Abstract
Copper tolerance among Arabidopsis ecotypes is inversely correlated with long-term K+ leakage and positively correlated with short-term K+ leakage (A. Murphy, L. Taiz [1997] New Phytol 136: 211–222). To probe the mechanism of the early phase of K+ efflux, we tested various channel blockers on copper and peroxide-induced K+ efflux from seedling roots. The K+ channel blockers tetraethyl ammonium chloride and 4-aminopyridine (4-AP) both inhibited short-term copper-induced K+ efflux. In contrast, peroxide-induced K+ efflux was insensitive to both tetraethyl ammonium chloride and 4-AP. Copper-induced lipid peroxidation exhibited a lag time of 4 h, while peroxide-induced lipid peroxidation began immediately. These results suggest that short-term copper-induced K+ efflux is mediated by channels, while peroxide-induced K+ efflux represents leakage through nonspecific lesions in the lipid bilayer. Tracer studies with 86Rb+ confirmed that copper promotes K+ efflux rather than inhibiting K+ uptake. Short-term K+ release is electroneutral, since electrophysiological measurements indicated that copper does not cause membrane depolarization. Short-term K+ efflux was accompanied by citrate release, and copper increased total citrate levels. Since citrate efflux was blocked by 4-AP, K+ appears to serve as a counterion during copper-induced citrate efflux. As copper but not aluminum selectively induces citrate production and release, it is proposed that copper may inhibit a cytosolic form of aconitase.
Copper, an abundant transition metal in soils, is an essential micronutrient for all living organisms. In plants, copper plays a vital role in both photosynthetic and respiratory electron transport, and functions as a cofactor for a variety of enzymes. The redox-active nature of the copper ion is crucial to its function in rapid cycling plastidic electron carriers such as plastocyanin and high-energy enzyme catalysts such as ascorbate oxidase and copper amine oxidases (Owen, 1982).
While the redox activity of copper makes it an ideal cofactor in cellular energy transfer reactions, it also confers the potential to cause oxidative damage when present in excess. Intracellular free copper ions can react with water to produce free radical hydroxyls, which in turn react to cause membrane lipid peroxidation (De Vos et al., 1989, 1991, 1993; Luna et al., 1994), cleavage of the sugar phosphate backbone of nucleic acids (Chubatsu and Meneghini, 1993), and protein denaturation resulting from the formation of disulfide linkages between Cys residues (Stohs and Bagchi, 1995). In addition, copper can displace other divalent cations coordinated with macromolecules, causing their inactivation or malfunction (Watkins and Ferguson, 1982; Lidon and Henriques, 1993). Copper also inhibits other cellular proteins, such as cell wall expansins (McQueen-Mason and Cosgrove, 1995).
One of the earliest physiological responses to excess copper is K+ efflux from the roots. Rapid K+ efflux has been widely interpreted as a symptom of toxicity resulting from copper-induced oxidative damage to the plasma membrane (De Vos et al., 1989, 1991; Strange and Macnair, 1991; Murphy and Taiz, 1997). In support of this hypothesis, long-term K+-efflux measurements in Silene cucubalis (De Vos et al., 1989, 1993), Mimulus guttatus (Strange and Macnair, 1991), and Arabidopsis (Murphy and Taiz, 1997) have shown that copper tolerance is inversely correlated with long-term K+ efflux. Surprisingly, short-term K+ efflux was positively correlated with copper tolerance in Arabidopsis seedlings (Murphy and Taiz, 1997). This finding raises the possibility that short- and long-term K+ efflux occur via different mechanisms. Rather than being a symptom of toxicity, short-term K+ efflux may actually be associated with a copper detoxification mechanism.
In principle, K+ efflux could occur either by diffusion across the lipid bilayer or through channels. Damage to membrane lipids resulting from peroxidation reactions has been documented in a number of plant species, most notably in Silene vulgaris (De Vos et al., 1989, 1993). Such oxidative damage might disrupt membranes, resulting in nonspecific K+ leakage out of the cell. Recent electrophysiological studies with the giant algal internode cells of Nitella flexilis have indicated that copper has four effects on the plasma membrane: (a) it increases the nonselective conductance; (b) it inhibits Cl− channels; (c) it inhibits the plasma membrane H+-ATPase; and (d) it promotes lipid peroxidation (Demidchik et al., 1997). Since Ca2+ could prevent the increase in nonspecific conductance, copper appeared to be competing with Ca2+ for binding sites on the membrane.
Based on preliminary evidence, we previously proposed that short-term copper-induced K+ efflux from Arabidopsis seedlings may be mediated by tetraethyl ammonium chloride (TEA)-sensitive K+ channels rather than by lipid peroxidation-induced membrane disruption (Murphy and Taiz, 1997). Here we present a more detailed analysis exploring the relationship between copper-induced K+ efflux, lipid peroxidation, and the effects of K+ channel blockers. The results of experiments testing the effects of various channel blockers and inhibitors on copper-treated Arabidopsis seedlings are consistent with K+ efflux via channels. However, K+ appears to be serving as a counterion for increased citrate release during the first 4 h of copper treatment. Citrate release has recently been implicated in aluminum tolerance in maize (Jorge and Arruda, 1997) and transgenic tobacco (de la Fuente et al., 1997). Thus, in Arabidopsis, citrate release may serve as a homeostatic response to excess copper in the external medium. This would account for the positive correlation previously observed between copper tolerance and short-term K+ efflux (Murphy and Taiz, 1997). A model is presented in which copper inhibits the cytosolic form of aconitase, thus stimulating citrate accumulation in root cells and subsequent citrate efflux.
