Abstract
Purpose
The oral mammalian target of rapamycin inhibitor everolimus demonstrated promising efficacy in a phase II study of pretreated advanced gastric cancer. This international, double-blind, phase III study compared everolimus efficacy and safety with that of best supportive care (BSC) in previously treated advanced gastric cancer.
Patients and Methods
Patients with advanced gastric cancer that progressed after one or two lines of systemic chemotherapy were randomly assigned to everolimus 10 mg/d (assignment schedule: 2:1) or matching placebo, both given with BSC. Randomization was stratified by previous chemotherapy lines (one v two) and region (Asia v rest of the world [ROW]). Treatment continued until disease progression or intolerable toxicity. Primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), overall response rate, and safety.
Results
Six hundred fifty-six patients (median age, 62.0 years; 73.6% male) were enrolled. Median OS was 5.4 months with everolimus and 4.3 months with placebo (hazard ratio, 0.90; 95% CI, 0.75 to 1.08; P = .124). Median PFS was 1.7 months and 1.4 months in the everolimus and placebo arms, respectively (hazard ratio, 0.66; 95% CI, 0.56 to 0.78). Common grade 3/4 adverse events included anemia, decreased appetite, and fatigue. The safety profile was similar in patients enrolled in Asia versus ROW.
Conclusion
Compared with BSC, everolimus did not significantly improve overall survival for advanced gastric cancer that progressed after one or two lines of previous systemic chemotherapy. The safety profile observed for everolimus was consistent with that observed for everolimus in other cancers.
INTRODUCTION
Gastric cancer is the fourth most common malignancy and second leading cause of cancer mortality worldwide, with 989,600 new cases and 738,000 deaths estimated to have occurred in 2008.1 Although resection may be curative in early-stage disease,2–4 approximately two thirds of patients present with inoperable or metastatic disease.5 The exceptions are Japan and Korea, where national screening programs lead to early-stage diagnosis in approximately one half of patients.6,7 Patients with advanced gastric cancer and distant metastases, who receive systemic treatment with regimens including fluorouracil and related compounds, platinum derivatives, taxanes, or irinotecan, have a 5-year survival rate of less than 5% and median overall survival (OS) less than 12 months.6,8–10 After failure of first-line therapy, there is little consensus on second- and third-line treatment options, and outcomes are poor2,8; in recent phase III trials of second-line chemotherapy for advanced gastric cancer, median OS was only 4.0 to 5.3 months.11,12 A need exists for effective therapy for patients with advanced gastric cancer whose disease progresses after first-line therapy.
Phosphatidylinositol 3-kinase (PI3K)/Akt and mammalian target of rapamycin (mTOR) are activated in 30% and 60% of human gastric carcinomas, respectively.13,14 PI3K/Akt/mTOR pathway dysregulation is also associated with chemotherapy resistance13 and decreased survival.15–17 These findings suggest the PI3K/Akt/mTOR pathway is frequently activated in gastric cancer and is directly linked to its progression.
The oral mTOR inhibitor everolimus has demonstrated clinical benefit and a tolerable safety profile in several human cancers and tumor syndromes.18–22 In preclinical models, everolimus inhibited downstream signaling molecules, cell proliferation, tumor growth and vascularization, and peritoneal metastasis.14,23–27
In a phase II study of everolimus 10 mg/d in 53 patients with advanced gastric cancer whose disease progressed after one or two previous chemotherapy lines, the disease control rate was 54.7%, median progression-free survival (PFS) per central radiology review was 2.7 months, and median OS was 10.1 months.28 The phase III GRANITE-1 (First Gastric Antitumor Trial With Everolimus; Clinical Trial No. NCT00879333) evaluated everolimus efficacy and safety in patients with advanced gastric cancer who experienced treatment failure after one or two lines of previous chemotherapy.
PATIENTS AND METHODS
Patients
Eligible patients were at least 18 years old with histologically or cytologically confirmed gastric adenocarcinoma, including that of the gastroesophageal junction, and had documented disease progression after one or two previous systemic chemotherapy lines for advanced disease. Additional inclusion criteria included Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 229 and adequate organ and hematologic function. Exclusion criteria included enteral feeding, malignant ascites requiring drainage, and chronic treatment with immunosuppressive agents.
All patients provided written informed consent before enrollment. The appropriate ethics committees at each participating center approved the protocol. The study was conducted in accordance with the protocol, good clinical practice principles, the Declaration of Helsinki, and all applicable local regulations. A steering committee supervised the conduct of the study. An independent data monitoring committee performed semiannual safety reviews and reviewed interim efficacy results.
Study Design and Assessment
Patients were randomly assigned at a 2:1 schedule to oral everolimus 10 mg/d or matching placebo. All patients received best supportive care (BSC), defined as care in accordance with local institutional practice, excluding anticancer therapy. Treatment continued until disease progression, unacceptable toxicity, or consent withdrawal. The protocol provided guidelines for dose interruption or reduction for adverse events (AEs). An initial dose reduction to 5 mg/d and a subsequent reduction to 5 mg every other day were permitted.
Treatment assignment was determined by a centralized interactive web response system that automated the random assignment of patient numbers to randomization numbers. Randomization numbers were linked to the treatment groups, which were in turn linked to medication numbers. The medication randomization list was produced by Novartis Drug Supply Management using a validated system. Randomization was stratified by the number of previous systemic chemotherapy lines (one v two) and region of enrollment (Asia [China, Hong Kong, Japan, Korea, Taiwan, and Thailand] v the rest of the world [ROW]). Aside from the independent data monitoring committee, all individuals involved in the study were blinded to treatment assignment.
Tumor response was assessed by the local investigator per the Response Evaluation Criteria in Solid Tumors, version 1.0,30 every 6 weeks until disease progression; complete (CR) or partial response (PR) required confirmation at least 4 weeks after initial observation. To determine the minimum and maximum concentrations of everolimus in whole blood (Cmin and Cmax, respectively), venous blood samples were collected predose and 1 and 2 hours postdose on day 1 of week 5. Hematology, biochemistry, and vital signs were assessed at baseline and at each visit. AEs were monitored continuously and assessed using the Common Terminology Criteria for Adverse Events, version 3.0.31
Statistical Analysis
All randomly assigned patients were assessed for efficacy; following the intent-to-treat principle, patients were analyzed per the treatment and stratum to which they were assigned on randomization. Safety was assessed in all patients who received at least one dose of study drug and had at least one postbaseline assessment.
Primary end point was OS, defined as the time from randomization to the time of death (any cause). Secondary end points included PFS, defined as the time from randomization to first documented disease progression or death (any cause); overall response rate (ORR); time to definitive deterioration of ECOG PS; time to definitive 5% deterioration in the global health status/quality of life (QoL) and physical, social, and emotional functioning scales of the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire; pharmacokinetics; and safety. (See Appendix [online-only] for information on how missing values were handled.)
Between-arm comparisons of OS and PFS were performed using log-rank tests stratified by the two randomization stratification factors at a one-sided cumulative 2.5% significance level. OS analyses were repeated in several patient subgroups (Appendix); no interaction test was performed. Comparisons of time to definitive deterioration in ECOG PS and time to definitive 5% deterioration in QoL were performed using log-rank tests stratified by the two randomization stratification factors at a two-sided 5% significance level. No other adjustments were performed. A hierarchical testing strategy was implemented such that formal significance for PFS could be declared only if the between-group difference in OS was significant. Subsequent levels of the hierarchy were deterioration in ECOG PS; deterioration in the QLQ-C30 global health status/QoL scale; and deterioration in the QLQ-C30 physical, social, and emotional functioning scales (successively compared). No statistical comparisons were performed for ORR or for pharmacokinetic or safety parameters. For all time-to-event end points, median values were estimated using the Kaplan-Meier method. Hazard ratios (HRs) and 95% CIs were derived from Cox proportional hazards models stratified by the two randomization stratification factors. Exact 95% CIs for ORR were calculated using the Clopper-Pearson method.
