHA was facilitated by the use of commercial standards and purified bacterial PHAs. 3-Hydroxyoctanoic acid, 3-hydroxydecanoic acid, 3-hydroxydodecanoic acid, and 3-hydroxytetradecanoic acid were purchased from Sigma (St. Louis), while 3-hydroxyhexanoic acid was obtained from D. Seebach (ETH, Zurich). PHA purified from Pseudomonas putida grown on linoleic acid, the monomer composition of which was determined by GC-MS as well as NMR, was obtained from G. Eggink (ATO-DLO, Wageningen, The Netherlands).
Seed or leaf fatty acid methyl-esters were prepared by heating intact or homogenized plant material at 80°C in a methanol solution containing 1 n HCl for 2 h. The fatty acid methyl-esters were extracted with 0.5 to 1 mL of hexane and 1 mL of 0.9% (w/v) NaCl and the organic phase was transferred to autoinjector vials. GC analysis was performed using a gas chromatograph equipped with a glass capillary column (model SP2330, Supelco, Bellefonte, PA) .
Triacylglycerides and diacylglycerides were separated by thin-layer chromatography (TLC). Seeds (50 mg) were homogenized in liquid nitrogen and lipases were inactivated by adding 1.2 mL of isopropanol and heating at 70°C for 10 min. After centrifugation, the powder was dried under nitrogen and the lipids were extracted in a chloroform/methanol mixture as described by Bligh and Dyer (1959). Lipids were separated on TLC plates (Silica 60, Merck, Darmstadt, Germany) developed with hexane: diethyl ether:formic acid (60:40:2, v/v). The lipids were visualized by staining with iodine vapor, and spots were scraped off the silica plates and trans-esterified in methanolic-HCl as described above.
RESULTS
Seed-Specific Expression of PhaC1 Synthase
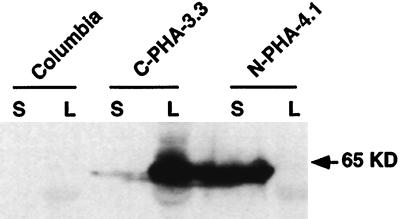
Plants transformed with the N-PHA construct (Fig. 1B) and expressing the PhaC1 gene under the napin promoter were screened by western analysis of 10- to 14-d-old siliques. Line N-PHA-4.1 was selected for further studies because it proved to have a single functional insert and a stable expression of the PhaC1 synthase. Western analysis showed strong accumulation of the PhaC1 protein in siliques, while no protein was detected in leaves (Fig. 2). In comparison, line C-PHA-3.3 transformed with the C-PHA construct (Fig. 1A) and expressing the PhaC1 gene under the CaMV 35S promoter showed only weak expression in siliques but strong expression in leaves (Fig. 2). Although protein extracts were made from whole siliques, it is to be expected that, according to the tissue specificity of the promoters used (Benfey et al., 1990; Höglund et al., 1992), the PhaC1 synthase gene is expressed almost exclusively in the seeds for line N-PHA-4.1, whereas the protein is found in both seeds and silique walls for line C-PHA-3.3.
Figure 2.
Western-blot analysis of PhaC1 synthase expression in siliques and leaves of transgenic plants. Twenty micrograms of total proteins from a rosette leaf (L) or 10- to 14-d-old siliques (S) from wild-type Columbia and transgenic lines C-PHA-3.3 and N-PHA-4.1 were loaded in each lanes.