MATERIALS AND METHODS
Plant Material and Reagents
Arabidopsis Wassilewskija (Ws-0) ecotype seeds were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). All reagents and growth media utilized were from Sigma (St. Louis), with the exception of 86RbCl, which was obtained from Amersham Pharmacia (Piscataway, NJ).
Growth Conditions
As it is not possible to monitor K+ efflux from seedlings grown on the vertical mesh transfer system used in previous studies (Murphy and Taiz, 1997), a different growth system was employed. Approximately 3,000 (60 mg) Arabidopsis seeds were placed in a 9-cm Petri dish on a polyester cloth mesh (0.5- × 0.5-mm mesh opening) spread over a ¼-strength Murashige and Skoog basal salt mixture (¼-MS) incorporated into 0.1% (w/v) Phytagel. Seeds were germinated under continuous fluorescent lighting (photon flux density = 130 μE s−2 m−2) at 20°C. After 10 d, the mesh with the seedlings was transferred to a Petri dish with 8 mL of ¼-MS pre-treatment solution. After 2 h, the mesh with seedlings was transferred to a new Petri dish with 10 mL of ¼-MS control, or the treatment specified made up in ¼-MS. Preliminary experiments indicated that this system yielded reproducible results for the first 6 h. However, assays could be reliably extended to 12 h, as shown in Figures 1 and 2, if conducted in an environmental chamber maintained at 95% relative humidity, if the growth medium was gently aerated, and if a starting volume of 20 mL was utilized.
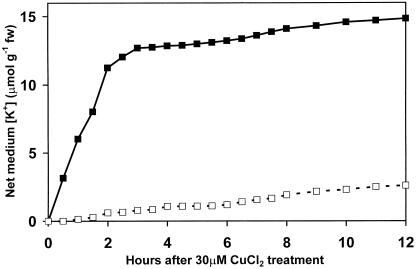
Figure 1.
Time course (12 h) of change in K+ content of growth medium bathing Arabidopsis seedling roots. ▪, Plus 30 μm CuCl2; □, 30 μm CuCl2 plus 5 mm TEA.
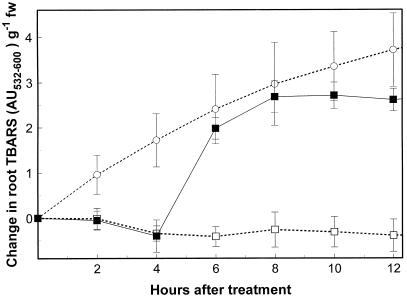
Figure 2.
Time course (12 h) of lipid peroxidation in Arabidopsis seedling roots in the presence of copper. □, Control (¼-MS); ○, ¼-MS plus 0.3 μm H2O2; ▪, ¼-MS plus 30 μm CuCl2.
K+ Efflux Measurements
After a brief equilibration and mixing, two 100-μL aliquots were removed from each dish and retained for K+ determination. Subsequently, two 100-μL aliquots were collected from each dish at the time points indicated. At the conclusion of the experiment, seedling roots were excised, blotted dry on paper towels for 2 min, weighed, dried in a 30°C drying oven, and weighed again. The K+ concentration of the aliquots was determined by flame photometry. Based on preliminary experiments with the system and on previously published results (Murphy and Taiz, 1997), samples from the inhibitor assays were collected at 0 and 4 h. The 4-h inhibitor experiments were repeated three times on different days. The results are expressed as means ± sd. The inhibitor concentrations used in the assays shown were chosen after empirically determining the lowest concentration that yielded the highest degree of inhibition of net K+ efflux in the presence of copper while having the least effect on K+ efflux in control seedlings.
Lipid Peroxidation Assays
Lipid peroxidation, as indicated by the formation of thiobarbituric acid reactive species (TBARS), was assayed using a method derived from those described by De Vos et al. (1989) and Cherif et al. (1996). Arabidopsis seedlings were grown in Petri dishes with control and copper treatments as described above. At 2-h time points, two Petri dishes of seedlings for each treatment were briefly rinsed in ice-cold distilled water, and then the roots were excised, blotted dry on paper towels for 2 min, and weighed. Root tissue (50 mg) from each set of plates was immediately placed in a hexane-washed 2-mL centrifuge tube and frozen in liquid nitrogen, and then 125 μL of a buffer consisting of 50 mm Tris-2-(N-morpholino)-ethanesulfonic acid (MES) (pH 7.1), 2% (w/v) SDS, and 2 μL of 1% (w/v) butylated hydroxytoluene in ethanol were added.