The study was designed to detect an improvement in median OS from 4.0 months with placebo to 5.4 months with everolimus (HR, 0.74). Considering the two-look Lan-DeMets group sequential design with an O'Brien-Fleming–type boundary,32 526 deaths were required at final analysis (90% power, stratified log-rank test, one-sided cumulative 2.5% significance). Assuming a 24-month recruitment period, 5% loss to follow-up, and 2:1 randomization in favor of everolimus, it was estimated that 633 patients would need to be enrolled. (See Appendix, online only, for results of interim analysis.)
RESULTS
Patient Disposition and Characteristics
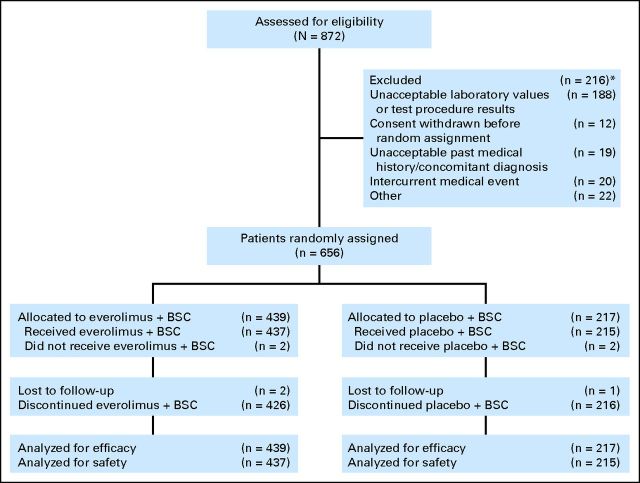
From July 2009 to November 2010, 656 patients from 137 centers in 23 countries were enrolled and received everolimus plus BSC (n = 439) or placebo plus BSC (n = 217; Fig 1). As of the analysis cutoff date (September 5, 2011), 11 patients (2.5%) in the everolimus arm and no patients in the placebo arm were still receiving study treatment. The most common reason for treatment discontinuation was disease progression (66.5% in the everolimus arm and 77.9% in the placebo arm). A higher percentage of patients discontinued everolimus because of AEs (21.4% v 15.7% with placebo) or consent withdrawal (4.6% v 3.2%). Median follow-up duration (ie, time from randomization date of median patient enrolled to date of data cutoff) was 14.3 months.
Fig 1.
CONSORT diagram. (*) Patients could be excluded for more than one reason. BSC, best supportive care.
Baseline demographics and disease characteristics were generally well balanced between treatment groups, although minor differences were observed (Table 1). Compared with the everolimus arm, more patients in the placebo arm had the proximal stomach tumor location, an ECOG PS of 2, and liver metastases. Overall, 47.7% of patients received one previous line of chemotherapy and 52.3% received two previous lines of chemotherapy (Table 1). The most commonly administered chemotherapy regimens contained fluoropyrimidines (96.0%), platinum derivatives (85.8%), and taxanes (38.4%). Other previous therapy included total (21.8%) and partial (28.4%) gastrectomy and radiotherapy (12.0%) (Table 1).
Table 1.
Baseline Patient Demographics and Disease Characteristics of All Randomly Assigned Patients
| Characteristic | Everolimus Plus BSC (n = 439) |
Placebo Plus BSC (n = 217) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 62 | 62 | ||
| Range | 20-86 | 26-88 | ||
| < 65 | 260 | 59 | 129 | 59 |
| ≥ 65 | 179 | 41 | 88 | 41 |
| Male sex | 322 | 73 | 161 | 74 |
| Race | ||||
| White | 166 | 38 | 75 | 35 |
| Black | 3 | < 1 | 1 | < 1 |
| Asian | 251 | 57 | 126 | 58 |
| Other | 19 | 4 | 15 | 7 |
| Region and No. of previous chemotherapy lines | ||||
| Asia, 1 line | 98 | 22 | 48 | 22 |
| Asia, 2 lines | 145 | 33 | 72 | 33 |
| Rest of the world, 1 line | 112 | 26 | 55 | 25 |
| Rest of the world, 2 lines | 84 | 19 | 42 | 19 |
| Time since initial diagnosis, months | ||||
| ≤ 12 | 176 | 40 | 93 | 43 |
| > 12 to ≤ 24 | 156 | 36 | 71 | 33 |
| > 24 | 107 | 24 | 53 | 24 |
| Anatomic site of cancer | ||||
| Proximal stomach | 162 | 37 | 94 | 43 |
| Distal stomach | 276 | 63 | 123 | 57 |
| Missing | 1 | < 1 | 0 | 0 |
| Gastroesophageal junction involvement | 118 | 27 | 69 | 32 |
| Histologic grade | ||||
| Well differentiated | 33 | 8 | 21 | 10 |
| Moderately differentiated | 137 | 31 | 69 | 32 |
| Poorly differentiated | 198 | 45 | 89 | 41 |
| Poorly differentiated/undifferentiated | 6 | 1 | 4 | 2 |
| Unknown | 65 | 15 | 34 | 16 |
| Measurable disease according to RECIST | 379 | 86 | 192 | 88 |
| Metastatic site | ||||
| Lung | 92 | 21 | 37 | 17 |
| Liver | 190 | 43 | 109 | 50 |
| ECOG performance status | ||||
| 0 | 144 | 33 | 70 | 32 |
| 1 | 269 | 61 | 120 | 55 |
| 2 | 25 | 6 | 27 | 12 |
| Missing | 1 | < 1 | 0 | 0 |
| Prior gastrectomy | ||||
| No | 216 | 49 | 111 | 51 |
| Partial | 126 | 29 | 60 | 28 |
| Total | 97 | 22 | 46 | 21 |
| Prior radiotherapy | 54 | 12 | 25 | 12 |
Abbreviations: BSC, best supportive care; ECOG, Eastern Cooperative Oncology Group.
Study Drug Exposure
Median duration of study drug exposure was 7.1 weeks for everolimus (range, 0.1 to 79.6 weeks) and 6.4 weeks for placebo (range, 0.4 to 90.9 weeks). Mean duration of exposure was 11.5 weeks (standard deviation [SD], 12.1 weeks) and 8.5 weeks (SD, 8.8 weeks), respectively. Median exposure was slightly longer in patients with versus without gastrectomy, patients at least 65 years old versus those younger than 65 years, Asians versus white patients or patients of other races, Japanese versus other ethnicities, and patients enrolled in Asia versus ROW (Table 2). Dose interruptions or reductions were more common with everolimus (48.5% v 16.7% with placebo). The most common reasons for dose interruption or reduction were AEs (34.6% and 11.6% with everolimus and placebo, respectively) and laboratory test abnormalities (14.0% and 0.5%, respectively). The median relative dose intensity was 1.0 for both treatment arms. The mean dose intensity was 8.9 mg/d with everolimus (SD, 1.7 mg/d) and 9.7 mg/d with placebo (SD, 1.0 mg/d).
Table 2.