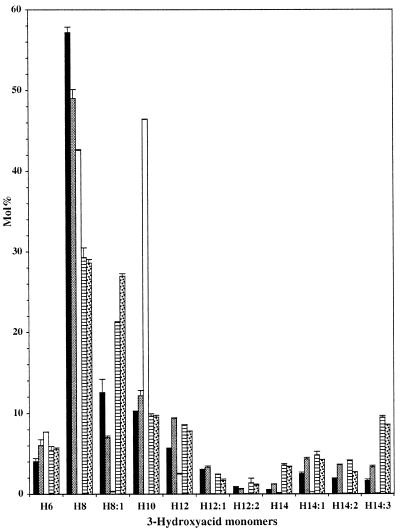
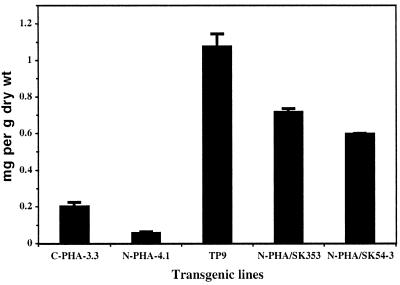
PHA was extracted from dry mature seeds of lines C-PHA-3.3 and N-PHA-4.1 (Fig. 3). Quantification by GC-MS of the saturated and unsaturated 3-hydroxyacid monomers containing 16 carbons was not possible in PHA purified from seeds because of the presence of contaminating products that co-migrate with these monomers. This problem was not encountered when PHA was purified from leaves or seedlings growing in liquid cultures. Thus, although 16-carbon monomers are most likely present in PHA from seeds, the amount of PHA was calculated only for 3-hydroxyacid monomers comprising between six and 14 carbons. On this basis, seeds from line N-PHA-4.1 accumulated 0.06 mg PHA g−1, while seeds from line C-PHA-3.3 accumulated 0.2 mg g−1 (Fig. 4). The monomer composition of the PHA produced in the two lines were significantly different, with N-PHA-4.1 generally containing a higher proportion of the 14-carbon monomers compared with C-PHA-3.3 (Fig. 3).
Figure 3.
Monomer composition of PHA accumulating in mature seeds of transgenic Arabidopsis. Transgenic lines C-PHA-3.3 and N-PHA-4.1 express the PHA synthase under the control of the CaMV 35S or napin promoters, respectively. The transgenic line TP9 expresses both the PHA synthase and the FatB3 thioesterase gene under the control of the napin promoter. Lines N-PHA/SK353 and N-PHA/SK54-3 are homozygous plants derived from a cross between transgenic line N-PHA-4.1 and the mutants SK353 and SK54-3, respectively. 3-Hydroxyacyl-CoA monomers are denoted by the prefix H. The monomer H10:1, present in trace amounts, is not shown. Values are ±sd. Black bars, C-PHA-3.3; shaded bars, N-PHA-4.1; white bars, TP9; striped bars, N-PHA/SK353; stippled bars, N-PHA/SK54–3.
Figure 4.
Quantity of PHA accumulating in mature seeds of transgenic plants. Values are ±sd.
Expression of the C. lanceolata FatB3 Thioesterase in Seeds
To determine whether expression of a medium-chain-length acyl-ACP thioesterase in developing seeds leads to an increased flow of fatty acids toward β-oxidation, the C. lanceolata FatB3 gene was expressed in Arabidopsis under the napin promoter. The FatB3 thioesterase has been shown to have minimal activity for 8:0-ACP, maximal activity for 10:0-ACP, and substantial activity (equivalent to 30% of the activity for 10:0-ACP) for 12:0-ACP, 14:0-ACP, and 16:0-ACP (Martini et al., 1999).
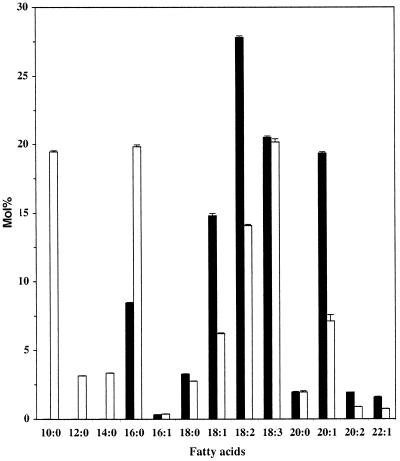
Plants transformed with the FatB3 gene were initially screened by analysis of fatty acid composition in seed lipids. Among 22 transgenic plants tested, line N-FatB3-4 was chosen for further analyses because it had a single functional insert and a high content of capric acid (19 mol %) in seed lipids. In addition to capric acid, seed lipids contained low amounts of lauric and myristic acids (3.1 and 3.3 mol %, respectively) and a greater than 2-fold increase in palmitic acid (Fig. 5). Expression of the PhaC1 synthase gene in developing seeds of line N-PHA-4.1 had no significant effects on the fatty acid composition of seed lipids (data not shown).
