Abstract
The present study aimed to investigate the effect of allyl isothiocyanate (AITC) on the viability and apoptosis of the human cervical cancer HeLa cell line in vitro, and to explore the potential underlying mechanisms of this. HeLa cells were treated with varying concentrations of AITC for different durations. The cell viability was then measured using a Cell Counting kit-8 assay and the apoptosis rate of the cells was detected using flow cytometry. Additionally, the B cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax) mRNA expression levels were determined by reverse transcription-quantitative polymerase chain reaction, while the Bax and Bcl-2 protein expression levels in cells were detected by western blot analysis. AITC was revealed to inhibit the viability of HeLa cells. AITC was revealed to induce the apoptosis of HeLa cells, as the apoptosis rate increased gradually with an increase in the dose. As the concentration of AITC increased, the Bax mRNA expression level increased, whilst the Bcl-2 mRNA expression level decreased. Furthermore, the Bax protein expression intensity increased whilst Bcl-2 protein expression intensity decreased, thereby resulting in a decrease in the ratio of Bcl-2/Bax proteins. AITC may inhibit cell viability by inducing the apoptosis of HeLa cells and this may be accounted for by the imbalance in the Bcl-2/Bax expression ratio.
Keywords: allyl isothiocyanate, HeLa cells, apoptosis, viability
Introduction
Globally, ~200,000 women succumb to mortality from cervical cancer every year and the age of onset of this disease has decreased over time (1), indicating that it presents a notable threat to the health of women. At present, the treatment methods for cervical cancer include surgical treatment, chemotherapy, radiotherapy, biological therapy and traditional Chinese medicine. In clinical practice, surgical treatment with radiotherapy or chemotherapy is the gold-standard. However, this treatment option has a number of shortcomings, including damage to the body and toxicity. Therefore, it is of interest to identify a novel treatment method for cervical cancer.
Allyl isothiocyanate (AITC) is widely distributed in cruciferous plants and their by-products, including mustard and horseradish (2,3). A number of studies have considered AITC to be associated with tumors. For instance, AITC may arrest cancer cells in mitosis, which in turn leads to apoptosis via B-cell lymphoma 2 (Bcl-2) protein phosphorylation (4–6). AITC may affect the proliferation of various tumor cells by inducing apoptosis and cell cycle arrest, or by inhibiting their invasion and metastasis, including in breast (7), bladder (8–10), lung (11), colon (12,13), liver (14,15) and prostate cancer cells (16). On the basis of the inhibitory effect AITC has on the proliferation of bladder cancer cells, population-based survey results have suggested that the intake of cruciferous plants may improve the survival rate of patients with bladder cancer (17), which may provide further evidence for the anticancer effect of AITC. However, few studies have reported on whether AITC has anticancer effects on cervical cancer. Therefore, in the present study, cervical cancer HeLa cells were treated with varying concentrations of AITC in order to investigate the effect of AITC on the viability and apoptosis of HeLa cells. In addition, the gene expression of Bcl-2 and Bcl-2-associated X protein (Bax), which are associated with apoptosis, was detected and the potential mechanisms underlying the effects of AITC on the apoptosis of HeLa cells was preliminarily discussed.
Materials and methods
Culture of HeLa cells
The cervical cancer HeLa cell line was sourced from the American Type Culture Collection (Manassas, VA, USA) and was cultured in Dulbecco's modified Eagle medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), prior to being incubated with 5% CO2 at 37°C. Cells were passaged every 2–3 days, during which the culture medium in the culture dish was removed and discarded, and the cells were rinsed with phosphate-buffered saline (PBS) twice, prior to being digested with trypsin for 1 min at 37°C. Subsequently, the adherent cells were pipetted into single cell suspensions in PRMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) and centrifuged at 1,000 × g for 5 min at 37°C. The supernatant was discarded. The cells were resuspended with 1 ml culture medium and then 200 µl was removed for subculture (subculture ratio 1:5).
