Introduction
There has been a precipitous increase in the number of squamous-cell carcinomas of the head and neck (HNCs), along with an increasing recognition of the connection between human papillomavirus (HPV) and oropharyngeal tumors.1,2 Despite a trend toward treatment intensification with concurrent chemoradiotherapy3–6 and multidrug induction chemotherapy,7 a significant number of individuals relapse after standard treatment.8,9 In addition, tumors that do not harbor HPV have a worse therapeutic response, and the survival of these patients is shorter than for patients in whom HPV status is positive.8
The phosphotidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is activated in many cancers,10 including head and neck tumors.11 Activation is often associated with mutations in the class IA PI3K catalytic subunit p110α (encoded by PIK3CA) and/or loss of the phosphate and tensin homolog (PTEN).10 Rapamycin inhibits mTOR, which is a protein kinase that controls cell growth by regulating many cellular processes, including protein synthesis and autophagy. mTOR regulates a cell-survival pathway that is aberrant in many cancers, particularly those with increased PI3K signaling as a result of mutations in PIK3CA or the tumor suppressor PTEN, with a mutation in the latter that results in a loss of expression. Previous studies have reported higher response rates for patients with PIK3CA mutations treated with PI3K/AKT/mTOR–pathway inhibitors than for patients without such mutations.12–14 We present the results of five patients with HNCs with squamous-cell histology and either PIK3CA mutations or PTEN loss who were treated with mTOR inhibitor-based therapy.
Case Reports
Five men with squamous-cell HNCs had either a loss of PTEN (n = 2) or PIK3CA mutation (n = 3; Table 1). Patients were treated on protocol with either temsirolimus (an ester of the mTOR inhibitor sirolimus) alone (two patients) or temsirolimus together with bevacizumab (anti–vascular endothelial growth factor [VEGF] antibody; three patients; Table 1). The median age of the patients was 56 years (range, 16 to 73 years). The median number of previous systemic therapies of patients in the metastatic setting was two (range, one to four). Responses were evaluated with the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.0).15 Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Informed consent was obtained in accordance with The University of Texas MD Anderson Cancer Center institutional review board guidelines. Histology was evaluated at the MD Anderson Cancer Center. PIK3CA mutations and PTEN loss were analyzed according to previously published methods.14 Testing was performed in our Clinical Laboratory Improvement Amendments–approved laboratory by using either Sanger sequencing for PIK3CA mutation or immunohistochemistry (Dako antibody; Dako, Glostrup, Denmark).
Table 1.
Clinical Characteristics and Responses of Patients With Squamous-Cell Carcinoma of the Head and Neck Treated With mTOR Inhibitor-Based Regimens
| Characteristic | Patient No. |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age at treatment, years | 16 | 56 | 73 | 49 | 59 |
| Previous treatments | Surgery and neck dissection | Radiation | Subtotal glossectomy and bilateral dissection | Surgery; radiation | Radiation |
| Chemoradiation with cisplatin | Interferon/vitamin E/13-cis-retinoic acid | Four therapeutic regimens including cisplatin, fluorouracil, docetaxel, cetuximab, everolimus, and erlotinib | Six therapeutic regimens including trastuzumab, cisplatin, paclitaxel, sorafenib, carboplatin, cixutumumab, and linsitinib | Two therapeutic regimens including: paclitaxel, carboplatin, brachytherapy, hyperthermia, cetuximab, docetaxel, cisplatin, and fluorouracil | |
| Carboplatin/docetaxel | Six therapeutic regimens including: cetuximab, carboplatin, paclitaxel, gemcitabine, docetaxel, and cetuximab | Radiation | Additional surgery | Additional radiation | |
| Additional surgeries | Additional surgeries | Surgeries | |||
| Disease site | Oral cavity | Tongue base | Tongue base | Oral tongue | Right tonsil |
| PIK3CA mutation | E542K | E542K | H1047R | No | No |
| PTEN | Present | Present | Not done | Absent | Absent |
| HPV Status | Negative | Negative | Negative | Negative | Positive |
| Treatment | Temsirolimus | Temsirolimus | Temsirolimus/bevacizumab | Temsirolimus/bevacizumab | Temsirolimus/bevacizumab |
| No. of treatment cycles | 2 | 2 | 2 | 5 | 16 |
| Time to tumor progression, months | 1.5 | 1.5 | 1.5+* | 3.3 | 11.5 |
| Overall survival, months | 2.9 | 1.5 | 1.5+* | 5.1 | 15.5 |
| Best RECIST response, % | 20 | 25 | −38 | −24 | −32 |
Abbreviations: HPV, human papillomavirus; mTOR, mammalian target of rapamycin; PTEN, phosphate and tensin homolog.