Figure 5.
Fatty acid composition of total lipids in mature seeds of wild-type Columbia (black bars) and of transgenic plants expressing the FatB3 thioesterase gene under the napin promoter (N-FatB3-4) (white bars). Values are ±sd.
Line N-FatB3-4 was crossed to line N-PHA-4.1, and a double homozygote plant called TP9 was selected. The fatty acid profile in total seed lipids was not different between TP9 and the parental line, N-FatB3-4 (data not shown). The amount of PHA accumulating in mature seeds of line TP9 was 18-fold higher than in the parental line N-PHA-4.1 (Fig. 4). Furthermore, the PHA monomer composition showed a 4-fold increase in the proportion of the 3-hydroxydecanoic acid (H10) monomer present in the polymer (Fig. 3). The proportion of all unsaturated monomers decreased strongly. In comparison, there was a more modest decrease in the proportion of other saturated monomers, including 3-hydroxytetradecanoic acid (H14) and 3-hydroxydodecanoic acid (H12), while 3-hydroxyoctanoic acid (H8) and 3-hydroxyhexanoic acid (H6) showed little change. Both the large increase in PHA synthesis and the high proportion of H10 monomer in the polymer indicated that expression of the FatB3 thioesterase in developing seeds leads to an increase in the flow of fatty acids toward β-oxidation, and that capric acid is the predominant fatty acid being degraded.
Flow of Fatty Acids toward β-Oxidation in Diacylglycerol Acyltransferase-Deficient Mutants
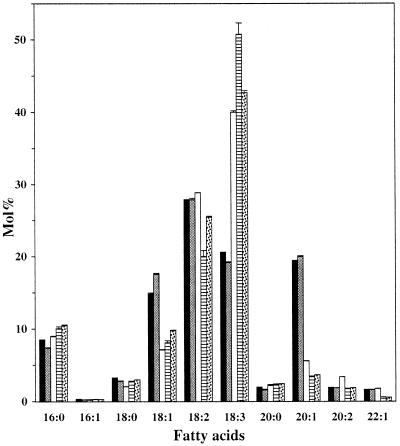
An Arabidopsis mutant showing a reduced sn-1,2-diacylglycerol acyltransferase (DAGAT) activity has been previously described (Katavic et al., 1995). In addition to a reduction in DAGAT activity, the original mutant, AS11, showed a reduction in total seed lipid and in the ratio of TAG to diacylglycerides (DAG) in developing and mature seeds. Furthermore, compared with the wild type, seed lipids of AS11 have a reduced proportion of 18:1 and 20:1 fatty acids and an increased proportion of 18:3 fatty acids. These pleiotropic effects have been assigned to a mutation at a single locus named TAG1. To study the flow of fatty acids toward β-oxidation in developing seeds of tag1 mutants, we have used two new independent mutant isolates, SK353 (Columbia ecotype) and SK54-3 (RLD ecotype), which were shown through complementation experiments to be new alleles of tag1 (L. Kunst, unpublished data). Fatty acid analysis indicated that, like AS11, SK353 and SK54-3 have an increased proportion of 18:3 and a decreased proportion of 18:1 and 20:1 fatty acids in total seed lipids compared with wild-type Arabidopsis seeds (Fig. 6). Furthermore, SK54-3 and SK353 also showed a reduced level of 22:1 in seeds lipids, which was not observed in AS11.
Figure 6.