After brief homogenization of the seedlings with a Teflon homogenizer, 700 μL of 0.8% (w/v) thiobarbituric acid in 10% (w/v) trichloroacetic acid was added. The entire contents were then vortexed for 1 min and incubated at 95°C for 15 min. The contents were again vortexed for 1 min and incubated again at 95°C for 15 min. Samples were cooled to room temperature and TBARS were extracted with 500 μL of n-butanol. TBARS were determined by reading spectroscopic A532 after nonspecific background absorbance (measured at 600 nm) was subtracted. Two replicate measurements were made at each time point. The experiments were repeated three times on different days and the results reported are the means plus sds.
86Rb Efflux and Uptake Assays
Studies of 86Rb release were performed as in K+ efflux assays above with the exception that seedlings were preincubated in 6 mL of ¼-strength Murishage and Skoog salts plus 20 μm 86RbCl (20 μCi mL −1) for 2 h (K+: 86Rb molar ratio = 250:1), and rinsed three times for 5 min in ¼-strength Murishage and Skoog salts with gentle shaking before the start of the assay. At each time point indicated, 100-μL samples were collected from the medium. At the conclusion of the experiment, roots were excised, blotted dry for 2 min, and weighed. Cherenkov radiation from each sample was counted in a liquid scintillation counter (model LS 5801, Beckman Instruments, Fullerton, CA). The experiment was repeated two times with two replicate samples collected at each time point.
Uptake of 86Rb was assayed as described by Hirsch et al. (1998). The assay utilized 500 10-d-old vertical mesh transfer-grown seedling roots in each sample and was repeated twice. Results represent means and sds of two experiments.
Electrophysiology Experiments
Ten-day-old plants were positioned horizontally on a polycarbonate support in a narrow acrylic chamber. The roots targeted for impalement were held in place with small pieces of closed-cell polyethylene foam positioned approximately 4 mm apart. The foam pieces, along with the polycarbonate support, immobilized the root without causing any damage. The chamber was attached to the stage of an BH-2 microscope (Olympus, Tokyo), which had been mounted on its back. This arrangement gave the root chamber an orientation such that the root was horizontally aligned and such that the electrode was being brought in from above. The chamber had a pressurized inflow system and gravity outflow, which allowed for a smooth exchange of solution and also did not expose the leaves to any of the experimental media. Flow rates were established to minimize perturbation to the roots, but also to allow for a rapid exchange of the experimental root chamber.
Electrodes (tip diameter of approximately 0.5 μm) were made from single-barreled borosilicate tubing (1-mm o.d./0.58-mm i.d., World Precision Instruments, Sarasota, FL) on a Sutter Instruments P-87 micropipette puller (Sutter Instruments, Novato, CA). Electrodes were filled with 3 m KCl (adjusted to pH 2 to reduce tip potentials), inserted into a microelectrode holder containing an Ag/AgCl electrochemical half-cell (World Precision Instruments), and then attached to the headstage of a World Precision Instrument KS-750 amplifier. The headstage was attached to a Leitz dual electrode holder, which was modified to work with a hydraulically driven micromanipulator (model MO–104, Narishige, Greenvale, NY). To reduce the potential for vibration the entire electrode assembly was positioned on a steel bar between a pair of damped rods (Newport Corp., Irvine, CA) both the microscope and electrode assemblies were placed on a vibration-free table (Micro-G, Woburn, MA). Output from the amplifier was recorded on a strip chart recorder.
In some experiments the plants were allowed to equilibrate in a solution of either 200 μm CaCl2 or 1.5 mm CaCl2, and 10 mm MES, pH 4.85 for several hours (usually 5–6 h). This was done to assess whether solutions with simple ionic composition and lower ionic strengths would affect the initial membrane potential measurement as well as the root's response to the introduction of 30 μm CuCl2. In this case, plants were removed from the gelled ¼-MS, rinsed in the solution of interest to remove any gelled media associated with the roots, and placed into a large covered watch glass in such a way that only the roots were exposed to the particular solution. During this equilibration phase, the solution was exchanged several times to ensure that the solution composition remained static.
Quantitation of Organic Acids
Silylated organic acids (OAs) from root extracts and root exudates were quantitated on an SRI 8610C GC (SRI Instruments, Torrance, CA) using a DB-5 0.25-mm × 30-m capillary column (J&W Scientific, Folsom, CA) according to the method of Adams et al. (1999). FID output was integrated using Peak Simple software from SRI Instruments. Arabidopsis seedlings were grown in Petri dishes as above, but a starting volume of 20 mL ¼-MS plus the treatment noted was utilized. Seedlings were grown and assayed under sterile conditions to control for microbial conversion of organic acids in exudates. For exudation assays, two 1-mL aliquots were collected at each time point from each treatment and immediately frozen in liquid nitrogen until addition to C18 Sep-Pak cartridges (Waters, Deerfield, IL), as described by Adams et al. (1999). After completion of the assay, seedling roots were excised, blotted on paper towels for 2 min, dried, and weighed as above. Exudate concentrations were normalized to net volume after removal of sample aliquots in each case.