Patient stopped treatment after 1.5 months and had 38% tumor regression at that point. The patient did not return to follow-up with us to determine when his tumor began to progress, but we were informed of his date of death.
Overall, two patients achieved a partial response, and one patient showed a tumor regression of 24% (cases 3, 4, and 5). The cases of these three patients are reviewed in more depth.
Patient 3 was a 73-year-old Asian man with tongue-based squamous-cell carcinoma (SCC) diagnosed in 2008. The patient was referred to the Clinical Center for Targeted Therapy in January 2010 after previous chemoradiotherapy, salvage surgery, and subsequent systemic chemotherapy with cisplatin, fluorouracil, docetaxel, and cetuximab and targeted therapy with everolimus and erlotinib. The last therapy was discontinued early as a result of a rash attributed to the everolimus. A PIK3CA mutation (exon 20, H1047R) was detected in a new SCC metastasis that involved subcutaneous and fibroconnective tissue with lymphovascular invasion and extranodal extension. HPV status was negative. Restaging computerized tomography (CT) images of the soft tissue of the neck and thorax showed a recurrent tumor that involved the left tongue base and floor of the mouth with invasion into the adjacent submental region and pre-epiglottic space. There were also extensive dermal and nodal metastases.
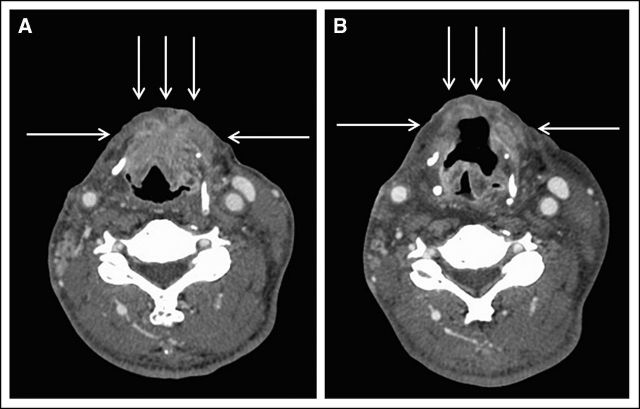
The patient was treated with temsirolimus 25 mg intravenously (IV) per week and bevacizumab 15 mg/kg IV on day 1 of a 21-day cycle with no serious toxic effects. The best response was a 38% decrease in measurable disease according to RECIST on radiographic imaging (Fig 1). The patient was having a second cycle of treatment when he withdrew consent.
Fig 1.
Patient 4 was a 49-year-old man diagnosed in 1999 with oral-cavity SCC. He was referred to the Clinical Center for Targeted Therapy in July 2009. Previous treatments included surgery and postoperative radiotherapy. Previous systemic regimens were as follows: trastuzumab, cisplatin, and paclitaxel; cisplatin and paclitaxel; sorafenib, carboplatin, and paclitaxel; cixutumumab; and erlotinib. For this patient, PIK3CA mutation was not detected, but according to immunochemistry, there was loss of PTEN staining. HPV was negative. Restaging CT images of the soft tissue of the neck and thorax showed progression of hypopharyngeal cancer and right posterior triangle, supraclavicular, and chest-wall metastases with visible skin lesions on the neck and upper back.