Fatty acid composition of total lipids in mature seeds of wild-type Columbia (black bars) and RLD (shaded bars) seeds, as well as in the AS11 (white bars), SK54-3 (striped bars), and SK353 (stippled bars) mutants. Values are ±sd.
The total lipid content in mature seeds of these mutants was reduced to 58% to 66% of the amount in wild type (Columbia 230 μg/mg dry weight; SK54-3, 130 μg/mg dry weight; SK353, 150 μg/mg dry weight), while Katavic et al. (1995) reported that lipid levels in AS11 reached 75% of wild type. The amount of fatty acids present in the DAG pool was highly elevated in mature seeds of AS11, SK353, and SK54-3, representing 4% to 6% of the total seed fatty acids, whereas it was 0.3% to 0.4% in wild-type seeds. No significant differences in the fatty acid composition were found in leaf lipids of the two mutants compared with wild type (data not shown). These data indicate that the overall phenotypes of the three tag1 mutants AS11, SK54-3, and SK353 are similar.
The transgenic plant N-PHA-4.1 was crossed to both SK353 and SK54-3, and plants homozygous for both PhaC1 synthase and tag1 were selected. The total amount of PHA accumulating in mature seeds of N-PHA/SK353 and N-PHA/SK54-3 double homozygote plants was approximately 10-fold higher than in the N-PHA-4.1 parent (Fig. 4). Furthermore, significant differences were found in the monomer composition of the PHA in the double mutants, with a 3-fold increase in the proportion of 3- hydroxytetradecatrienoic acid (H14:3), 3-hydroxytetradecanoic acid (H14:0), and 3-hydroxyoctenoic acid (H8:1) (Fig. 3). There was also a small reduction in the proportion of the monomer 3-hydroxydodecenoic acid (H12:1) and a small increase in the proportion of the monomer 3-hydroxydodecadienoic acid (H12:2).
DISCUSSION
PHA accumulating in plants expressing the P. aeruginosa PhaC1 synthase in the peroxisomes is synthesized from the 3-hydroxyacyl-CoA intermediates of the β-oxidation of fatty acids (Mittendorf et al., 1998a, 1998b). It has been previously shown that the amount of PHA synthesized in plants is linked to the activity of the β-oxidation cycle, being higher during germination and senescence and lower during photosynthetic growth of the plant (Mittendorf et al., 1998a, 1998b). Only a small proportion of the 3-hydroxyacyl-CoAs intermediates generated by the peroxisomal β-oxidation pathway is incorporated into PHA. One potential reason could be the poor availability of the intermediates to the PHA synthase because of substrate channeling between the β-oxidation enzymes. Alternatively, since the normal β-oxidation intermediate is the S-isomer of 3-hydroxyacyl-CoA, and the PHA synthase only uses the R-isomer, a low rate of epimerization of 3-hydroxyacyl-CoAs may restrict PHA synthesis (Mittendorf et al., 1998b).