For root extracts, roots from approximately 3,000 seedlings were collected at each time point, excised with a razor blade, and washed two times for 5 min in ice-cold 1.5 m CaCl2. The roots were then blotted dry on paper towels for 2 min, frozen in liquid nitrogen, lyophilized to dryness and weighed before preparation, and assay as described by Adams et al. (1999). All assays were repeated three times and results represent means and sds of those experiments.
Molecular masses and relative quantities of peaks identified by comparison to GC standards were verified by ES+ LC-MS using a Quattro II quadrupole MS (Micromass, Beverly, MA). It should be noted that, in our hands, oxalate and pyruvate derivatized poorly, even when samples were dried extensively. Therefore, LC-MS utilizing anthracene as an internal standard was used for the quantitation of these compounds.
Statistical Analysis
The significance of control versus copper-treated assays for each inhibitor was analyzed in pairwise fashion using Student's t test. The comparison of net K+ efflux (30μm CuCl2, control) of inhibitors versus inhibitor controls was evaluated by the Neuman-Keuls ANOVA method. All statistical calculations were made using Sigma Stat (SPSS, Chicago).
RESULTS
Arabidopsis seedlings were grown in Petri dishes as described in the presence or absence of 30 μm CuCl2, and the change in medium K+ concentration, expressed in micromoles per gram fresh weight, was assayed over a 12-h period. Copper treatment caused a rapid efflux of K+ from Arabidopsis seedlings during the first 3 h before leveling off (Fig. 1). This was followed by a slow rate of release beginning at about 6 h of treatment. The rapid copper-induced K+ efflux was completely prevented by the inclusion of the K+ channel blocker TEA (5 mm) in the incubation medium, although TEA did not prevent the slow release of K+ seen at 6 h (Fig. 1).
The effects of a variety of channel blockers and other inhibitors on the rapid phase of copper-induced K+ efflux from the roots of Arabidopsis seedlings at 4 h are summarized in Table I. For each inhibitor tested, the table presents the amount of K+ released into the medium in the presence or absence of 30 μm Cu2+, along with the significance of the differences between the two treatments based on Student's t test. In addition, the determination of significance by ANOVA at P < 0.05 of the net values compared to the inhibitor (30 μm Cu2+, ¼-MS) control is given in the last column. The only compounds that had a significant effect on copper-induced K+ efflux, as evaluated by ANOVA, were the two K+ channel blockers, TEA and 4-aminopyridine (4-AP), which inhibited K+ efflux by about 80% and 70%, respectively. Although both niflumic acid, a general inhibitor of anion channels, and trifluoperizine, a calmodulin antagonist, reduced K+ efflux in copper-treated seedlings by about 40%, due to large sum sds, the inhibition was not statistically significant when analyzed by ANOVA. Neither 3,4,5-trimethoxybenzoic acid 8-(diethylamino)-octyl ester, which blocks intracellular Ca2+ channels, nor the anion channel blockers 4,4′diisothiocyanatostilbene (fast anion channels) or 9- anthracenecarboxylic acid (slow anion channels), interfered with copper-induced K+ release.
Table I.
K+ leakage from Arabidopsis roots at 4 h in response to 30 μm copper with or without the addition of specific inhibitors
| Raw Values Treatment | Inhibitor Target | Control | 30 μm Copper | Significance (30 μm Copper versus Control)
|
Net Value Significant? | |
|---|---|---|---|---|---|---|
| t | p | |||||
| μmol/g dry wt | ||||||
| Inhibitor control | 1 ± 1.6 | 83 ± 11.4 | 12.344 | <.001 | ||
| 5 mm TEA | K+ Channels | 6 ± 4.1 | 17 ± 11.5 | 1.57 | 0.190 | Y |
| 50 μm 4-AP | K+ Channels | 3 ± 5.6 | 24 ± 2.4 | 5.913 | 0.004 | Y |
| 40 μm DIDS | Fast anion | 19 ± 1.6 | 104 ± 4.0 | 34.093 | <.001 | N |
| 10 μm 9-AC | Slow Cl− | 0 ± 7.2 | 74 ± 6.4 | 13.233 | <.001 | N |
| 20 μm Niflumic acid | Anion channels | −5 ± 1.6 | 48 ± 24.0 | 3.802 | 0.019 | N |
| 1 μm Trifluoperazine | Calmodulin | 0 ± 5.6 | 50 ± 4.4 | 12.102 | <.001 | N |
| 50 μm TMB-8 | Intracellular Ca2+ | 34 ± 39.2 | 106 ± 17.6 | 2.902 | 0.044 | N |
SDs values are means ± sd. The significance of pairwise comparisons of control and copper treatments as determined by Student's t test are indicated in the t and p columns. Net change in K+ concentration (30 μm copper–control) for each treatment were used to determine ANOVA values. DIDS, 4,4′ Diisothiocyanatostilbene; TMB-8, 3,4,5-trimethoxybenzoic acid 8-(diethylamino)-octyl ester; 9-AC, 9 anthracenecarboxylic acid.