Exposure to Study Treatment in the Everolimus Plus Best Supportive Care Treatment Arm in the Safety Population
| Characteristic | No. of Patients | Duration of Exposure (weeks) |
Mean Dose Intensity (mg/d) | |
|---|---|---|---|---|
| Median | Range | |||
| Overall population | 437 | 7.1 | 0.1-79.6 | 8.9 |
| Gastrectomy | ||||
| Yes | 224 | 8.0 | 0.9-70.7 | 8.8 |
| No | 213 | 6.7 | 0.1-79.6 | 9.1 |
| Sex | ||||
| Male | 322 | 7.1 | 0.4-79.6 | 8.9 |
| Female | 115 | 7.0 | 0.1-74.7 | 8.9 |
| Age, years | ||||
| < 65 | 258 | 6.9 | 0.1-79.6 | 9.1 |
| ≥ 65 | 179 | 8.0 | 0.9-58.3 | 8.6 |
| Race | ||||
| Asian | 251 | 8.0 | 0.1-79.6 | 8.8 |
| White | 164 | 6.6 | 0.9-74.7 | 9.1 |
| Other | 22 | 6.1 | 0.9-42.4 | 9.5 |
| Ethnicity | ||||
| Chinese | 110 | 6.4 | 0.1-53.0 | 9.1 |
| Japanese | 74 | 11.4 | 1.0-70.7 | 8.3 |
| Hispanic/Latino | 35 | 7.0 | 0.9-46.3 | 9.1 |
| Indian | 2 | 7.4 | 6.3-8.4 | 7.8 |
| Mixed | 1 | 6.4 | — | 10.0 |
| Other | 215 | 7.1 | 0.6-79.6 | 9.0 |
| Region | ||||
| Asia | 243 | 7.9 | 0.1-79.6 | 8.9 |
| ROW | 194 | 6.8 | 0.9-74.7 | 9.0 |
Abbreviation: ROW, rest of world.
Median everolimus Cmin and Cmax were 13.8 ng/mL and 67.4 ng/mL, respectively, for patients who received everolimus 10 mg/d (Appendix Table A1). There was no apparent difference in steady-state everolimus concentrations between patients enrolled in Asia and ROW or those with and without gastrectomy (Appendix Table A1).
Efficacy
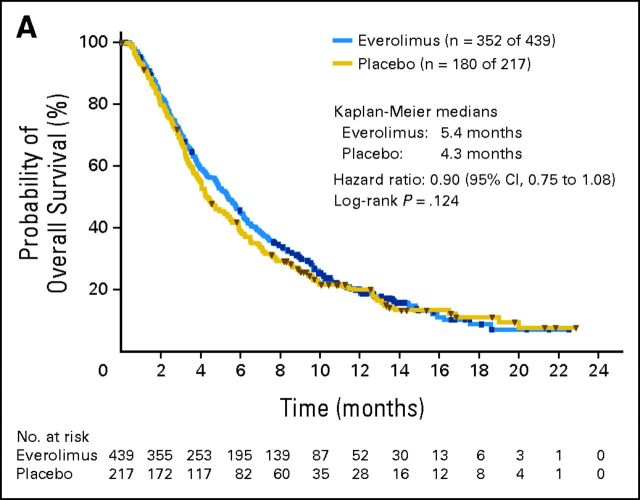
The estimated median OS was 5.4 months with everolimus plus BSC (95% CI, 4.8 to 6.0 months) and 4.3 months with placebo plus BSC (95% CI, 3.8 to 5.5 months; HR for OS, 0.90; 95% CI, 0.75 to 1.08; P = .124; Fig 2A). A trend for reduction in the risk of death was observed with everolimus in patients enrolled in ROW (15% reduction in risk) and patients enrolled in ROW with two previous chemotherapy lines (26% reduction in risk; Fig 2B); these trends in ROW seemed to be driven by patients enrolled outside of Europe (Fig 2B). Across the remaining subgroups analyzed, results were consistent with those of the overall population (Fig 2B). The percentage of patients who started other antineoplastic therapy after study treatment discontinuation was slightly higher with placebo (45.2% v 39.2% with everolimus; Appendix Table A2).
Fig 2.
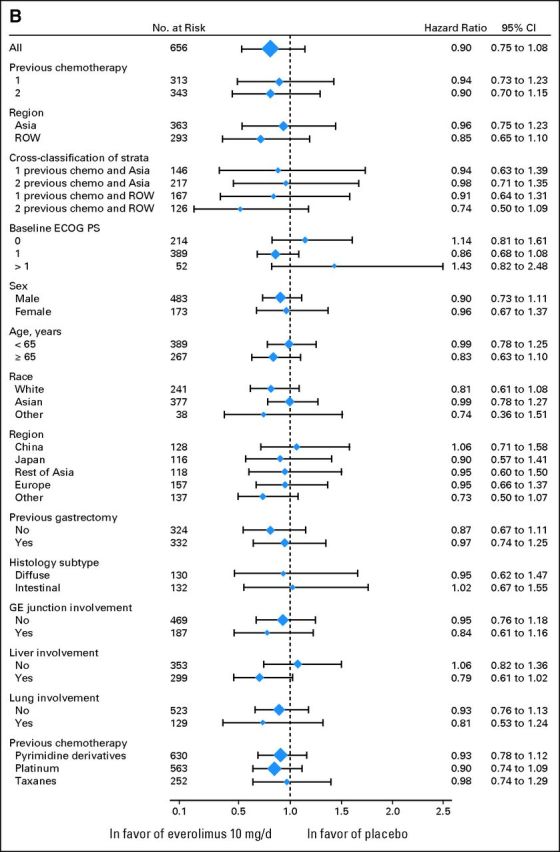
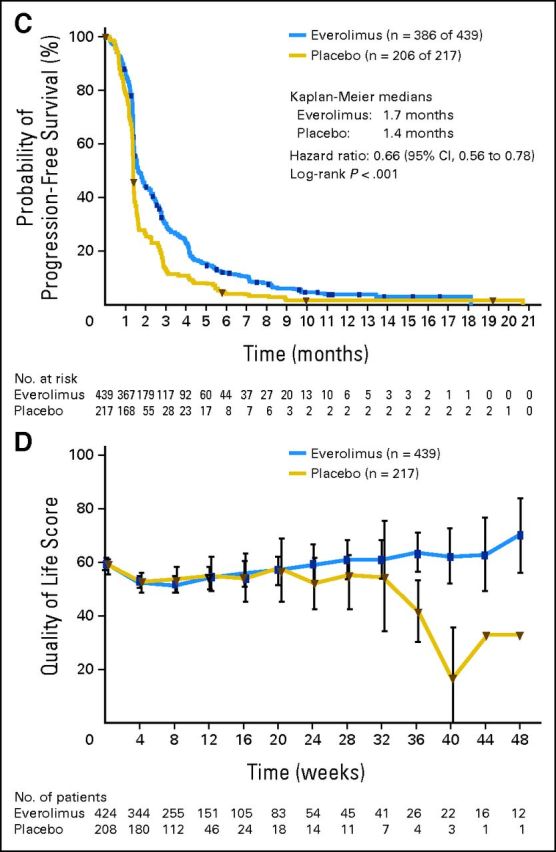
Overall and progression-free survival for all randomly assigned patients. (A) Kaplan-Meier plot of overall survival. (B) Forest plot of overall survival in subgroups. (C) Kaplan-Meier plot of progression-free survival. (D) Longitudinal mean scores of the global health status/quality-of-life scale of the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire. ECOG PS, European Cooperative Oncology Group performance status; GE, gastroesophageal; n, number of patients with event (of the number of patients at risk); ROW, rest of world. Appendix Table A3 (online only) lists details on the events experienced.