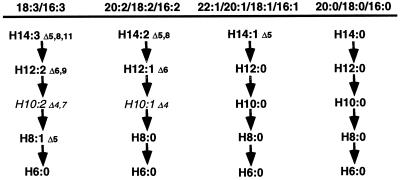
Despite this limitation, we have recently shown that the amount of PHA synthesized in plants growing in liquid media can be increased by approximately 10-fold by adding fatty acids in the form of Tween conjugates (Mittendorf et al., 1999). Thus, the amount of PHA synthesized in peroxisomes can be directly influenced by the flow of fatty acids toward β-oxidation. These feeding experiments have also shown that the monomer composition of the PHA can be used as an indicator of which fatty acids are predominantly targeted for β-oxidation. This is because β-oxidation of fatty acids generates a number of intermediates, many of which are unique to a particular group of fatty acids (Fig. 7). For example, degradation of 18:1 or 16:1 fatty acids generates six different 3-hydroxyacyl-CoA intermediates that can be included into PHA: 3-hydroxyhexadecenoic acid (H16:1), 3-hydroxytetradecenoic acid (H14:1), 3-hydroxydodecanoic acid (H12:0), 3-hydroxydecanoic acid (H10:0), 3-hydroxyoctanoic acid (H8:0), and 3-hydroxyhexanoic acid (H6:0). Of those monomers, only two are unique to the degradation of 18:1 and 16:1 fatty acids, H16:1 and H14:1; the other monomers are also generated by the degradation of saturated fatty acids (Fig. 7). Thus, not only the quantity of PHA synthesized can be used as an indicator of the relative amount of flow of fatty acids toward β-oxidation, but relative changes in the monomer composition of the PHA can also give an indication of the nature of the flow, i.e. what kind of fatty acids are targeted toward β-oxidation. For example, feeding PHA-producing plants with Tween-oleate results in a substantial increase in the proportion of both H16:1 and H14:1 monomers (Mittendorf et al., 1999). Although there are no studies reporting the affinity of the P. aeruginosa PHA synthase for the different 3-hydroxyacyl-CoAs, PHA synthases from pseudomonads have a very broad substrate specificity: they are able to incorporate nearly 100 different 3-hydroxyacids into PHA (Steinbuchel and Valentin, 1995). Thus, although the P. aeruginosa PHA synthase may have different affinities for various 3-hydroxyacyl-CoAs generated by the β-oxidation of plant fatty acids, these differences are likely to be relatively small.
Figure 7.
Spectrum of 3-hydroxyacyl-CoAs generated by the degradation of the four major groups of plant fatty acids through the β-oxidation cycle. All the potential 3-hydroxyacid moieties ranging from six to 14 carbons are incorporated into plant peroxisomal PHAs, except for H10:2 (undetectable) and H10:1 (trace amount) (indicated in small italic letters). It is hypothesized that the PhaC1 synthase cannot use 3-hydroxyacyl-CoAs having a double bond at the Δ4 position.
The amount of PHA accumulating in seeds of N-PHA-4.1 (0.06 mg/g) was approximately 3-fold less than for line C-PHA-3.3 (0.2 mg/g), despite the fact that the use of the napin promoter leads to a much higher level of PhaC1 expression during the stage of maximal lipid synthesis compared with the CaMV 35S promoter (Fig. 2). This indicates that a substantial amount of fatty acids are being degraded through β-oxidation either in tissues or at stages of seed development where the napin promoter is poorly active or not active compared with CaMV 35S (Benfey et al., 1990; Höglund et al., 1992).
Analysis of the PHA monomer composition reveals an approximately 2-fold increase in the proportion of all 14-carbon monomers and H12, as well as a 2-fold decrease in the proportion of the H8:1 monomer in PHA from seeds of line N-PHA-4.1 compared with seeds from line C-PHA-3.3 (Fig. 3). The changes in monomer composition cannot be simply explained by a change in the flow of a particular fatty acid toward β-oxidation. For example, while the proportion of H8:1 is decreased, the proportion of H14:3, which is also derived from the degradation of tri-unsaturated fatty acids, is increased (Figs. 3 and 7). Interpretation of the significance of these changes is particularly difficult since the two promoters used (CaMV 35S versus napin) have quite distinct tissue specificity and strength, and that PHA monomer composition may be different in various tissues within the seed (e.g. endosperm versus cotyledon). Nevertheless, the global increase in the proportion of all 14-carbon monomers in PHA from line N-PHA-4.1 suggests that the availability of long-chain 3-hydroxyacyl-CoAs to the PHA synthase is enhanced, perhaps due to the higher expression of the PhaC1 synthase in the seeds of this line (Fig. 2).
Plants expressing the C. lanceolata FatB3 thioesterase under the napin promoter accumulated in their seed lipids capric acid (19 mol %), lauric acid (3.1 mol %), and myristic acids (3.4 mol %), and showed a 2-fold increase in palmitic acid (Fig. 5). Similar fatty acid profiles have been obtained in transgenic B. napus expressing the FatB3 gene under the control of the napin promoter and are in agreement with the acyl-ACP thioesterase activity measured in vitro (Martini et al., 1999).