The inhibition of K+ efflux by K+ channel blockers is consistent with a model in which early copper-induced K+ release is mediated by channels. Alternatively, K+ efflux might be due to membrane damage caused by copper-induced lipid peroxidation. To test the latter hypothesis, the kinetics of copper-induced lipid peroxidation in Arabidopsis seedling roots, as measured by the formation of TBARS, was determined. As shown in Figure 2, copper caused a large increase in lipid peroxidation, but only after a 4-h lag period. In contrast, treatment of seedling roots with 0.3% (v/v) peroxide resulted in a steady increase in TBARS, with no detectable lag period. These results suggest that the rapid efflux of K+ during the first 4 h of copper treatment is not due to copper-induced lipid peroxidation.
Although the early phase of copper-induced K+ leakage appears to be mediated by K+ channels rather than by lipid peroxidation, it is possible that the channels are being regulated by oxidation. If so, short-term peroxide-induced K+ leakage should also be prevented by the same K+ channel blockers that inhibit copper-induced leakage. As shown in Table II, treatment of Arabidopsis seedling roots with 0.3% (v/v) H2O2 caused massive K+ efflux. However, in contrast to the results with copper, none of the inhibitors tested significantly reduced K+ efflux compared with peroxide controls. These results demonstrate that short-term K+ leakage induced by peroxide is insensitive to channel blockers, which is consistent with a lipid peroxidation model.
Table II.
K+ leakage from Arabidopsis roots at 4 h in response to 0.3% peroxide with or without the addition of specific inhibitors
| Raw Values | Control | 0.3% Peroxide | Significance (0.3% Peroxide versus Control)
|
Net Value Significant? | |
|---|---|---|---|---|---|
| t | p | ||||
| μmol/g dry wt | |||||
| Inhibitor control | 0 ± 3.7 | 149 ± 21.6 | 11.775 | <0.001 | |
| 5 mm TEA | 6 ± 6.5 | 113 ± 6.5 | 12.781 | <0.001 | N |
| 50 μm 4-AP | 3 ± 5.6 | 108 ± 16.5 | 10.368 | <0.001 | N |
| 40 μm DIDS | 19 ± 1.6 | 195 ± 7.2 | 41.331 | <0.001 | N |
| 10 μm 9-AC | 0 ± 7.2 | 213 ± 8.8 | 32.417 | <0.001 | N |
| 20 μm Niflumic acid | −5 ± 1.6 | 171 ± 22.4 | 13.574 | <0.001 | N |
| 1 μm Trifluoperazine | −1 ± 5.6 | 146 ± 9.2 | 23.608 | <0.001 | N |
| 50 μm TMB-8 | 34 ± 39.2 | 131 ± 48.0 | 2.728 | 0.053 | N |
Values are means ± sd. The significance of pairwise comparisons of control and copper treatments as determined by Student's t test are indicated in the t and p columns. Net change in K+ concentration (0.3% peroxide–control) for each treatment were compared with the inhibitor control by ANOVA. The significance of that difference (P < 0.05) is indicated in the last column. DIDS, 4,4′ Diisothiocyanatostilbene; TMB-8, 3,4,5-trimethoxybenzoic acid 8-(diethylamino)-octyl ester; 9-AC, 9 anthracenecarboxylic acid.
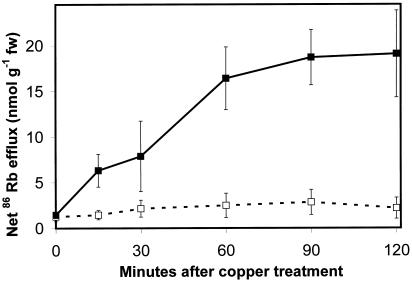
Although the increase in K+ concentration in the medium surrounding copper-treated Arabidopsis roots has been interpreted as an increase in K+ efflux, it could also be due to a decrease in K+ influx. To determine the effect of copper on efflux only, seedlings were preincubated with the K+ analog 86Rb, and then transferred to control or copper-containing media. Aliquots were collected at 20-min intervals for 2 h and assayed for Cherenkov radiation. The results are shown in Figure 3. Control seedlings exhibited a negligible amount of 86Rb efflux, while efflux from copper-treated seedling roots increased to seven times control levels within 2 h of the start of treatment.
Figure 3.
Uptake and efflux of the K+ analog 86 Rb. Net efflux of 86Rb from Arabidopsis seedling roots. □, Control; ▪, plus 30 μm copper.
We also determined the effect of copper on 86Rb uptake from the external medium during a 10-min incubation period. Both high- and low-affinity uptake systems were tested. Copper treatment had no effect on high-affinity K+ uptake in the presence of 0.01 mm 86Rb (9 ± 1.0 nmol g−1 fresh weight h−1 for copper-treated versus 8 ± 0.1 nmol g−1 fresh weight h−1 for controls). At 86Rb concentrations of 0.1 mm, copper inhibited uptake by 30% (49 ± 7.5 versus 72 ± 5.3 nmol g−1 fresh weight h−1), while at 1 mm 86Rb (low affinity uptake system), copper inhibited uptake by 11% (164 ± 21.1 versus 183 ± 2.7 nmol g−1 fresh weight h−1). Since the effect of copper on influx is considerably less than its effect on efflux, we conclude that copper-induced K+ release into the medium is primarily due to a promotion of efflux.