The patient was treated with temsirolimus 25 mg IV per week and bevacizumab 10 mg/kg IV on day 1 of a 21-day cycle with no serious toxic effects. CT scans after the first cycle showed evidence of a response in hypopharyngeal and upper esophageal disease as well as improvement in skin lesions of the neck. The best response was a 24% decrease in measurable disease by RECIST. the patient was having his fifth cycle of treatment when he died of unknown causes.
Patient 5 was a 59-year-old man with laryngeal SCC initially diagnosed in 2006, who was referred to the Clinical Center for Targeted Therapy in January 2010. Previous treatments included surgery, radiation with concomitant weekly paclitaxel and carboplatin and a combination of docetaxel, cisplatin, and fluorouracil with a subsequent substitution for cisplatin by carboplatin. Tissue was negative for PIK3CA mutation but showed a loss of PTEN. HPV was positive by in situ hybridization for high-risk type 16. Restaging CT images of the soft tissue neck and thorax showed metastatic adenopathy in the right superior mediastinum that extended into the lower mediastinum and bilateral pulmonary nodules with no local recurrence at the laryngectomy site.
The patient was treated with temsirolimus 25 mg IV per week and bevacizumab 15 mg/kg IV on day 1 of a 21-day cycle. The patient eventually developed grade 3 proteinuria but had no other serious toxic effects. The maximum response was a 32% decrease in disease. The patient continued to be administered treatment for just less than 1 year (16 completed cycles) before discontinuation as a result of disease progression.
Discussion
Although patients with early-stage HNC, especially if they are HPV positive, have excellent cure rates with the use of surgery, radiation, and/or platinum-based chemotherapy, the prognosis remains poor for many patients with HPV-negative disease and even for some patients with HPV-associated advanced-stage disease.8,16 Furthermore, there is no standard of care for patients with platinum-refractory, recurrent, or metastatic disease, and treatment options for such patients are limited.9 The addition of cetuximab, which is a recombinant monoclonal antibody directed against the epithelial growth factor receptor, to platinum/fluorouracil chemotherapy in the EXTREME (Erbitux in First-line Treatment of Recurrent of Metastatic Head and Neck Cancer) protocol resulted in longer overall and progression-free survival as well as higher objective response rates without an increased incidence of adverse events.17 However, questions remain about the long-term disease control with this regimen.9
Observations emerging from the use of phosphor-specific antibodies that detect the activated state of signaling molecules in tissue arrays indicate that the AKT/mTOR pathway is frequently activated in squamous cell cancers of the head and neck.11 Of interest, such activation may be independent from that of the epidermal growth factor receptor. Activation may occur via a PIK3CA mutation. Alternatively, because PTEN is a repressor of the PI3K/AKT/mTOR pathway, its loss, which is generally mediated by the presence of a mutation, also activates the pathway.18 Indeed, molecular analyses of our patients revealed a PIK3CA mutation in three individuals, and two patients had PTEN loss. Furthermore, Molinolo et al11 identified a small subgroup of patients in whom the mTOR pathway was activated but not AKT, which suggested the existence of an AKT-independent signaling route as well.
In our study, treatment with the mTOR-inhibitor temsirolimus, in combination with bevacizumab, was well tolerated by our patients with advanced-stage squamous-cell HNCs. Grade 1 and 2 events, which were possibly drug related, included fatigue (patients 4 and 5) and hypertriglyceridemia (patient 5). There was one grade 3 event (proteinuria; patient 5).
The three patients who responded to temsirolimus also received the VEGF inhibitor bevacizumab. The exact role of VEGF inhibition in the tumor regression of these patients is unclear. Hypoxia-inducible factor 1 alpha (HIF-1α) mediates adaptive responses to hypoxic conditions induced by antiangiogenic therapy, and temsirolimus attenuates HIF-1α.19 Therefore, more than one mechanism may be operative for this combination. Our findings suggest that the role of mTOR inhibitor-based treatment in squamous-cell HNC warrants additional investigation. Finally, because of our initial experience, others researchers have suggested that the assessment of the phosphorylation status of S6 kinase20 or eukaryotic translation initiation factor 4E-binding protein21 might serve as a clinically useful marker to correlate with the response to mTOR inhibitor-based therapy. As such, we plan to incorporate these biomarkers into future studies that will target the PI3K/PTEN/mTOR pathway for patients with HNC.