Co-expression of the FatB3 thioesterase with the peroxisomal PHA synthase in line TP9 led to a 18-fold increase in the amount of PHA accumulating in mature seeds compared with the parental line N-PHA-4.1 expressing only the PHA synthase (Fig. 4). Furthermore, PHA in seeds of line TP9 showed a large decrease in the proportion of all unsaturated monomers, as well a smaller decrease in saturated monomer with 12 and 14 carbons (Fig. 3). These changes in monomer composition are almost totally compensated for by an increase in the H10 monomer, going from 12 mol % in N-PHA-4.1 to 46 mol % in the double-transgenic TP9. These data can be explained by an increased flow of fatty acids toward β-oxidation in thioesterase-expressing plants, with capric acid representing a high proportion of these degraded fatty acids.
These results are in agreement with the work of Eccleston and Ohlrogge (1998), which showed that in transgenic B. napus lines expressing a high level of lauroyl-ACP thioesterase in developing seeds, a substantial portion of the fatty acid produced in these seeds is recycled to acetyl-CoA and Suc through the β-oxidation and glyoxylate pathways. This present work extends these findings and shows that it is the medium-chain-length fatty acids that contribute largely to the flow toward β-oxidation. In B. napus expressing the lauroyl-ACP thioesterase in developing seeds, increased β-oxidation was observed only for lines accumulating approximately 60 mol % laurate in seed lipids. Conversely, a line accumulating 40 mol % laurate showed no induction of the β-oxidation enzyme, although the rate of incorporation of 14C-acetate into Suc was not tested for this line (Eccleston and Ohlrogge, 1998). Despite the relatively low level of medium-chain-length fatty acids found in seed lipids of plants expressing the C. lanceolata FatB3 thioesterase (19 mol % capric acid), there is still a substantial increase in the flow of fatty acids toward β-oxidation. Thus, there is a significant amount of fatty acid futile cycling even in plants accumulating a relatively low amount of medium-chain-length fatty acids.
In developing seeds of plants expressing a thioesterase, degradation of fatty acids is thought to be due, at least in part, to the inability of the plant acyltransferases to efficiently incorporate medium-chain-length fatty acids into TAG, leading to an increased pool of either free or CoA-esterified medium-chain-length fatty acids. Free fatty acids are toxic to plant cells, and it is likely that incorporation of medium-chain-length fatty acids into membrane lipids would disrupt membrane function. In this context, we were interested in examining whether a reduction in the incorporation of the usual long-chain fatty acids (as opposed to the unusual medium-chain-length fatty acids) into TAG would also lead to an increased flow of fatty acids toward β-oxidation. We have thus examined the accumulation of PHA in developing seeds of the tag1 mutant.
The Arabidopsis tag1 mutant shows a decrease in DAGAT activity, as well as a decrease in the TAG to DAG ratio in developing and mature seeds (Katavic et al., 1995). In addition, the fatty acid composition of total seed lipids showed an increase in 18:3 and a decrease in 18:1 and 20:1. These changes could be explained through a reduction in DAGAT activity leading to accumulation of 20:1-CoA, resulting in feedback inhibition of 18:1 elongation and making more 18:1 available for desaturation to 18:3. Since an Arabidopsis gene homologous to the mouse DAGAT gene (Cases et al., 1998) maps to the same area as TAG1 on chromosome two, near marker mi139, it is likely that TAG1 encodes the DAGAT gene.