Cell viability using a Cell Counting kit-8 (CCK8) assay
The HeLa cells, which had been digested and counted during the logarithmic growth phase, were seeded onto 96-well plates at a density of 1×104 cells/well. After 12 h of incubation, the cells were treated with varying concentrations (0, 5, 15 and 45 µM) of AITC (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The treatment duration was set as 24, 48 and 72 h at a temperature of 37°C. A total of 5 replicate wells were used for each concentration and a blank control group was treated with an equivalent volume of PBS (7). Following treatment, the cells were rinsed using PBS three times and 100 µl CCK8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well (the ratio of CCK8 reagent to medium was 1:10). The cells were incubated at 37°C in the dark for 2 h. Subsequently, the absorbance values were determined using an enzyme microplate reader at a wavelength of 450 nm and the cell viability rate was calculated according to following expression: Cell viability rate=(Atreatment group-Ablank control group)/(Acontrol group-Ablank control group), where A denotes the absorbance value.
Apoptosis detection by flow cytometry
The HeLa cell suspension was added to a 6-well plate at a density of 1×105 cells/well. After 12 h of incubation, the cells were treated with varying concentrations of AITC (0, 5, 15 and 45 µM) for 48 h. A total of 3 parallel samples were used for each concentration. Following completion of the treatment, the cells were digested with trypsin for 1 min at 37°C, washed twice with PBS, centrifuged at 1,000 × g for 5 min at 37°C and resuspended in PBS. Subsequently, 1×105 cells were counted and centrifuged again at 1,000 × g for 5 min at 37°C. The supernatant was discarded and 195 µl Annexin V-fluorescein isothiocyanate (FITC), as part of a cell apoptosis detection kit (Beyotime Institute of Biotechnology, Haimen, China) was added for resuspension and agitated gently to mix following the addition of 5 µl Annexin V-FITC. The mixture was gently agitated to mix again following the addition of 10 µl propidium iodide staining solution (part of the aforementioned cell apoptosis detection kit). Next, the mixture was incubated at room temperature in the dark for 20 min, prior to being placed in an ice bath at 0°C for 20 min. The apoptosis rate was subsequently detected using a Gallios™ flow cytometer (Version no. A75199AA; BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FCS Express 3.0 (DeNovo software, Glendale, CA, USA) and a cell apoptosis detection kit (Beyotime Institute of Biotechnology, Haimen, China).
Detection of Bax and Bcl-2 mRNA levels
The concentrations of AITC were set to 0, 5, 15 and 45 µM. After 48 h of treatment at 4°C, total RNA was extracted from the HeLa cells using the TRIzol RNA extraction reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The purity and content of RNA were detected using a nucleic acid and protein analyzer. At the same time, the integrity of RNA was identified by 1% agarose gel electrophoresis (Abcam, Cambridge, MA, USA). RNA (1 µg) was used for cDNA synthesis through reverse transcription using a reverse transcription-quantitative polymerase chain reaction (RT-qPCR) kit (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's protocols. The primers of all genes were as follows: Bax forward, TCCTCATCGCCATGCTCAT and reverse, CCTTGGTCTGGAAGCAGAAGA; Bcl-2 forward, GATGACCGAGTACCTGAACC and reverse, CAGGAGAAATCGAACAAAGGC; and β-actin forward, TGCTGTGTTCCCATCTATCG and reverse, TTGGTGACAATACCGTGTTCA. The 2−∆∆Cq method was used for quantification (18), and an RT-qPCR reaction system was established according to the following conditions: 5 µl 2 X SYBR-Green mixture (Qiagen GmbH, Hilden, Germany), 0.5 µl cDNA, 0.5 µl primer and 4 µl purified H2O. Reaction conditions were as follows: Following pre-denaturation at 95°C for 10 min (denaturation at 95°C for 15 sec, annealing and extension at 60°C for 60 sec), 40 PCR cycles were performed on a ViiA7 quantitative fluorescence PCR machine (ABI Corporation, Lee's Summit, MO, USA) at 95°C for 5 sec, 55°C for 30 sec and 72°C for 20 sec. A total of 3 parallel samples were set for each experiment, and β-actin was used as the internal control gene.
