Abstract
The present study aimed to determine the levels of prothrombin induced by vitamin K absence-II (PIVKA-II) according to the Barcelona Clinic Liver Cancer (BCLC) staging system, to develop an appropriate strategy for managing hepatocellular carcinoma (HCC), particularly early HCC, and to investigate the value of PIVKA-II for predicting prognosis-associated pathological parameters. Clinical information of 117 patients with hepatitis B-associated HCC was retrospectively collected. Preoperative serum PIVKA-II and α-fetoprotein (AFP) levels were measured using a chemiluminescence method. The efficiency of PIVKA-II levels for predicting pathological parameters was evaluated using step-wise logistic regression. The receiver operator characteristic curve was used to evaluate the predictive performance of PIVKA-II levels. It was demonstrated that except for the difference between stages B and C HCC (P=0.923), serum PIVKA-II levels significantly increased according to BCLC stage (P<0.050), however AFP levels did not. In early HCC (stage 0+A), the correlation between PIVKA-II and AFP levels (dual-positive, 64.70% in stage 0; 46.97% in stage A) was relatively weak (r=0.410). PIVKA-II >40 mAU/ml was an independent predictor of microvascular invasion [hazard ratio (HR), 3.77; 95% confidence interval (CI), 1.31–10.88; P=0.014; and high Ki67 expression in situ (HR, 2.99; 95% CI, 1.19–7.52; P=0.020). Combined analysis of PIVKA and AFP levels may contribute to an effective strategy for the management of patients with early HCC, as high PIVKA-II levels indicated a more aggressive tumor phenotype. Further investigation of PIVKA-II levels may provide novel insights into the mechanism underlying the metastasis of HCC cells and facilitate the development of novel therapeutic strategies for HCC.
Keywords: hepatocellular carcinoma, PIVKA-II, microvascular invasion, proliferation, Ki67
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies leading to death worldwide (1). Although the diagnosis and treatment of HCC have been improved, prognosis is poor (2). The death rate of patients with HCC was nearly constant from 2000 to 2011 (3). Moreover, HCC was estimated to represent the third-leading cause in China of malignancy-related death subsequent to 2011 (3). Poor prognosis is partly related to the initial diagnosis of HCC when the tumor stage is advanced, indicating the likelihood that curative resection is not possible (4–7). Further, the high incidence of metastasis and recurrence after curative resection significantly impairs the efficacy of treatment (8–10). Therefore, there is an urgent need to develop an accurate tool to aid early diagnosis as well as to predict prognosis to improve the outcomes of patients with HCC.
Serum proteins are considered to the most clinically applicable markers for routine analyses, because they offer the advantages of noninvasiveness, cost-effectiveness, and high reproducibility (11). Currently, α-fetoprotein (AFP) is the most frequently used biomarker for HCC surveillance (4,5). However, because of its unsatisfactory performance, even using the optimal cutoff value (10–20 ng/ml) (12), AFP is not recommended as an adjunct to abdominal ultrasound (4,13). Prothrombin induced by vitamin K absence-II (PIVKA-II), also known as des-γ-carboxy prothrombin, is a useful marker for diagnosing HCC, and elevated levels of PIVKA-II are associated with poor prognosis of patients undergoing different treatments (14–18). Moreover, PIVKA-II performs better compared with AFP for the diagnosis and prognosis of HCC (18,19) and therefore is considered a promising biomarker for managing HCC. However, the clinical significance of PIVKA-II was mainly assessed by studies of Japanese patients with HCC (20–22), and the use of PIVKA-II levels to assess Chinese patients is limited. Moreover, most Chinese HCC patients are infected with hepatitis B virus (HBV). In contrast, the majority of patients in Japan are infected with hepatitis C virus (HCV), which may bias the interpretation of PIVKA-II data acquired by studies of a Japanese cohort (23). Therefore, it is essential to conduct a study mainly focusing on Chinese patients with HBV-related HCC to evaluate the clinical significance of PIVKA-II levels.
Pathological parameters such as differentiation, microvascular invasion (MVI), and Ki67 expression level are major risk factors for tumor recurrence and mortality of patients with HCC (24–26). However, these parameters are detectable only through microscopic examination of surgical specimens, and correct interpretation of the findings requires experienced pathologists. Moreover, predicting such parameters is a major issue in assessing the prognosis of patients with HCC. Therefore, the discovery of a serum biomarker for predicting prognosis-related pathological parameters will likely enhance management of HCC. Unfortunately, we are unaware of any relevant published data for Chinese patients with HBV-associated HCC.
Therefore, to develop an appropriate management strategy, we conducted a retrospective study of 117 Chinese patients with HBV-associated HCC to determine the distribution of serum PIVKA-II concentrations according to Barcelona Clinic Liver Cancer (BCLC) stages (4), particularly stage 0-A (defined as early HCC), and assessed the ability of PIVKA-II levels to predict prognosis-related pathological parameters.