Estimated median PFS was 1.7 months with everolimus (95% CI, 1.5 to 1.9 months) and 1.4 months with placebo (95% CI, 1.4 to 1.5 months). Although everolimus reduced the risk of disease progression or death compared with placebo (HR, 0.66; 95% CI, 0.56 to 0.78; P < .001; Fig 2C), formal statistical significance could not be declared per the hierarchical testing strategy. The estimated percentage of patients progression free at 6 months was approximately three times greater with everolimus (12.0%; 95% CI, 9.0% to 15.4%; v 4.3%; 95% CI, 2.1% to 7.7%).
Among patients with measurable disease at baseline, one patient in the everolimus arm experienced a CR, versus no patients in the placebo arm (Table 3). The ORR (percentage of patients with CR or PR) was 4.5% with everolimus (95% CI, 2.6% to 7.1%) and 2.1% with placebo (95% CI, 0.6% to 5.3%). The disease control rate (percentage of patients with CR, PR, or stable disease) was approximately two-fold higher with everolimus (everolimus: 43.3%; 95% CI, 38.2% to 48.4%; v placebo: 22.0%; 95% CI, 16.3% to 28.5%). Tumor shrinkage was observed in approximately three times as many patients treated with everolimus (37.8% v 12.3% with placebo).
Table 3.
Best Overall Tumor Response According to RECIST for Patients With Measurable Disease
| Response | Everolimus Plus BSC (n = 379) |
Placebo Plus BSC (n = 191) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Best overall response | ||||
| CR | 1 | < 1 | 0 | 0 |
| PR | 16 | 4 | 4 | 2 |
| SD | 147 | 39 | 38 | 20 |
| PD | 157 | 41 | 119 | 62 |
| Unknown* | 58 | 15 | 30 | 16 |
| ORR (CR and PR) | 17 | 4 | 4 | 2 |
| DCR (CR, PR, and SD) | 164 | 43 | 42 | 22 |
Abbreviations: BSC, best supportive care; CR, complete response; DCR, disease control rate; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Tumor response data not available.
Time to deterioration of ECOG PS did not differ significantly between treatment arms (median time to deterioration, 2.3 months for everolimus v 2.2 months for placebo; HR, 0.96; 95% CI, 0.76 to 1.20; P = .693). A trend for a slightly longer time to ≥ 5% deterioration in global QoL was observed for everolimus (median time to ≥ 5% deterioration, 1.51 months v 1.45 months; HR, 0.84; 95% CI, 0.69 to 1.03; P = .094). Over time and versus placebo, everolimus recipients had higher mean scores for the global health status/QoL scale of the QLQ-C30 questionnaire (Fig 2D).
Safety
Almost all patients experienced at least one AE (99.1% in the everolimus arm and 96.7% in the placebo arm). The most common AEs (any grade) reported with everolimus were decreased appetite, stomatitis, fatigue, and nausea (Table 4). AEs that occurred in at least 10% of everolimus recipients were decreased appetite, stomatitis, thrombocytopenia, rash, diarrhea, and decreased weight. The most common grade 3/4 AEs with everolimus were anemia, decreased appetite, and fatigue (Table 4). The proportion of patients who experienced grade 3/4 AEs was similar in all patient subgroups assessed (Table 5). All-grade and grade 3/4 pneumonitis were relatively uncommon, with incidences in the everolimus arm of 3.0% (n = 13) and 0.7% (n = 3), respectively. Pneumonitis was not observed in the placebo arm.
Table 4.
Adverse Events Irrespective of Relationship to Study Treatment With ≥ 10% Incidence in the Everolimus Plus BSC Treatment Arm in the Safety Population
| Adverse Event | Everolimus Plus BSC (n = 437) |
Placebo Plus BSC (n = 215) |
||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade 3/4 |
Any Grade |
Grade 3/4 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Decreased appetite | 208 | 48 | 48 | 11 | 78 | 36 | 12 | 6 |
| Stomatitis | 174 | 40 | 20 | 5 | 23 | 11 | 0 | 0 |
| Fatigue | 150 | 34 | 34 | 8 | 65 | 30 | 11 | 5 |
| Nausea | 132 | 30 | 16 | 4 | 69 | 32 | 8 | 4 |
| Diarrhea | 115 | 26 | 15 | 3 | 33 | 15 | 2 | 1 |
| Anemia | 114 | 26 | 70 | 16 | 42 | 20 | 27 | 13 |
| Abdominal pain | 107 | 24 | 21 | 5 | 57 | 27 | 13 | 6 |
| Vomiting | 107 | 24 | 13 | 3 | 62 | 29 | 9 | 4 |
| Constipation | 91 | 21 | 3 | < 1 | 42 | 20 | 3 | 1 |
| Rash | 87 | 20 | 1 | < 1 | 19 | 9 | 0 | 0 |
| Weight decreased | 86 | 20 | 11 | 3 | 19 | 9 | 0 | 0 |
| Pyrexia | 81 | 19 | 3 | < 1 | 24 | 11 | 2 | 1 |
| Thrombocytopenia | 80 | 18 | 22 | 5 | 5 | 2 | 3 | 1 |
| Asthenia | 70 | 16 | 20 | 5 | 22 | 10 | 9 | 4 |
| Dyspnea | 61 | 14 | 18 | 4 | 23 | 11 | 9 | 4 |
| Upper abdominal pain | 53 | 12 | 6 | 1 | 27 | 13 | 2 | 1 |
| Peripheral edema | 53 | 12 | 1 | < 1 | 23 | 11 | 2 | 1 |
| Hypokalemia | 52 | 12 | 26 | 6 | 9 | 4 | 2 | 1 |
| Insomnia | 51 | 12 | 2 | < 1 | 22 | 10 | 0 | 0 |
| Cough | 50 | 11 | 1 | < 1 | 17 | 8 | 0 | 0 |
| Back pain | 48 | 11 | 10 | 2 | 16 | 7 | 2 | 1 |
| Neutropenia | 47 | 11 | 17 | 4 | 6 | 3 | 1 | < 1 |
| Pruritus | 47 | 11 | 0 | 0 | 9 | 4 | 0 | 0 |
NOTE. All data are sorted by descending frequency in the everolimus plus BSC treatment group.
Abbreviation: BSC, best supportive care.
Table 5.
Incidence of Grade 3/4 Adverse Events by Patient Subgroup in the Safety Population
| Patient Subgroup | Everolimus Plus BSC |
Placebo Plus BSC |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Overall population | 437 | 71 | 215 | 53 |
| Gastrectomy | ||||
| Yes | 224 | 70 | 107 | 48 |
| No | 213 | 72 | 108 | 59 |
| Sex | ||||
| Male | 322 | 69 | 161 | 55 |
| Female | 115 | 76 | 54 | 48 |
| Age, years | ||||
| < 65 | 258 | 71 | 128 | 54 |
| ≥ 65 | 179 | 71 | 87 | 53 |
| Race | ||||
| Asian | 251 | 67 | 125 | 44 |
| White | 164 | 77 | 74 | 64 |
| Other | 22 | 77 | 16 | 81 |
| Ethnicity | ||||
| Chinese | 110 | 62 | 56 | 48 |
| Japanese | 74 | 70 | 41 | 39 |
| Hispanic/Latino | 35 | 74 | 15 | 60 |
| Indian | 2 | 50 | 0 | 0 |
| Mixed | 1 | 0 | 3 | 67 |
| Other | 215 | 76 | 100 | 61 |
| Region | ||||
| Asia | 243 | 65 | 119 | 45 |
| ROW | 194 | 78 | 96 | 65 |
Abbreviations: BSC, best supportive care; ROW, rest of world.