Detection of Bax and Bcl-2 protein levels using western blot analysis
The concentrations of AITC were set to 0, 5, 15 and 45 µM. Following 48 h of treatment at 37°C, the cells were added to cell lysis solution (155 mM ammonium chloride, 10 mM sodium bicarbonate and 0.5 mM EDTA; Miltenyi Biotec, Inc., Auburn, CA, USA) for 2 h at 37°C, and placed in a homogenizer for homogenization. Total cell protein was extracted using a total protein extraction kit (BestBio Co., Shanghai, China), according to the manufacturer's protocol, and was centrifuged at 1,000 × g for 5 min at 37°C, prior to the supernatant being obtained. The amount of protein was determined using the Coomassie brilliant blue protein assay kit (Shanghai Majorbio Pharmaceutical Technology Co., Ltd., Shanghai, China), according to the manufacturer's protocol. Next, the Laemmli sample buffer, containing 60 mM Tris-Cl (pH 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol and 0.01% bromophenol blue (Sigma-Aldrich; Merck KGaA), was added and boiled at 100°C. SDS-PAGE gel (6–12%; Sigma-Aldrich; Merck KGaA) was prepared as follows: The protein sample (50 µg) was loaded for electrophoretic separation for 3 h and then placed into the electric transducers, in which the transfer buffer was added to the transmembrane for 1.5 h and the target protein was transferred onto nitrocellulose (NC) membranes. Non-specific binding was blocked using PBS with Tween (PBST; Sigma-Aldrich; Merck KGaA) containing 5% skimmed milk powder, and agitated at room temperature for 2 h. Bcl-2 (dilution, 1:100; cat. no. MS-123-A1; Biotium, Inc., Freemont, CA, USA), Bax (dilution, 1:100; cat. no. Rb-1486-R1; Biotium, Inc.) and GAPDH antibodies (dilution, 1:100; cat. no. MS-168-P1; Biotium, Inc.) were utilized. The membrane was washed 3 times with PBST and the horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (dilution, 1:1,000; cat. no. A-21422; Sigma-Aldrich; Merck KGaA) was added and incubated in a 4°C refrigerator overnight. The membrane was washed using PBST for 30 min, and the secondary horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (dilution, 1:1,000; cat. no. A-2418, Sigma-Aldrich; Merck KGaA), prepared with PBST (containing 2.5% skimmed milk powder), was added and was incubated in a shaker at 58°C for 60 min. The NC membranes were washed with PBST 3 times and was then evenly coated with reagents from an enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA) in a darkroom for 5 min. Following washing, the film was placed in a fixing solution (40% methanol and 7% acetic acid) for 5 min at 37°C, and hung to dry following flushing. The gel imaging system was sourced from ABI Corporation. The optical densities of the protein bands of Bax, Bcl-2 and GADPH were analyzed using the GIS-2020D gel image analysis system (Sigma-Aldrich; Merck KGaA), while the expression intensities of Bax and Bcl-2 proteins were expressed as the ratio of the optical density of the Bax protein band to the optical density of the β-actin protein band and the ratio of the optical density of the Bcl-2 protein band to the optical density of the β-actin protein band by using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
Statistical methods
Data were statistically analyzed using SPSS 19.0 software (IBM Corp.). Normally distributed data are presented as the mean ± standard deviation. The normally distributed data in different groups were compared using one-way analysis of variance and the data of two groups were compared using Fisher's least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of AITC on the viability of Hela cells
HeLa cells were treated with varying concentrations of AITC for different durations, and a CCK8 assay was conducted to determine the effect of AITC on the viability of HeLa cells, the results of which are presented in Table I and Fig. 1. The results revealed that AITC inhibited the viability of HeLa cells, an effect that was most significant when the cells were treated with 45 µM AITC for 72 h (P=0.024).
Table I.
Effect of AITC on the viability of HeLa cells.
| Treatment duration, h | |||
|---|---|---|---|
| Treatment concentration, µM | 24 | 48 | 72 |
| 0 | 1.00±0.042 | 1.00±0.043 | 1.00±0.041 |
| 5 | 0.98±0.036 | 0.88±0.040a,b | 0.79±0.042a,b |
| 15 | 0.89±0.043a | 0.71±0.037a,b | 0.60±0.041a,b |
| 45 | 0.76±0.045a | 0.62±0.038a,b | 0.48±0.039a,b |
P<0.05 vs. 0 µM AITC
P<0.05 vs. 24 h. AITC, allyl isothiocyanate.