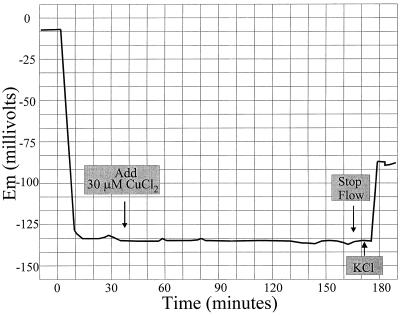
How does copper promote K+ efflux from root cells? One possibility is that it causes membrane depolarization by opening anion channels, by inhibiting the plasma membrane H+-ATPase, or by both mechanisms. To test this directly, the effect of copper on the membrane potential of Arabidopsis root epidermal cells was measured. As shown in Figure 4, no effect of 30 μm CuCl2 on the membrane potential was detected. The addition of 1 mm KCl after 2 h rapidly depolarized the membrane, indicating that the cell was still viable. Reductions of the ionic strength of the external medium by either dilution of the ¼-MS or replacement with 200 μm CaCl2 had little or no effect on the membrane potential (data not shown). Thus, in roots, short-term copper-induced K+ efflux does not appear to be caused by membrane depolarization. Instead, K+ release is electroneutral.
Figure 4.
Sample trace of membrane potential (Em) measurement after copper treatment. At 172 min, one drop of 100 mm KCl was added to the area surrounding the seedling root to bring the external medium to approximately 1 mm KCl.
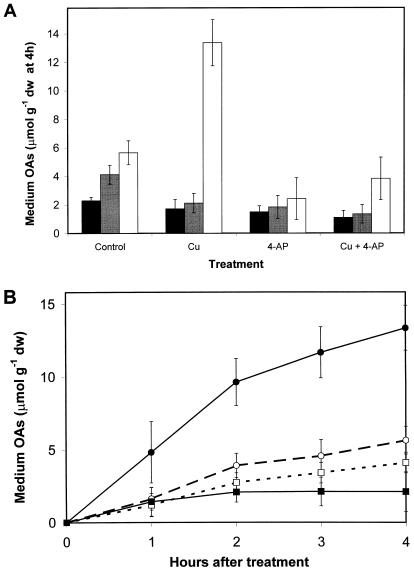
Since electroneutral release of K+ by roots requires either the uptake of a compensating cation or, more likely, the release of a counteranion, organic acid levels in roots and root exudates of control and copper-treated plants were determined during the rapid phase (0–4 h) of K+ efflux. First, the organic acid constituents of root exudates were measured after a 4-h exposure to control medium, 30 μm copper, 50 μm 4-AP, or 30 μm copper plus 50 μm 4-AP. The results are presented in Figure 5A. The release of citrate into the external medium of copper-treated roots increased more than 2-fold over controls (P < 0.001), but was reduced in both control and copper treatments in the presence of the K+ channel inhibitor 4-AP. Copper-induced citrate release in the presence of 4-AP was reduced to approximately 1.5 times control levels, although the plus and minus copper treatments remained statistically different (P < 0.022). Malate and succinate levels in exudates were slightly, but significantly, reduced by copper treatment (P = 0.002, 0.031, respectively), and were not significantly different from 4-AP controls when copper was added. At 4 h, the concentrations of oxalate, pyruvate, and lactate were below the limit of detection by the method used, although pyruvate and lactate levels increased to measurable levels after 5 h (data not shown).
Figure 5.
Organic acid efflux from Arabidopsis seedling roots. A, Levels in external medium at 4 h after treatment with copper, the K+ channel inhibitor 4-AP, or 30 μm copper plus 4-AP. Black bars, Succinate; gray bars, malate; white bars, citrate. B, Time course of citrate and malate efflux with and without copper treatment. □, Malate control; ▪, malate plus 30 μm copper; ○, citrate control; ●, citrate plus 30 μm copper.
The kinetics of citrate and malate efflux were examined for the first 4 h after copper treatment. After transferring seedlings to fresh media with or without copper at the beginning of the assay, the measured levels of citrate and malate in the medium increased in both control and copper-treated seedlings during the first 2 h (Fig. 5B). The controls released low levels of citrate and malate into the medium over the 4-h time period. Copper caused a doubling in the rate of citrate efflux, while malate efflux was slightly reduced.
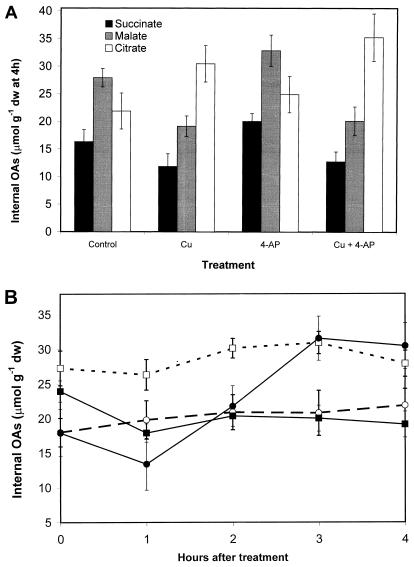
To determine whether the release of organic acids reflects increased production within the roots, the organic acid concentrations of root extracts were also measured. As shown in Figure 6A, at 4 h the malate and succinate concentrations in copper-treated roots decreased by approximately 25% (P < 0.02), while citrate levels increased by approximately 35% (P = 0.017). When treated with the K+ channel blocker 4-AP, however, all three internal organic acid levels increased (P < 0.01), while treatment with copper plus 4-AP resulted in a 35% decrease in malate levels and a 40% increase in citrate levels (P < 0.03).