Acknowledgment
We thank Joann Aaron, MA, and Rekiya Wallace, BS, for editing and reviewing the manuscript and providing assistance in the preparation of figures.
Footnotes
Clinical trial information: NCT00610493.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Filip Janku, Novartis; Razelle Kurzrock, Genentech, Roche Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Chaturvedi AK Engels EA Pfeiffer RM, etal: Human papillomavirus and rising oropharyngeal cancer incidence in the United States J Clin Oncol 29:4294–4301,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML Koch WM Capone RB, etal: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers J Natl Cancer Inst 92:709–720,2000. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA Harari PM Giralt J, etal: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck N Engl J Med 354:567–578,2006. [DOI] [PubMed] [Google Scholar]
- 4.Brizel DM Albers ME Fisher SR, etal: Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer N Engl J Med 338:1798–1804,1998. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA Goepfert H Maor M, etal: Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer N Engl J Med 349:2091–2098,2003. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere AA Maor M Weber RS, etal: Long-term results of Intergroup RTOG 91-11: A phase III trial to preserve the larynx—Induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy J Clin Oncol 24: 284s,2006. suppl abstr 5517 [Google Scholar]
- 7.Posner MR Hershock DM Blajman CR, etal: Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer N Engl J Med 357:1705–1715,2007. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK Harris J Wheeler R, etal: Human papillomavirus and survival of patients with oropharyngeal cancer N Engl J Med 363:24–35,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin DM, Khuri FR: Advances in the management of recurrent or metastatic squamous cell carcinoma of the head and neck Head Neck 2011.10.1002/hed.21910 [epub ahead of print on November 2, 2011] [DOI] [PubMed] [Google Scholar]
- 10.Engelman JA: Targeting PI3K signalling in cancer: Opportunities, challenges and limitations Nat Rev Cancer 9:550–562,2009. [DOI] [PubMed] [Google Scholar]
- 11.Molinolo AA Hewitt SM Amornphimoltham P, etal: Dissecting the Akt/mammalian target of rapamycin signaling network: Emerging results from the head and neck cancer tissue array initiative Clin Cancer Res 13:4964–4973,2007. [DOI] [PubMed] [Google Scholar]
- 12.Janku F Tsimberidou AM Garrido-Laguna I, etal: PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors Mol Cancer Ther 10:558–565,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janku F Wheler JJ Westin SN, etal: PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations J Clin Oncol 30:777–782,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piha-Paul SA, Cohen PR, Kurzrock R: Salivary duct carcinoma: Targeting the phosphatidylinositol 3-kinase pathway by blocking mammalian target of rapamycin with temsirolimus J Clin Oncol 29:e727–e730,2011. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P Arbuck SG Eisenhauer EA, etal: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92:205–216,2000. [DOI] [PubMed] [Google Scholar]
- 16.Langer CJ: Targeted therapy in head and neck cancer: State of the art 2007 and review of clinical applications Cancer 112:2635–2645,2008. [DOI] [PubMed] [Google Scholar]
- 17.Vermorken JB Mesia R Rivera F, etal: Platinum-based chemotherapy plus cetuximab in head and neck cancer N Engl J Med 359:1116–1127,2008. [DOI] [PubMed] [Google Scholar]
- 18.Wu X Senechal K Neshat MS, etal: The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway Proc Natl Acad Sci U S A 95:15587–15591,1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Bufalo D Ciuffreda L Trisciuoglio D, etal: Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus Cancer Res 66:5549–5554,2006. [DOI] [PubMed] [Google Scholar]
- 20.Magnuson B, Ekim B, Fingar DC: Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks Biochem J 441:1–21,2012. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh AC Costa M Zollo O, etal: Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E Cancer Cell 17:249–261,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]