Figure 1.
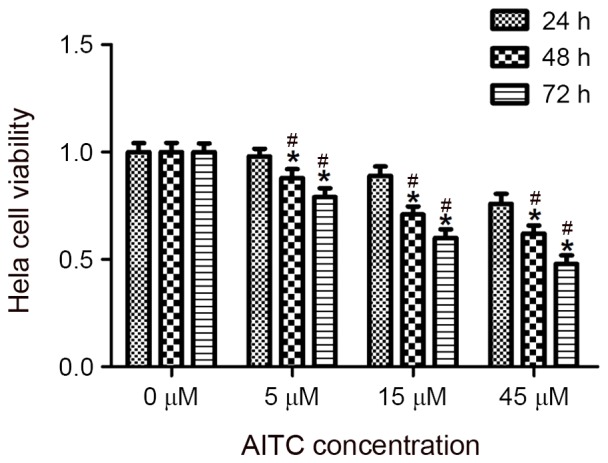
Effect of AITC on the viability of HeLa cells. *P<0.05, vs. 0 µM AITC; #P<0.05 vs. 24 h treatment group. AITC, allyl isothiocyanate.
Effect of AITC on the apoptosis of HeLa cells
HeLa cells were treated with 0, 5, 15 and 45 µM AITC for varying durations and the apoptosis rates of HeLa cells in different groups were detected using flow cytometry, the results of which are presented in Fig. 2 (in which the right upper quadrant presents the results for late apoptotic cells and the right lower quadrant presents the results for early apoptotic cells) and Fig. 3. As determined by flow cytometry, the apoptosis rate in the control group was 5.08±1.12%, which was significantly lower compared with 10.65±1.98, 17.49±1.68 and 24.26±1.83% in the 5, 15 and 45 µM AITC treatment groups, respectively. The apoptosis rates in the treatment groups were significantly higher than the apoptosis rate in the control group at all drug concentrations (P<0.05).
Figure 2.
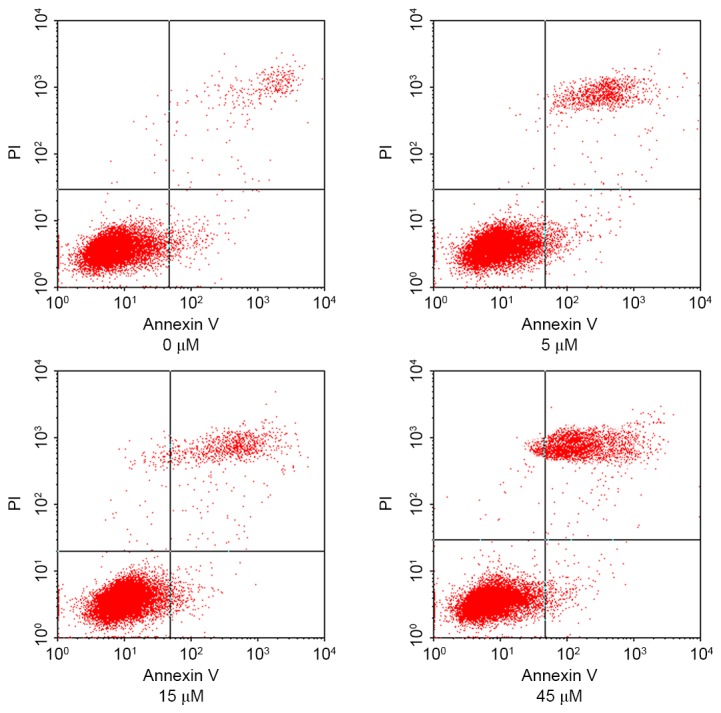
Effect of differing concentrations of allyl isothiocyanate on the apoptosis rate of cells. PI, propidium iodide.
Figure 3.