AEs leading to study drug discontinuation occurred in 21.5% of everolimus and 15.8% of placebo recipients; those leading to dose adjustments/interruptions occurred in 55.4% of everolimus and 21.4% of placebo recipeints. The most common AEs leading to study drug discontinuation (everolimus v placebo) were fatigue (2.1% v 1.4%), gastrointestinal hemorrhage (1.4% v 0.9%), and abdominal pain (1.1% v 0.5%). The AEs most commonly leading to dose adjustment or interruption were thrombocytopenia (everolimus: 10.3% v placebo: 0.5%), stomatitis (everolimus: 7.8% v placebo: 0.5%), and neutropenia (everolimus: 6.6% v placebo: 0%). Three patients in the everolimus arm died and their deaths were suspected to be a result of study treatment (n = 1 each for sudden death, grade 3 pneumonitis, and grade 4 gastrointestinal hemorrhage). In the placebo arm, two patients died and their deaths were suspected to be a result of study treatment (n = 1 each for multiorgan failure and cerebrovascular accident).
DISCUSSION
GRANITE-1 did not demonstrate a significant survival benefit for everolimus versus BSC in patients with advanced gastric cancer whose disease progressed on one or two lines of previous systemic chemotherapy. The lack of significant benefit for everolimus may be partially attributable to the slightly higher percentage of placebo recipients who initiated antineoplastic therapy after study drug discontinuation (45.2% v 39.2% for everolimus). OS results were consistent across subgroups, although a trend toward a reduced risk of death with everolimus was noted for patients enrolled in ROW (15% reduction in risk) and patients enrolled in ROW who received two previous systemic chemotherapy lines (26% reduction in risk). These trends, which may be a result of chance alone, were mostly driven by patients enrolled outside Europe. A 34% reduction in the risk of disease progression or death with everolimus was observed. Notably, the estimated percentage of patients remaining progression free at 6 months was higher with everolimus (12.0% v 4.3%), as were the disease control rate (43.3% v 22.0%) and the tumor shrinkage rate (37.8% v 12.3%). These results suggest everolimus has activity in this heavily pretreated population.
Identification of specific biomarkers for various patient subpopulations with advanced gastric cancer may help define those patients who would receive the most benefit from everolimus treatment. Despite extensive efforts, including those of a phase II study of everolimus in gastric cancer,33 identification of gastric cancer biomarkers predictive of benefit from everolimus has been elusive. Results of ongoing biomarker analyses of GRANITE-1 are eagerly awaited.
Advanced gastric cancer, particularly that which progresses after systemic chemotherapy, is associated with a poor prognosis. The fact that 96.7% of placebo recipients in our study experienced at least one AE highlights the large number of comorbidities and overall high level of underlying risk in patients with heavily pretreated advanced gastric cancer. Although cross-study comparisons should be performed with caution, it is interesting that the median OS reported for everolimus in our trial (5.4 months) is similar to, or even longer than, that reported for second-line chemotherapy in two recent phase III studies, whereas the median OS reported for placebo in our study (4.3 months) is similar to, or even longer than, that reported for the control arms.11,12 In a study of irinotecan versus BSC in 40 patients with advanced gastric cancer previously treated with only one line of systemic chemotherapy, irinotecan significantly reduced the risk of death (HR, 0.48; 95% CI, 0.25 to 0.92; P = .012).11 Median OS was 4.0 months with irinotecan and 2.4 months with BSC; the disease control rate was 53% with irinotecan but was not reported for BSC. In the second study, 202 patients with advanced gastric cancer previously treated with one chemotherapy regimen that included both a fluoropyrimidine and platinum derivative or two chemotherapy regimens, of which one contained a fluoropyrimidine derivative and the other a platinum derivative, were randomly assigned to receive chemotherapy (docetaxel or irinotecan) or BSC.12 Results of this study showed that second-line chemotherapy significantly reduced the risk of death (HR, 0.66; 95% CI, 0.49 to 0.89; P = .007). Median OS was 5.3 months with second-line chemotherapy versus 3.8 months with BSC. These results highlight the need to standardize chemotherapy regimens when designing clinical trials following first-line therapy. Notably, the use of post–first-line chemotherapy and types of regimens used differ owing to between-country differences in approved/preferred agents and reimbursement systems.
The everolimus AE profile observed in our study was generally consistent with that previously observed for everolimus in cancer, with no new safety signals identified.18–20,28 Although stomatitis and pneumonitis, AEs commonly associated with everolimus, were observed in 39.8% and 3.0% of patients, respectively, they led to treatment discontinuation in only three patients (n = 2 for stomatitis, n = 1 for pneumonitis). The median duration of everolimus exposure was longer in patients with versus without gastrectomy, patients age at least 65 years versus those younger than 65 years, Asian versus white patients or patients of other races, Japanese versus other ethnicities, and patients enrolled in Asia versus ROW. AE incidence was mostly similar across patient subgroups.
In conclusion, the phase III GRANITE-1 study did not meet its primary objective of demonstrating a significant survival benefit for everolimus compared with BSC in patients with advanced gastric cancer whose disease progressed after one or two lines of previous systemic chemotherapy. The everolimus AE profile was consistent with that observed for everolimus in other cancers.
Acknowledgment
We thank the patients who participated in the GRANITE-1 trial; the investigators, the study nurses, and the clinical research associates from the individual trial centers who provided ongoing support; Melanie Leiby, PhD (ApotheCom, Yardley, PA), for assistance with manuscript preparation; and Novartis Pharmaceuticals for supporting this trial and for funding medical editorial assistance on the manuscript.
Appendix
Supportive methodology: Handling of missing values.
For the primary end point of overall survival, if a patient was not known to have died, survival was censored at the date of last contact. For the secondary end point of progression-free survival (PFS), if a patient was not known to have died or experienced disease progression at the date of the analysis cutoff or when he/she received further antineoplastic therapy, PFS was censored at the time of the last adequate tumor assessment before the analysis cutoff date or the date of the start of new antineoplastic therapy, whichever occurred first. If a PFS event was observed after at least two missing tumor assessments, then the date of progression was censored at the date of the last adequate tumor assessment. If a PFS event occurred after a single missing tumor assessment, the actual date of disease progression was used. For the secondary end points of time to definitive deterioration of Eastern Cooperative Oncology Group (ECOG) performance status and time to definitive 5% deterioration in the global health status/quality of life scale of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30, if a patient died before definitive deterioration but within 8 weeks (ie, twice the planned period between two assessments), the date of death was considered as the event date; patients who died after more than 8 weeks were censored at the date of their last available assessment. If definitive deterioration was observed after at least two missing assessments, the event was backdated to the first missing assessment before deterioration. For each EORTC QLQ-C30 subscale, the raw scores were standardized as described in the third edition of the EORTC QLQ-C30 manual (Fayers P et al: The EORTC QLQ-C30 Scoring Manuscript [ed 3]. Brussels, Belgium, EORTC, 2001). No specific methodology was applied to handle individual missing answers to specific questions of the EORTC QLQ-C30.
Subgroup analyses.