Expression of the peroxisomal PhaC1 synthase in the two mutants SK353 and SK54-3, representing novel alleles of tag1, led to a 10-fold increase in the amount of PHA accumulating in mature seeds (Fig. 4). Furthermore, the composition of the PHA produced in the tag1 background showed an increase in the proportion of monomers derived from the degradation of 18:3, namely H14:3, H12:2, and H8:1. These changes in PHA composition can be explained by the increased proportion of 18:3 going toward β-oxidation (Fig. 7), which in turn reflects the principal fatty acid that increases in mature seeds of tag1 mutants (Fig. 6) (Katavic et al., 1995). Surprisingly, there is also an 3-fold increase in the proportion of H14:0 monomer in the PHA produced in tag1. Although this monomer is uniquely derived from saturated fatty acids, the total amount of saturated fatty acids in SK353 and SK54-3 does not deviate by more then 1.3-fold from the wild type (Fig. 6). This indicates that the flow of saturated fatty acids toward β-oxidation during seed development may be higher then could be deduced from the fatty acid composition of mature seeds.
This work demonstrates that in developing seeds of plants showing a reduction in the incorporation of fatty acids into TAG, either due to a decrease of DAGAT activity or because of the synthesis of unusual fatty acids, re-cycling of fatty acids toward β-oxidation is enhanced. Considering that the amount of PHA accumulating in 7-d-old germinating seedlings, representing a time when the degradation of fatty acids and the β-oxidation cycle are high, is 4 mg g−1 dry weight (Mittendorf et al., 1998b), accumulation 0.6 to 1.1 mg PHA g−1 dry weight during the development of seeds deficient in DAGAT activity or expressing a medium-chain-length thioesterase represents a substantial flow of fatty acids toward β-oxidation for a tissue that is normally devoted to the synthesis of lipids.
B. napus expressing a high level of lauroyl-ACP thioesterase in developing seeds was shown to compensate the loss of fatty acids toward β-oxidation by an increase in the expression of several proteins involved in fatty acid biosynthesis (Eccleston and Ohlrogge, 1998). Furthermore, Shintani and Ohlrogge (1995) have shown that addition of fatty acids (in the form of Tween conjugate) to the medium of a tobacco suspension cell culture resulted in the feedback inhibition of fatty acid biosynthesis through the biochemical or posttranslational modification of the acetyl-CoA carboxylase. These studies, in combination with the present work demonstrating an increased flow of fatty acids toward β-oxidation in developing seeds either producing unusual medium-chain-length fatty acids or deficient in DAGAT activity, indicate that plant cells have mechanisms that sense levels of free or esterified fatty acids and respond through the activation or repression of the fatty acid biosynthetic and degradation pathways. Furthermore, the present study shows that synthesis of PHA in plant peroxisomes can be used as a powerful tool to study the genetic and metabolic factors affecting both the quantity and quality of the fatty acid flow toward peroxisomal β-oxidation in various tissues.
ACKNOWLEDGMENTS
The authors thank Ljerka Kunst (University of British Columbia) for providing the seeds for the mutants SK353 and SK54-3. We are also grateful to Stéphanie Stolz for her skilled technical assistance and to Christiane Nawrath for critical reading of the manuscript.
NOTE ADDED IN PROOF
The TAG1 gene from Arabidopsis has recently been cloned and was shown to encode a diacylglycerol acyltransferase (J. Zou, Y. Wei, C. Jako, A. Kumar, G. Selvaraj, D.C. Taylor [1999] Plant J 19: 645–653; D.H. Hobbs, C. Lu, M.J. Hills [1999] FEBS Lett 452: 145–149).
Footnotes
This work was supported in part by a grant from the Herbette Foundation. D.C. was a recipient of a fellowship from the Firmenich Foundation (no. 82FI–053519).