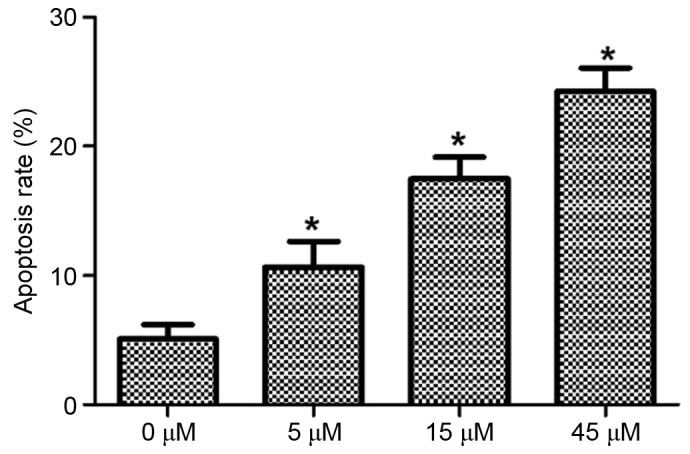
Comparison of the apoptosis rate with differing treatment concentrations. *P<0.05 vs. 0 µM. Allyl isothiocyanate.
Effects of AITC on Bax and Bcl-2 mRNA expression in HeLa cells
With the 0 µM AITC treatment group serving as a control and β-actin acting as an internal reference gene, Bax and Bcl-2 mRNA expression levels in HeLa cells were detected using a semi quantitative method following 48 h of AITC treatment, the results of which are presented in Fig. 4. Compared with the control group, the Bcl-2 mRNA expression level was significantly decreased in the 15 and 45 µM treatment groups (P<0.05). The differences between the treatment groups (at all concentrations) and the control group were statistically significant (P<0.05).
Figure 4.
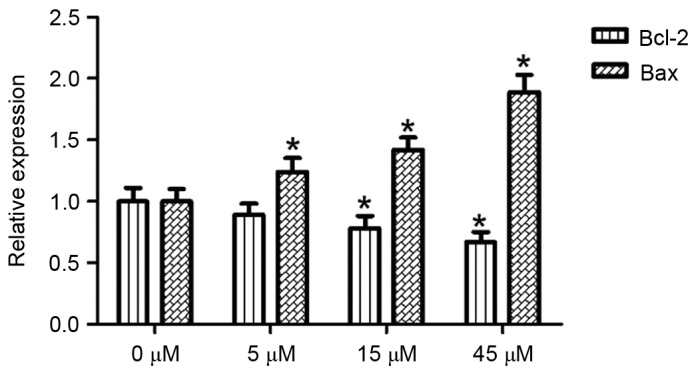
Effects of AITC treatment on the Bax and Bcl-2 mRNA expression levels *P<0.05 vs. 0 µM AITC. AITC, allyl isothiocyanate; Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein.
Effects of AITC on Bax and Bcl-2 protein expression in HeLa cells
Following treatment with 0, 5, 15 and 45 µM AITC for 48 h, Bax and Bcl-2 protein expression levels in HeLa cells were detected. As illustrated in Fig. 5 and Table II, compared with the control group, the expression level of Bax protein was significantly upregulated, whereas the expression level of Bcl-2 protein was significantly downregulated following AITC treatment (all P<0.001).
Figure 5.
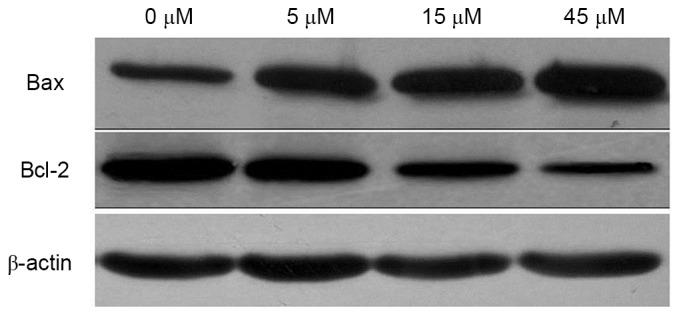
Effects of allyl isothiocyanate treatment on Bax and Bcl-2 protein expression levels. Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein.
Table II.
Effects of AITC treatment on Bax and Bcl-2 protein expression levels.
| Concentration, µM | |||||
|---|---|---|---|---|---|
| Protein | 0 | 5 | 15 | 45 | P-value |
| Bax | 0.58±0.07 | 0.71±0.06a | 0.83±0.04a | 1.02±0.08a | <0.001 |
| Bcl-2 | 0.98±0.09 | 0.89±0.08 | 0.65±0.07a | 0.50±0.05a | <0.001 |
| Bcl-2/Bax | 1.69±0.14 | 1.25±0.13a | 0.78±0.11a | 0.49±0.12a | <0.001 |
P<0.05 vs. 0 µM AITC. AITC, allyl isothiocyanate; Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X apoptosis regulator.