For the primary end point of overall survival, analyses were performed for the following subgroups: number of prior chemotherapy lines (1 or 2), region (Asia or rest of world [ROW]), cross-classification of the number of prior chemotherapy lines and region (one prior regimen plus Asia; two prior regimens plus Asia; one prior regimen plus ROW, or two prior regimens plus ROW), baseline ECOG performance status (0, 1, or ≥ 2), sex (male or female), age (< 65 years or ≥ 65 years), race (white, Asian, or other), specific region (China, Japan, rest of Asia, Europe, or other), prior gastrectomy (yes or no), histology subtype (diffuse or intestinal), gastroesophageal junction involvement (yes or no), liver involvement (yes or no), lung involvement (yes or no), and prior chemotherapy (pyrimidine derivatives, platinum, or taxanes).
Results of the interim analysis.
A single interim analysis was performed after approximately 60% of the number of deaths required for final analysis was observed. At the time of the interim analysis, which occurred after 382 deaths were observed, the observed hazard ratio was 0.93 (95% CI, 0.75 to 1.16), and the P value from the stratified log-rank test was .266. This P value was greater than the .008 threshold required to stop the study for outstanding efficacy.
GRANITE-1 investigators.
Steering committee members: Atsushi Ohtsu (co-chair), Eric Van Cutsem (co-chair), Jaffer Ajani, Yung-Jue Bang, Lin Shen, Kun-Huei Yeh, Chiara Costantini, Syed Rizvi, Tarek Sahmoud, Heind Smith. Independent data monitoring committee members: Roberto Labianca, Ichinosuke Hyodo, Ian Ford. GRANITE-1 investigators: Argentina: G. Mendez, Hospital de Gastroenterologia Dr Carlos Bonorino Udaondo, Buenos Aires; N. Pilnik, Clínica Viedma SA, Sarmiento; R. Kowalyszyn, Fundación Rusculleda, Cordoba. Australia: N. Tebbutt, Austin Hospital, Heidelberg; T. Price, North Adelaide Oncology–Calvary North Adelaide Hospital, North Adelaide; P. Cooray, Box Hill Hospital, Box Hill and The Alfred Hospital, Prahran; L. Lipton, Western Hospital, Melbourne; D. Yip, The Canberra Hospital, Garran; A. Strickland, Monash Medical Centre, Clayton; M. Eastgate, Royal Brisbane & Women's Hospital, Herston; D. Kotasek, Ashford Cancer Centre, Ashford. Belgium: E. Van Cutsem and H. Prenen, University Hospitals Leuven and KU Leuven, Leuven; S. Laurent, Universitair Ziekenhuis Gent, Gent; M. Polus, C.H.U. Sart-Tilman, Liège; J.-L. Canon, Grand Hôpital de Charleroi, Charleroi. Canada: C. Brezden-Masely, St. Michael's Hospital, Oncology Clinical Research Group, Toronto; H. Lim, BC Cancer Agency, Vancouver Cancer Centre, Vancouver; J. Alcindor, McGill University, Montréal; S. Berry, Sunnybrook Health Sciences Centre, Toronto; G. Bebb, Tom Baker Cancer Centre, Calgary; J. Asselah, Centre Hospitalier Universitaire de Sherbrooke/CHUS–Hôpital Fleurimont, Sherbrooke. People's Republic of China: Y.-X. Bai, Tumor Hospital of Harbin Medical University, Harbin; H.-M. Pan, Sir Run Run Shaw Hospital, Hangzhou; J. Li, Cancer Hospital of Fudan University, Shanghai; J.-M. Xu, 307 Hospital of PLA, Beijing; S.-K. Qin, The 81st Hospital of PLA, Nanjing; R.-H. Xu, Sun Yat-Sen University Cancer Centre, Guangzhou; F. Bi, West China Hospital of Sichuan University, Chengdu; W. Liu, Hebei Medical University Fourth Hospital, Shijiazhuang; L. Shen, Peking University Cancer Hospital, Beijing; Y.-Z. Yuan, Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai; M. Tao, The First Affiliated Hospital of Soochow University, Jiangsu; J.-J. Wang, Shanghai Changzheng Hospital, Shanghai; R.-C. Luo, Nanfang Hospital, Guangzhou; Y.-P. Liu, The First Hospital of China Medical University, Shenyang. Colombia: C. Narvaez, Instituto Oncológico de Pasto, Nariño. France: O. Bouché, Hôpital Robert Debré, Paris; E. Francois, Centre Antoine Lacassagne, Nice; L. Mineur, Institut Sainte Catherine, Avignon; M. Ducreux, Institut Gustave Roussy, Villejuif; E. Terrebonne, Hôpital de Haut-Lévêque, Pessac; D. Pezet, CHU Hôtel Dieu, Nantes; J.F. Seitz, CHU La Timone, Marseille; M. Ychou, Centre Val d'Aurelle–Paul Lamarque, Montpellier; A. Ferru, CHU Poitiers, Poitiers; G. Lledo, Hôpital Privé Jean Mermoz, Lyon; E. Raymond, Hôpital Beaujon, Clichy; J. Taieb, Hôpital Georges Pompidou, Paris. Germany: S.-E. Al-Batran, Krankenhaus Nordwest Studienzentrale Haematologie/Onkologie, Frankfurt; P. Thuss-Patience, Charité Universitätsmedizin Berlin: Campus Virchow-Klinikum, Berlin; S. Probst, Städtische Kliniken Bielefeld, Bielefeld; M. Ebert, Klinikum rechts der Isar der TU München, Munich; M. Clemens, Krankenanstalt Mutterhaus der Borromäerinnen, Trier; M. Moehler, Johannes Gutenberg-Universtät Mainz, Mainz; R. Hofheinz, Universitätsmedizin Mannheim, Mannheim. Hong Kong, Special Administrative Region, People's Republic of China: K.