Patients and methods
Patients
The present retrospective study included 117 patients with HBV-associated HCC who underwent curative resection at Zhongshan Hospital (Fudan University, Shanghai, China). HCC was defined according to the results of imaging studies and biochemical assays, and diagnosis was confirmed using histopathology according to the criteria of the guidelines of the American Association for the Study of Liver Diseases (4). Staging was determined according to the BCLC system (27), and tumor differentiation was determined using the Edmondson grading system (23). Patients undergoing vitamin K or warfarin treatment were excluded (19). The Research Ethics Committee of Zhongshan Hospital granted approval for the use of human subjects, and informed consent was obtained from each patient.
Sample collection, storage, and measurements
Peripheral blood samples were collected 3 days before curative resection. Blood samples were centrifuged immediately after collection to separate serum, and approximately 3 ml of serum was collected from each patient. After separation, serum samples were stored at −80°C. PIVKA-II concentrations were measured using a Lumipulse G1200 automated immunoassay instrument (Fujirebio, Inc., Tokyo, Japan), and AFP concentrations were determined using a Cobas e 601 module (Roche Diagnostics, GmbH, Mannheim, Germany).
Pathological parameters
We collected data for the pathological parameters MVI (28), Ki67 (29), glypican 3 (GPC3) (30), heat shock protein 70 (HSP70) (31,32), and cytokeratin 19 (CK19) (10,33,34). MVI was defined as the presence of a tumor thrombus in a vascular space lined by endothelial cells in the tumor parenchyma or stroma (19). Immunohistochemistry was used to analyze the expression of Ki67, GPC3, HSP70, and CK19, and two independent pathologists assessed expression levels. KI67 expression was determined according to the median percentage of positive tumor cells within the stroma, and the cutoff value in the present study was defined as 20.00%.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Values of continuous variables are expressed as the mean ± standard error of the mean. The chi-squared test, Fisher's exact probability tests, and Student's t-test were used, as appropriate, to evaluate the significance of differences in data between groups. If the differences within groups were not normally distributed, the nonparametric Mann-Whitney test or the Wilcoxon signed-rank test was used. A receiver operating characteristic (ROC) curve and area under curve (AUC) were used to evaluate the predictive value of clinical variables, including PIVKA-II levels. To identify independent predictors for MVI and high Ki67 expression, variables with P<0.05 (chi-squared test) were included in the logistic regression model, using a stepwise selection procedure (19), and P<0.05 was considered significant.
Results
Patient characteristics
The clinical characteristics of the 117 patients are summarized in Table I. According to the BCLC staging system, 83 patients were classified as early-stage HCC (stage 0, n=17; stage A, n=66), and the other 34 patients were classified as intermediate-stage HCC (stage B, n=24) or advanced HCC (stage C, n=10).
Table I.
Correlation between preoperative serum PIVKA and clinicopathological characteristics.
| Clinical characteristics | No. of patients (n=117) | PIVKA-II≤40 (mAU/ml) | PIVKA-II>40 (mAU/ml) | P-value |
|---|---|---|---|---|
| Age, years | ||||
| ≤50 | 31 | 6 | 25 | 0.350 |
| >50 | 86 | 24 | 62 | |
| Sex | ||||
| Female | 14 | 7 | 7 | 0.026 |
| Male | 103 | 23 | 80 | |
| ALT, U/l | ||||
| ≤40 | 87 | 24 | 63 | 0.412 |
| >40 | 30 | 6 | 24 | |
| AST, U/l | ||||
| ≤40 | 89 | 25 | 64 | 0.279 |
| >40 | 28 | 5 | 23 | |
| AFP, ng/ml | ||||
| ≤20 | 48 | 14 | 34 | 0.466 |
| >20 | 69 | 16 | 53 | |
| No. of tumors | ||||
| Single | 82 | 22 | 60 | 0.652 |
| Multiple | 35 | 8 | 27 | |
| Tumor size, cm | ||||
| ≤5 | 70 | 25 | 45 | 0.002 |
| >5 | 47 | 5 | 42 | |
| Tumor border | ||||
| Clear | 109 | 29 | 80 | 0.371 |
| Unclear | 8 | 1 | 7 | |
| Satellite lesion | ||||
| No | 95 | 27 | 68 | 0.152 |
| Yes | 22 | 3 | 19 | |
| Macrovascular invasion | ||||
| No | 107 | 30 | 77 | 0.052 |
| Yes | 10 | 0 | 10 | |
| Microvascular invasion | ||||
| No | 64 | 24 | 40 | 0.001 |
| Yes | 53 | 6 | 47 | |
| Edmondson stage | ||||
| I–II | 47 | 14 | 33 | 0.400 |
| III–IV | 70 | 16 | 54 | |
| BCLC stage | ||||
| 0+A | 83 | 26 | 57 | 0.028 |
| B+C | 34 | 4 | 30 | |
| CK19 | ||||
| Negative | 72 | 18 | 54 | 0.841 |
| Positive | 45 | 12 | 33 | |
| GPC3 | ||||
| Negative | 36 | 11 | 25 | 0.470 |
| Positive | 81 | 19 | 62 | |
| HSP70 | ||||
| Negative | 27 | 6 | 21 | 0.643 |
| Positive | 90 | 24 | 66 | |
| Ki67 | ||||
| Low | 62 | 21 | 41 | 0.030 |
| High | 55 | 9 | 46 |
Bold values indicate P<0.05. Ki67 expressions were stratified according to median expression level of the study population. aIndicated Fisher exact tests. PIVKA-II, prothrombin induced by vitamin K absence-II; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CK19, cytokeratin 19; GPC3, glypican 3; HSP70, heat shock protein 70.