Discussion
At present, the drugs used for the treatment of cervical cancer are often highly toxic and induce severe side effects, which may cause damage to patient tissues during the treatment of cancer and may reduce the chance of survival (2,3). Therefore, the identification of a safe, effective and low toxicity anticancer drug has attracted interest from researchers, and the development of these drugs from natural sources has become an important strategy in the development of antitumor drugs. As a type of plant chemical that is prevalent in the natural diet of humans, AITC may affect the proliferation of a variety of tumor cells (19,20), and has attracted extensive attention from researchers. The anticancer effect of AITC has been verified by studies in vivo and in vitro (21,22), but the function of AITC in the treatment of cervical cancer has not been reported. Therefore, the present study confirmed the effect of AITC on cervical cancer cells through cell line experiments and preliminarily explored the underlying mechanisms of this.
The inhibitory effect on cell viability was most significant when the cells were treated with 45 µM AITC for 72 h (P=0.024). At present, the effect of AITC on HeLa cells has not previously been reported. However, a similar study was reported by Hasegawa et al (23), which revealed that isothiocyanate, including phenyl-ethyl isothiocyanate and benzyl isothiocyanate, may inhibit HeLa cell viability through cell cycle arrest. AITC is a type of isothiocyanate, which means that the conclusion of the present study is consistent with that of Hasegawa et al (23). In addition, when HeLa cells were treated with AITC for 48 h, the apoptosis rate of cells exhibited a dose-dependent increase, suggesting that AITC may inhibit HeLa cell viability by inducing cell apoptosis. However, the mechanism of AITC inhibiting cell viability is complex. For example, AITC may inhibit HeLa cell viability through cell cycle arrest and the induction of cell apoptosis.
Bax and Bcl-2 have attracted attention due to their regulation of cell apoptosis, and their function in promoting or inhibiting cell apoptosis may be realized through their protein expression (24). Bax and Bcl-2 are a pair of positive-negative apoptosis regulating genes. In addition to promoting cell apoptosis, Bax may also reduce the inhibitory effect of Bcl-2 on cell apoptosis by forming a dimer with Bcl-2 (25). Therefore, the occurrence of cell apoptosis is associated with an imbalance of Bcl-2/Bax expression. The present study revealed that AITC may induce the apoptosis of HeLa cells. By detecting Bcl-2 and Bax expression levels, it was demonstrated that, with an increase in the concentration of AITC, the expression levels of Bax mRNA and protein increased whilst Bcl-2 mRNA and protein expression levels decreased, resulting in a decrease in the ratio of Bcl-2/Bax proteins. The results of the present study suggested that the apoptosis of HeLa cells induced by AITC may be associated with an imbalance in Bcl-2/Bax expression. Sávio et al (8) also revealed that the apoptosis induced by AITC was associated with a Bcl-2/Bax expression imbalance.