-M. Chu, Queen Mary Hospital, Hong Kong. Israel: A. Shani, Sheba Medical Center, Tel Hashomer; E. Idilevich, Kaplan Hospital, Rehovot; A. Hubert, Hadassah Medical Organization–Ein Karem, Jerusalem; B. Brenner, Rabin Medical Center, Petah Tikva. Italy: G. Luppi, Azienda Ospedaliero Universita Policlinico di Modena and Universita Studi Modena e Reggio Emilia, Modena; A. Santoro, Istituto Clinico Humanitas, Milan; S. Del Prete, Ospedale San Giovanni di Dio, Frattamaggiore; F. Di Costanzo, Azienda Ospedaliero Universitaria Careggi, Florence; S. Frustaci, Centro di Riferimento Oncologico, Aviano. Japan: A. Ohtsu, T. Doi, National Cancer Center Hospital East, Kashiwa; K. Chin, Cancer Institute Hospital of JFCR, Tokyo; K. Muro, Aichi Cancer Center Hospital, Nagoya; Y. Hamamoto, E. Warita, Tochigi Cancer Center, Utsunomiya; T. Satoh, T. Kudo, Kinki University Hospital, Osaka; T. Nishina, National Hospital Organization Shikoku Cancer Center, Matsuyama; J. Furuse, Kyorin University Hospital, Tokyo; K. Yamaguchi, Saitama Cancer Center, Saitama; Y. Komatsu, Hokkaido University Hospital, Sapporo; H. Takiuchi, Osaka Medical College Hospital, Osaka; S. Kato, Tohoku University Hospital, Sendai; W. Koizumi, Kitasato University East Hospital, Sagamihara; Y. Yamada, National Cancer Center Hospital, Tokyo; K. Nakamura, Kyushu University Hospital, Fukuoka. Korea: H.-C. Chung, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul; Y.H. Kim, Korea University Anam Hospital, Seoul; J. Lee, Samsung Medical Center, Seoul; E.-K. Song, Chonbuk National University Hospital, Gwangju; Y.-J. Bang, Seoul National University Hospital, Seoul; M.H. Ryu, Asan Medical Center, Seoul; J.-G. Kim, Kyungpook National University Hospital, Daegu. Mexico: J. Gonzalez, Fundacion Rodolfo Padilla Padilla A.C., León; G. Calderillo, Insituto Nacional de Cancerología, Mexico City. Netherlands: D.J. Richel, Academisch Medisch Centrum Universiteit van Amsterdam, Amsterdam. New Zealand: M. Findlay, Cancer Trials New Zealand, Auckland. Peru: E. Alarcon, Clinica Anglo Americana, Lima; W. Rodriguez, Clinica Ricardo Palma, Lima; M. Olivera, Oncosalud, Lima. Russia: A. Garin, Russian Cancer Research Centre, Moscow; V. Moiseenko, N. N. Petrov Research Institute of Oncology, St Petersburg. Spain: J.M. Tabernero, Hospital Vall d'Hebron, Barcelona. Taiwan, Republic of China: K.-H. Yeh, National Taiwan University Hospital, Taipei; C.-P. Li, Taipei Veterans General Hospital, Taipei; Y.-Y. Chen, Chang Gung Memorial Hospital Kaohsiung, Kaohsiung City; J.-S. Chen, Chang Gung Memorial Hospital Linkou, Linkou; W.-T. Huang, Chi-Mei Hospital, Liouying. Thailand: V. Srimuninnimit, Siriraj Hospital, Bangkok; E. Sirachainan, Ramathibodi Hospital, Bangkok; P. Sunpaweravong, Prince of Songkla University, Phuket. United Kingdom: D. Ferry, New Cross Hospital, Wolverhampton; W. Mansoor, Christie NHS Foundation Trust, Manchester; J. Bridgewater, University College Hospital, London; R. Glynne-Jones, Mount Vernon Centre for Cancer Treatment, Northwood; R. Roy, Castle Hill Hospital, Cottingham; D. Cunningham, Royal Marsden Hospital, London. United States: J. Ajani, University of Texas MD Anderson Cancer Center, Houston, TX; P. Gada, University of Minnesota, Minneapolis, MN; J.T. Beck, Highlands Oncology Group, Fayetteville, AR.
Table A1.
Everolimus Steady-State Blood Concentrations by Actual Dose in the Safety Population
| Population | Cmin (ng/mL) |
Cmax (ng/mL) |
||||
|---|---|---|---|---|---|---|
| No. of Patients | Median | Range | No. of Patients | Median | Range | |
| Everolimus, 10 mg/d | ||||||
| Overall | 201 | 13.8 | 0-81.8 | 218 | 67.5 | 15.3-282.0 |
| Asia | 127 | 15.1 | 0-54.9 | 132 | 69.4 | 18.3-167.0 |
| ROW | 74 | 11.4 | 2.2-81.8 | 86 | 63.7 | 15.3-282.0 |
| With gastrectomy | 118 | 13.8 | 0-81.8 | 125 | 72.9 | 19.9-282.0 |
| Without gastrectomy | 83 | 13.2 | 2.6-60.3 | 93 | 53.8 | 15.3-157.0 |
| Everolimus, 5 mg/d | ||||||
| Overall | 18 | 9.3 | 2.1-24.3 | 16 | 34.7 | 6.3-98.9 |
| Asia | 11 | 9.8 | 2.1-21.2 | 10 | 29.1 | 6.3-81.0 |
| ROW | 7 | 6.3 | 4.0-24.3 | 6 | 34.7 | 12.3-98.9 |
| With gastrectomy | 10 | 9.0 | 4.9-24.3 | 9 | 41.9 | 14.8-81.0 |
| Without gastrectomy | 8 | 10.0 | 2.1-17.2 | 7 | 12.3 | 6.3-98.9 |
Abbreviations: Cmax, maximum concentration in whole blood; Cmin, minimum concentration in whole blood; ROW, rest of world.
Table A2.
Antineoplastic Therapies Since Discontinuation of Study Treatment in the Full Analysis Set
| Type of Therapy | Everolimus Plus BSC (n = 439) |
Placebo Plus BSC (n = 217) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Any | 172 | 39.2 | 98 | 45.2 |
| Type of therapy* | ||||
| Chemotherapy | 155 | 35.3 | 89 | 41.0 |
| Immunotherapy | 1 | 0.2 | 0 | 0 |
| Radiation therapy | 13 | 3.0 | 6 | 2.8 |
| Surgery | 0 | 0 | 1 | 0.5 |
| Targeted therapy | 5 | 1.1 | 1 | 0.5 |
| Other | 3 | 0.7† | 4 | 1.8‡ |
Abbreviation: BSC, best supportive care.
Patients could receive > 1 type of therapy.
Includes Chinese traditional medicine (n = 2) and Java Brucea fruit fat injection (n = 1).
Includes Chinese traditional medicine (n = 1), antineoplastic agents (n = 1), fluorouracil (n = 1), and PDK1 inhibitor (n = 1).
Table A3.
Analysis of Survival in the Full Analysis Set
| Survival | Everolimus Plus BSC (n = 439) |
Placebo Plus BSC (n = 217) |
Hazard Ratio | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||||
| Overall survival | .1244 | ||||||
| Deaths | 352 | 80.2 | 180 | 82.9 | 0.90 | 0.75 to 1.08 | |
| Censored | 87 | 19.8 | 37 | 17.1 | — | ||
| PFS | |||||||
| Total events | 386 | 87.9 | 206 | 94.9 | 0.66 | 0.56 to 0.78 | < .001 |
| Progression | 315 | 71.8 | 174 | 80.2 | — | ||
| Deaths | 71 | 16.2 | 32 | 14.7 | — | ||
| Censored | 53 | 12.1 | 11 | 5.1 | — | ||
Abbreviations: BSC, best supportive care; PFS, progression-free survival.
Footnotes
Supported by Novartis Pharmaceuticals.