Analysis of PIVKA-II and AFP levels
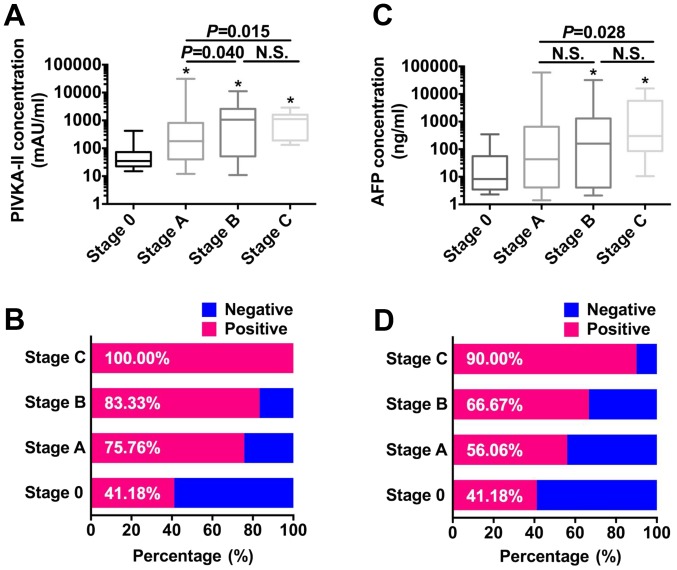
The median PIVKA-II levels increased from very early (stage 0) to advanced (stage C) (Fig. 1A). PIVKA-II levels of patients with stage 0 were significantly lower compared with those of patients with stage A, B, or C HCC (all P<0.050). PIVKA-II levels increased significantly from BCLC stages A to B (P=0.040). Similar results were obtained when the levels of PIVKA-II of patients with stage A HCC were compared with those of patients with stage C HCC (P=0.015) (Fig. 1A). However, there was no significant difference between PIVKA-II levels of patients with stages B or C HCC (P=0.923). When the cutoff was defined as 40 mAU/ml, the optimal value for HCC diagnosis of Chinese patients (16), the positive rate of detection of PIVKA-II increased from very-early to advanced HCC (41.18–100.00%) (Fig. 1B), and all patients with stage C HCC were positive for PIVKA-II. In contrast, the median AFP level did not increase, and there were significant differences only between stages 0 and B or C (both P<0.050) and between stages A and C (P=0.028) (Fig. 1C). However, similar to the PIVKA-II data, the rates of AFP-positive samples increased with tumor progression (41.18–90.00%) when cutoff value for AFP was defined as 20 ng/ml (23) (Fig. 1D).
Figure 1.
Analyses of the rates of detection of PIVKA-II and AFP in patients with different BCLC stages. (A) PIVKA-II levels. (B) Positive rates of PIVKA-II. (C) Distribution of AFP levels. (D) Positive rates of AFP detection. *P<0.05 vs. stage 0. PIVKA-II, prothrombin induced by vitamin K absence-II; AFP, α-fetoprotein; BCLC, Barcelona clinic liver cancer.
Evaluating the potential significance of combining PIVKA-II with AFP values for patients with early-stage HCC
The rate of samples positive for PIVKA-II or AFP was relatively low, particularly for BCLC stage 0 (<50.00% each) (Fig. 1B and D). Therefore, we investigated the potential value of combining the PIVKA-II and AFP data for patients with early-stage HCC. This analysis revealed a significant correlation between their levels, although the Pearson's r value was 0.41 (Fig. 2A), indicating a weak correlation between these two biomarkers and the potential value of combining AFP and PIVKA-II values. Therefore, we further analyzed the significance of combining PIVKA-II and AFP rates for patients with BCLC stages 0 and A.