The present study demonstrated that AITC may inhibit HeLa cell viability through the induction of cell apoptosis and that the imbalance of Bcl-2/Bax expression may be the mechanism accounting for AITC-induced cell apoptosis, which may provide evidence for further research for novel anticancer drugs for cervical cancer. However, the results of the present study remain far from being used for clinical applications and require further verification in future studies.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Xu K, Thornalley PJ. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol. 2000;60:221–231. doi: 10.1016/S0006-2952(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya A, Li Y, Wade KL, Paonessa JD, Fahey JW, Zhang Y. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis. 2010;31:2105–2110. doi: 10.1093/carcin/bgq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng F, Tang L, Li Y, Yang L, Choi KS, Kazim AL, Zhang Y. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J Biol Chem. 2011;286:32259–32267. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 6.Wu CL, Huang AC, Yang JS, Liao CL, Lu HF, Chou ST, Ma CY, Hsia TC, Ko YC, Chung JG. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 7.Bo P, Lien JC, Chen YY, Yu FS, Lu HF, Yu CS, Chou YC, Yu CC, Chung JG. Allyl isothiocyanate induces cell toxicity by multiple pathways in human breast cancer cells. Am J Chin Med. 2016;44:415–437. doi: 10.1142/S0192415X16500245. [DOI] [PubMed] [Google Scholar]
- 8.Sávio AL, da Silva GN, Salvadori DM. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil) Mutat Res. 2015;771:29–35. doi: 10.1016/j.mrfmmm.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya A, Li Y, Geng F, Munday R, Zhang Y. The principal urinary metabolite of allyl isothiocyanate, N-acetyl-S-(N-allylthiocarbamoyl) cysteine, inhibits the growth and muscle invasion of bladder cancer. Carcinogenesis. 2012;33:394–398. doi: 10.1093/carcin/bgr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savio AL, da Silva GN, de Camargo EA, Salvadori DM. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 gene expression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil) Mutat Res. 2014;762:40–46. doi: 10.1016/j.mrfmmm.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Tripathi K, Hussein UK, Anupalli R, Barnett R, Bachaboina L, Scalici J, Rocconi RP, Owen LB, Piazza GA, Palle K. Allyl isothiocyanate induces replication-associated DNA damage response in NSCLC cells and sensitizes to ionizing radiation. Oncotarget. 2015;6:5237–5252. doi: 10.18632/oncotarget.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau WS, Chen T, Wong YS. Allyl isothiocyanate induces G2/M arrest in human colorectal adenocarcinoma SW620 cells through down-regulation of Cdc25B and Cdc25C. Mol Med Rep. 2010;3:1023–1030. doi: 10.3892/mmr.2010.363. [DOI] [PubMed] [Google Scholar]
- 13.Lai KC, Lu CC, Tang YJ, Chiang JH, Kuo DH, Chen FA, Chen IL, Yang JS. Allyl isothiocyanate inhibits cell metastasis through suppression of the MAPK pathways in epidermal growth factor-stimulated HT29 human colorectal adenocarcinoma cells. Oncol Rep. 2014;31:189–196. doi: 10.3892/or.2013.2865. [DOI] [PubMed] [Google Scholar]
- 14.Hwang ES, Kim GH. Allyl isothiocyanate influences cell adhesion, migration and metalloproteinase gene expression in SK-Hep1 cells. Exp Biol Med (Maywood) 2009;234:105–111. doi: 10.3181/0806-RM-190. [DOI] [PubMed] [Google Scholar]
- 15.Garcia A, Haza AI, Arranz N, Rafter J, Morales P. Protective effects of isothiocyanates alone or in combination with vitamin C towards N-nitrosodibutylamine or N-nitrosopiperidine-induced oxidative DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J ApplToxicol. 2008;28:196–204. doi: 10.1002/jat.1270. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, Nair S, Kong AN. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 17.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Louhivuori LM, Bart G, Larsson KP, Louhivuori V, Näsman J, Nordström T, Koivisto AP, Akerman KE. Differentiation dependent expression of TRPA1 and TRPM8 channels in IMR-32 human neuroblastoma cells. J Cell Physiol. 2009;221:67–74. doi: 10.1002/jcp.21828. [DOI] [PubMed] [Google Scholar]
- 20.Chen NG, Chen KT, Lu CC, Lan YH, Lai CH, Chung YT, Yang JS, Lin YC. Allyl isothiocyanate triggers G2/M phase arrest and apoptosis in human brain malignant glioma GBM 8401 cells through a mitochondria-dependent pathway. Oncol Rep. 2010;24:449–455. doi: 10.3892/or_00000878. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Zhuang JX, Wang Q, Zhang HY, Yang P. Inhibitory effect of benzyl isothiocyanate on proliferation in vitro of human glioma cells. Asian Pac J Cancer Prev. 2013;14:2607–2610. doi: 10.7314/APJCP.2013.14.4.2607. [DOI] [PubMed] [Google Scholar]
- 22.Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: Recent advances. Mol Nutr Food Res. 2014;58:1685–1707. doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa T, Nishino H, Iwashima A. Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anticancer Drugs. 1993;4:273–279. doi: 10.1097/00001813-199304000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 25.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]