Figure 6.
Organic acid content of Arabidopsis seedling root extracts. A, Levels at 4 h after treatment with copper, the K+ channel inhibitor 4-AP, or 30 μm copper plus 4-AP. Black bars, Succinate; gray bars, malate; white bars, citrate. B, Time course of citrate and malate efflux with and without copper treatment. □, Malate control; ▪, malate plus 30 μm copper; ○, citrate control; ●, citrate plus 30 μm copper.
When extract citrate and malate levels were examined at 1-h intervals over a 4-h incubation period, citrate levels nearly doubled between the 1st and 3rd h of treatment, while malate levels declined by almost 40% in response to copper (Fig. 6B).
DISCUSSION
The results presented here suggest that the early phase of copper induced K+ efflux is mediated by K+ channels rather than by nonspecific lesions in the membrane caused by lipid peroxidation. Two main findings support this conclusion. First, the early phase of copper-induced K+ leakage precedes copper-induced lipid peroxidation in the roots, as measured by TBARS formation, by 3 to 4 h. Second, the K+ channel blockers TEA and 4-AP both significantly inhibit copper-induced K+ release without interfering with copper-induced lipid peroxidation. Niflumic acid, an inhibitor of organic acid efflux, was also marginally effective in preventing K+ loss from roots, suggesting an indirect effect on K+ release resulting from inhibition of organic acid release (Jones, 1998).
Exogenous peroxide also induced K+ efflux, but this efflux was insensitive to all of the inhibitors tested, indicating that the majority of the K+ efflux induced by peroxide occurs through nonspecific oxidative damage to membrane constituents. Consistent with this observation, H2O2-induced lipid peroxidation exhibited no lag period.
Several properties of short-term copper-induced K+ leakage were determined. First, using 86Rb as a tracer, we confirmed that the increase in K+ in the medium is due to a stimulation of K+ efflux rather than an inhibition of K+ influx by copper. Unlike the copper-induced membrane depolarization reported in Nitella flexilis (Demidchik et al., 1997), in our studies no copper-induced membrane depolarization could be detected during the first 2 h under conditions similar to those used in the efflux studies. This means that short-term K+ efflux is electroneutral under physiological conditions.
Measurements of organic acids indicated that copper induced a 2-fold increase in the release of citrate into the medium, while other organic acids decreased slightly. The kinetics of citrate release was correlated closely with the kinetics of short-term K+ release from Arabidopsis roots. Moreover, the K+ channel blocker 4-AP blocked the release of citrate. The number of moles of citrate released was approximately 17% that of the net K+ efflux measured by flame photometry and approximately 27% of the K+ efflux indicated by radiotracer experiments. Therefore, assuming a valence of −2 to −3, citrate serves as a counterion for 50% to 75% of the K+ released. Presumably, other released anions make up the difference.
The finding that copper induces citrate release from Arabidopsis roots has important implications for studies of copper tolerance. Citrate is an effective chelator of metal ions and citrate release has recently been implicated in aluminum tolerance in maize, tobacco, and papaya (Jorge and Arruda, 1997; de la Fuente et al., 1997). However, in Arabidopsis, total citrate levels decrease in aluminum-treated roots (Larsen et al., 1998). We previously showed that short-term K+ release was positively correlated with decreased ecotypic sensitivity in Arabidopsis, which was difficult to explain if one assumes that K+ release is a sign of membrane degradation. The solution to the apparent paradox appears to be that K+ is exiting through channels rather than nonspecific holes, and is actually serving as a counterion for citrate release. We therefore propose that citrate release can ameliorate the toxicity of excess copper, as has been shown with aluminum in other plant species.
What is the mechanism of copper-induced citrate release? Measurements of internal citrate indicated that copper causes a 40% increase in the total citrate level of the root. Although the release of organic acids, in general, is likely due to the regulation of an organic acid channel, the additional release of citrate in the presence of copper may be the result of an enhancement of intracellular citrate concentration. Recent studies have shown that aconitase (EC 4.2.1.3), an enzyme containing an Fe-S cluster that catalyzes the conversion of citrate to isocitrate, is strongly inhibited by copper (Murakami and Yoshino, 1997). In those studies, treatment of yeast with copper specifically inhibited aconitase activity, causing an accumulation of citrate. In animals and yeast, there are two forms of the enzyme: a mitochondrial version that participates in the Krebs cycle, and a cytosolic form whose physiological function is poorly understood. The cytosolic aconitase of animals serves both as an aconitase and as an iron-responsive element-binding protein (IRE-BP), proteins that have been shown to bind to mRNA and either stimulate or inhibit the translation of proteins involved in iron homeostasis (O'Halloran, 1993). An Arabidopsis aconitase with homology to mammalian IRE-BPs has been cloned (Peyret et al., 1995). We postulate that the increase in total citrate in copper-treated seedlings may be due to an inhibition of aconitase, presumably the cytosolic form of the enzyme. The rise in cytosolic citrate levels would result in a higher rate of citrate efflux, with K+ serving as the counterion.