LITERATURE CITED
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- Benfy PN, Ren L, Chua NH. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO. 1990;9:1677–1684. doi: 10.1002/j.1460-2075.1990.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem. 1959;37:911–915. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Brouquisse R, Pradet A, Raymond P. Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol. 1992;99:595–600. doi: 10.1104/pp.99.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston VS, Cranmer AM, Voelker TA, Ohlrogge JB. Medium-chain fatty acid biosynthesis and utilization in Brassica napus plants expressing lauroyl-acyl carrier protein thioesterase. Planta. 1996;198:46–53. [Google Scholar]
- Eccleston VS, Ohlrogge JB. Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell. 1998;10:613–621. doi: 10.1105/tpc.10.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart B. Catabolism of fatty acids (α- and β-oxidation) In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 527–565. [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund AS, Rödin J, Larsson E, Rask L. Distribution of napin and cruciferin in developing rape seed embryos. Plant Physiol. 1992;98:509–515. doi: 10.1104/pp.98.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MA, Fleming Y, Larson TR, Graham IA. No induction of β-oxidation in leaves of Arabidopsis that over-produce lauric acid. Planta. 1999;207:385–392. doi: 10.1007/s004250050496. [DOI] [PubMed] [Google Scholar]
- Ismail I, Debellis L, Alpi A, Smith SM. Expression of glyoxylate cycle genes in cucumber roots responds to sugar supply and can be activated by shading or defoliation of the shoot. Plant Mol Biol. 1997;35:633–640. doi: 10.1023/a:1005840522049. [DOI] [PubMed] [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, MacKenzie SL, Covello PS, Kunst L. Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. 1995;108:399–409. doi: 10.1104/pp.108.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls of tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chae HS, Lee TK, Kim SH, Shin SH, Cho BH, Cho SH, Kang BG, Lee WS. Ethylene-mediated phospholipid catabolic pathway in glucose-starved carrot suspension cells. Plant Physiol. 1998;116:223–229. [Google Scholar]
- Martini N, Schell J, Abbadi A, Spener F, Töpfer R. Rapsöl mit mittelkettigen Fettsäuren. Voträge für Pflanzenzüchtung. 1999;45:133–154. [Google Scholar]
- Mittendorf V, Bongcam V, Allenbach L, Coullerez G, Martini N, Poirier Y. Polyhydroxyalkanoate synthesis in transgenic plants as a new tool to study carbon flow through β-oxidation. Plant J. 1999;20:45–55. doi: 10.1046/j.1365-313x.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Mittendorf V, Krüger N, Steinbüchel A, Poirier Y. Biosynthesis of medium-chain-length polyhydroxyalkanoates in transgenic Arabidopsis plants expressing the PhaC1 and PhaC2 synthases from Pseudomonas aeruginosa. In: Steinbüchel A, editor. The Biochemical Principles and Mechanisms of Biosynthesis and Biodegradation of Polymers. Weinheim, Germany: Wiley-VCH; 1998a. pp. 368–375. [Google Scholar]
- Mittendorf V, Robertson EJ, Leech RM, Krüger N, Steinbüchel A, Poirier Y. Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid β-oxidation. Proc Natl Acad Sci USA. 1998b;95:13397–13402. doi: 10.1073/pnas.95.23.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistelli L, Nieri B, Smith SM, Alpi A, DeBellis L. Glyoxylate cycle enzyme activities are induced in senescent pumpkin fruits. Plant Sci. 1996;119:23–29. [Google Scholar]
- Poirier Y. Production of new polymeric compounds in plants. Curr Opin Biotechnol. 1999;10:181–185. doi: 10.1016/s0958-1669(99)80032-9. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Dennis DE, Klomparens K, Somerville C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992;256:520–523. doi: 10.1126/science.256.5056.520. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio-Technology. 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- Shintani DK, Ohlrogge JB. Feedback inhibition of fatty acid synthesis in tobacco suspension cells. Plant J. 1995;7:577–587. [Google Scholar]
- Stålberg K, Ellerström M, Josefsson LG, Rask L. Deletion analysis of a 2S seed storage protein promoter of Brassica napus in transgenic tobacco. Plant Mol Biol. 1993;23:671–683. doi: 10.1007/BF00021523. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Füchtenbusch B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998;16:419–427. doi: 10.1016/s0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Valentin HE. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 1995;128:219–228. [Google Scholar]