Presented at the 48th Annual Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology, San Francisco, CA, January 19-21, 2012, the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012, and the 14th World Congress on Gastrointestinal Cancer, Barcelona, Spain, June 27-30, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00879333.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Atsushi Ohtsu, Bayer (C); Tarek Sahmoud, Novartis Pharmaceuticals (C); Heind Smith, Novartis Pharmaceuticals (C); Chiara Costantini, Novartis Pharma AG (C); Syed Rizvi, Novartis Pharmaceuticals (C); Syed Rizvi, Novartis Pharma KK (C); David Lebwohl, Novartis Pharmaceuticals (C) Consultant or Advisory Role: Atsushi Ohtsu, Taiko (C), Chugai-Roche (C), Novartis (C), GlaxoSmithKline (C), Takeda (C); Yung-Jue Bang, Novartis (C); Kun-Huei Yeh, Novartis (U); David Ferry, Novartis (C); Salah-Eddin Al-Batran, Novartis (C), sanofi-aventis (C), Roche Pharma AG (C) Stock Ownership: Tarek Sahmoud, Novartis; Heind Smith, Novartis; Syed Rizvi, Novartis; David Lebwohl, Novartis Honoraria: Yung-Jue Bang, Novartis; Kun-Huei Yeh, Novartis; David Ferry, Novartis; Salah-Eddin Al-Batran, Novartis, sanofi-aventis, Roche Pharma AG Research Funding: Jaffer A. Ajani, Novartis; Yung-Jue Bang, Novartis; Yeul Hong Kim, Novartis; Niall C. Tebbutt, Novartis; Salah-Eddin Al-Batran, Novartis, sanofi-aventis; Eric Van Cutsem, Novartis Expert Testimony: None Patents: None Other Remuneration: Heind Smith, Novartis
AUTHOR CONTRIBUTIONS
Conception and design: Atsushi Ohtsu, Yung-Jue Bang, Tarek Sahmoud, Lin Shen, Kun-Huei Yeh, Heind Smith, Syed Rizvi, Eric Van Cutsem
Provision of study materials or patients: Jaffer A. Ajani, Yu-Xian Bai, Hyun-Cheol Chung, Lin Shen, Kei Muro, David Ferry, Salah-Eddin Al-Batran, Eric Van Cutsem
Collection and assembly of data: Atsushi Ohtsu, Jaffer A. Ajani, Yu-Xian Bai, Yung-Jue Bang, Hyun-Cheol Chung, Hong-Ming Pan, Tarek Sahmoud, Lin Shen, Kun-Huei Yeh, Keisho Chin, Kei Muro, Yeul Hong Kim, Niall C. Tebbutt, Salah-Eddin Al-Batran, Heind Smith, Syed Rizvi, David Lebwohl, Eric Van Cutsem
Data analysis and interpretation: Atsushi Ohtsu, Yung-Jue Bang, Tarek Sahmoud, Lin Shen, Kun-Huei Yeh, David Ferry, Niall C. Tebbutt, Chiara Costantini, Heind Smith, Syed Rizvi, Eric Van Cutsem
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A Bray F Center MM, etal: Global cancer statistics CA Cancer J Clin 61:69–90,2011 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer (including cancer in the proximal 5cm of the stomach), version 2.2012. http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- 3.Okines A Verheij M Allum W, etal: Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 21:v50–v54,2010suppl 5 [DOI] [PubMed] [Google Scholar]
- 4.Sasako M Inoue M Lin JT, etal: Gastric Cancer Working Group report Jpn J Clin Oncol 40:i28–i37,2010suppl 1 [DOI] [PubMed] [Google Scholar]
- 5.Howlander N Noone AM Krapcho M, etal: SEER Cancer Statistics Review, 1975-2008 http://seer.cancer.gov/csr/1975_2008
- 6.Inoue M, Tsugane S: Epidemiology of gastric cancer in Japan Postgrad Med J 81:419–424,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS Lee H Jeung HC, etal: Advanced detection of recent changing trends in gastric cancer survival: Up-to-date comparison by period analysis Jpn J Clin Oncol 41:1344–1350,2011 [DOI] [PubMed] [Google Scholar]
- 8.Catalano V Labianca R Beretta GD, etal: Gastric cancer Crit Rev Oncol Hematol 71:127–164,2009 [DOI] [PubMed] [Google Scholar]
- 9.Jemal A Siegel R Xu J, etal: Cancer statistics, 2010 CA Cancer J Clin 60:277–300,2010 [DOI] [PubMed] [Google Scholar]
- 10.Wagner AD Unverzagt S Grothe W, etal: Chemotherapy for advanced gastric cancer Cochrane Database Syst Rev 3:CD004064,2010 [DOI] [PubMed] [Google Scholar]
- 11.Thuss-Patience PC Kretzschmar A Bichev D, etal: Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer 47:2306–2314,2011 [DOI] [PubMed] [Google Scholar]
- 12.Kang JH Lee SI Lim do H, etal: Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone J Clin Oncol 30:1513–1518,2012 [DOI] [PubMed] [Google Scholar]
- 13.Oki E Baba H Tokunaga E, etal: Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer Int J Cancer 117:376–380,2005 [DOI] [PubMed] [Google Scholar]
- 14.Lang SA Gaumann A Koehl GE, etal: Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model Int J Cancer 120:1803–1810,2007 [DOI] [PubMed] [Google Scholar]
- 15.Xu DZ Geng QR Tian Y, etal: Activated mammalian target of rapamycin is a potential therapeutic target in gastric cancer BMC Cancer 10:536,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu G Wang J Chen Y, etal: Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer Clin Cancer Res 15:1821–1829,2009 [DOI] [PubMed] [Google Scholar]
- 17.An JY Kim KM Choi MG, etal: Prognostic role of p-mTOR expression in cancer tissues and metastatic lymph nodes in pT2b gastric cancer Int J Cancer 126:2904–2913,2010 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ Escudier B Oudard S, etal: Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors Cancer 116:4256–4265,2010 [DOI] [PubMed] [Google Scholar]
- 19.Yao JC Shah MH Ito T, etal: Everolimus for advanced pancreatic neuroendocrine tumors N Engl J Med 364:514–523,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baselga J Campone M Piccart M, etal: Everolimus in postmenopausal hormone receptor-positive advanced breast cancer N Engl J Med 366:520–529,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franz DN Belousova E Sparagana S, etal: Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicenter, randomised, placebo-controlled phase 3 trial Lancet 381:125–132,2013 [DOI] [PubMed] [Google Scholar]
- 22.Bissler JJ Kingswood JC Radzikowska E, etal: Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: A multicenter, randomized, double-blind, placebo-controlled trial Lancet 10.1016/S0140-6736(12)61767-X [DOI] [PubMed] [Google Scholar]
- 23.Taguchi F Kodera Y Katanasaka Y, etal: Efficacy of RAD001 (everolimus) against advanced gastric cancer with peritoneal dissemination Invest New Drugs 29:1198–1205,2011 [DOI] [PubMed] [Google Scholar]
- 24.Jaeger-Lansky A Cejka D Ying L, etal: Effects of vatalanib on tumor growth can be potentiated by mTOR blockade in vivo Cancer Biol Ther 9:919–927,2010 [DOI] [PubMed] [Google Scholar]
- 25.Yeh KH Chiang YW Lin CS, etal: Chemosensitizing effects and sustained G1-S cell cycle arrest by low-dose RAD001 (everolimus) for cisplatin and 5-fluorouracil in human gastric cancer cells Proc Am Assoc Cancer Res 58:957,2007abstr 4043 [Google Scholar]
- 26.Cejka D Preusser M Fuereder T, etal: mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo Anticancer Res 28:3801–3808,2008 [PubMed] [Google Scholar]
- 27.Lee KH Hur HS Im SA, etal: RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase Cancer Lett 299:22–28,2010 [DOI] [PubMed] [Google Scholar]
- 28.Doi T Muro K Boku N, etal: Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer J Clin Oncol 28:1904–1910,2010 [DOI] [PubMed] [Google Scholar]
- 29.Oken MM Creech RH Tormey DC, etal: Toxicity and response criteria of the Eastern Cooperative Oncology Group Am J Clin Oncol 5:649–655,1982 [PubMed] [Google Scholar]
- 30.Therasse P Arbuck SG Eisenhauer EA, etal: New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92:205–216,2000 [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute. Cancer Therapy Evaluation Program: Common terminology criteria for adverse events, version 3.0, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 32.Lan KKG, DeMets DL: Discrete sequential boundaries for clinical trials Biometrika 70:659–663,1983 [Google Scholar]
- 33.Yoon DH Ryu MH Park YS, etal: Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum Br J Cancer 106:1039–1044,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]