Early inhibition of aconitase by copper resulting in citrate and K+ release is also consistent with the comparatively higher initial rates of copper accumulation and higher rates of short-term K+ release found in relatively copper-tolerant Arabidopsis ecotypes (Murphy and Taiz, 1997). Further studies of copper inhibition of plant aconitase activity and possible IRE-BP activity are warranted in order to evaluate the citrate release model for copper tolerance in Arabidopsis.
Footnotes
This research was supported by grant nos. 94–37100–0755 (to A.M. and L.T.) and 98–35100–6105 (J.S. and L.K.) from the U.S. Department of Agriculture.
LITERATURE CITED
- Adams M, Chen Z, Landman P, Colmer T. Simultaneous determination by capillary gas chromatography of organic acids, sugars, and sugar alcohols in plant tissue extracts as their trimethylsilyl derivatives. Anal Biochem. 1999;266:77–84. doi: 10.1006/abio.1998.2906. [DOI] [PubMed] [Google Scholar]
- Cherif M, Nodet P, Hagege D. Malondialdehyde cannot be related to lipoperoxidation in habituated sugar beet plant cells. Phytochemistry. 1996;41:1523–1526. [Google Scholar]
- Chubatsu L, Meneghini R. Metallothionein protects DNA from oxidative damage. Biochem J. 1993;291:193–198. doi: 10.1042/bj2910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Ramirez-Rodriguez V, Cabrera-Ponce J, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Sokolik A, Yurin V. The effect of Cu2+ on ion transport systems of the plant cell plasmalemma. Plant Physiol. 1997;114:1313–1325. doi: 10.1104/pp.114.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos C, Schat H, De Waal M, Vooijs R, Ernst W. Increased resistance to copper induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol Plant. 1991;82:523–528. [Google Scholar]
- De Vos C, Schat H, Vooijs R, Ernst W. Copper induced damage to the permeability barrier in roots of Silene cucubalus. J Plant Physiol. 1989;135:164–165. [Google Scholar]
- De Vos C, Ten Bookum W, Vooijs R, Schat H, DeKok L. Effect of copper on fatty acid composition and peroxidation of lipids in the roots of copper tolerant and sensitive Silene cucubalus. Plant Physiol Biochem. 1993;31:151–158. [Google Scholar]
- Hirsch R, Lewis B, Spalding E, Sussman M. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Jones D. Organic acids in the rhizosphere: a critical review. Plant Soil. 1998;205:25–44. [Google Scholar]
- Jorge R, Arruda P. Aluminum-induced organic acids exudation by roots of an aluminum-tolerant tropical maize. Phytochemistry. 1997;45:675–681. [Google Scholar]
- Larsen P, Degenhardt J, Tai C, Stenzler L, Howell S, Kochian L. Aluminum resistant Arabidopsis mutants that exhibit altered patterns off aluminum accumulation and organic acid release from roots. Plant Physiol. 1998;117:9–18. doi: 10.1104/pp.117.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidon F, Henriques F. Copper mediated inhibition on protein synthesis in rice shoots. J Plant Nutr. 1993;16:1619–1630. [Google Scholar]
- Luna C, Gonzalez C, Trippi V. Oxidative damage caused by an excess of copper in oat leaves. Plant Cell Physiol. 1994;35:11–15. [Google Scholar]
- McQueen-Mason S, Cosgrove D. Expansin mode of action on cell walls: analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Yoshino M. Inactivation of aconitase in yeast exposed to oxidative stress. Biochem Mol Biol Int. 1997;41:481–486. doi: 10.1080/15216549700201501. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. Correlation between potassium efflux and copper sensitivity in ten Arabidopsis ecotypes. New Phytol. 1997;136:211–222. [Google Scholar]
- O'Halloran T. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Owen C. Biochemical Aspects of Copper. Park Ridge, NJ : Noyes; 1982. [Google Scholar]
- Peyret P, Perez P, Alric M. Structure, genomic organization, and expression of the Arabidopsis thaliana aconitase gene. J Biol Chem. 1995;270:8131–8137. doi: 10.1074/jbc.270.14.8131. [DOI] [PubMed] [Google Scholar]
- Stohs S, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Strange J, Macnair M. Evidence for a role for the cell membrane in copper tolerance of Mimulus guttatus fischer ex DC. New Phytol. 1991;119:383–388. [Google Scholar]
- Watkins C, Ferguson I. The interaction of copper and zinc with calcium in apple fruit. Sci Hortic. 1982;17:319–325. [Google Scholar]