Figure 2.
Overlap between PIVKA-II and AFP was low in early HCC. (A) Correlation between PIVKA-II and AFP levels in early HCC. Although there was a significant correlation between these biomarkers, the value of the Pearson correlation coefficient was low. (B) Positive rates of PIVKA-II and AFP detection in different subgroups with early HCC. PIVKA-II, prothrombin induced by vitamin K absence-II; AFP, α-fetoprotein; HCC, hepatocellular carcinoma
The rates of positive detection using the combination of PIVKA-II and AFP levels were 58.82 and 92.42% for stages 0 and A, respectively (Fig. 2B). In the stage 0 group, the PIVKA-II-positive rate of AFP-negative patients was 30.00%, the AFP-positive rate of PIVKA-II-negative patients was 30.00% (Fig. 2B). For the stage A group, the PIVKA-II-positive rate of AFP-negative patients was 82.76%, the AFP-positive rate of PIVKA-II-negative patients was 68.75%. Together, these results reveal low overlap between PIVKA-II and AFP levels in patients with early HCC and indicate the promise of combining PIVKA-II and AFP levels for managing these patients.
Correlation between serum PIVKA-II levels and patients' clinicopathological characteristics
The correlations between the PIVKA-II serum levels and patients' clinical characteristics are shown in Table I. Patients with high serum PIVKA-II levels were more likely to have a larger tumor (>5 cm, P=0.002) and an advanced tumor stage (P=0.028). Further, we found that male patients were more likely to have higher serum PIVKA-II levels (P=0.026), suggesting that sex ratio bias should be considered when using PIVKA-II as a biomarker for HCC. When we evaluated the correlation between PIVKA-II levels and pathological parameters MVI, CK19, GPC3, HSP70, and Ki67, we found that the PIVKA-II level correlated significantly with MVI (P=0.001) and high Ki67 expression (P=0.030), indicating that patients with high serum PIVKA-II levels likely have a more aggressive tumor phenotype.
Serum PIVKA-II levels serve as an independent predictor of MVI and tumor cell proliferation
We next investigated whether PIVKA-II served as an independent predictor for MVI and tumor cell proliferation, which indicate the potential of HCC to progress. When we first assessed the correlation between clinicopathological characteristics and MVI, we found that age, AFP level, tumor size, satellite lesions, macrovascular invasion, BCLC stage, and PIVKA-II level correlated significantly with MVI (all P<0.050) (Table II). Multivariate analysis revealed that PIVKA-II level [hazard ratio (HR), 3.77; 95% confidence interval (CI), 1.31–10.88; P=0.014], age (HR, 0.36; 95% CI, 0.14–0.92; P=0.034), AFP level (HR, 2.88; 95% CI, 1.14–7.25; P=0.025), and BCLC stage (HR, 2.91; 95% CI, 1.16–7.26; P=0.022) were independent predictors for MVI (Table II). The AUC of the PIVKA-II level for predicting MVI was 0.634 (95% CI, 0.533–0.735) with 88.70% sensitivity and 38.10% specificity (Fig. 3A).
Table II.
Predictive factors of microvascular invasion in hepatocellular carcinomas.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinical characteristics | MVI− | MVI+ | P-value | HR | 95% CI | P-value |
| Age, years | ||||||
| ≤50 | 11 | 20 | 0.012 | 0.36 | 0.14–0.92 | 0.034 |
| >50 | 53 | 33 | ||||
| Sex | ||||||
| Female | 7 | 7 | 0.706 | N.A. | ||
| Male | 57 | 46 | ||||
| ALT, U/l | ||||||
| ≤40 | 50 | 37 | 0.305 | N.A. | ||
| >40 | 14 | 16 | ||||
| AST, U/l | ||||||
| ≤40 | 52 | 37 | 0.149 | N.A. | ||
| >40 | 14 | 16 | ||||
| AFP, ng/ml | ||||||
| ≤20 | 36 | 12 | 0.001 | 2.88 | 1.14–7.25 | 0.025 |
| >20 | 28 | 41 | ||||
| No. of tumors | ||||||
| Single | 49 | 33 | 0.093 | N.A. | ||
| Multiple | 15 | 20 | ||||
| Tumor size, cm | ||||||
| ≤5 | 45 | 25 | 0.011 | N.S. | ||
| >5 | 19 | 28 | ||||
| Tumor border | ||||||
| Clear | 62 | 47 | 0.085 | N.A. | ||
| Unclear | 2 | 6 | ||||
| Satellite lesion | ||||||
| No | 57 | 38 | 0.017 | N.S. | ||
| Yes | 7 | 15 | ||||
| Macrovascular invasion | ||||||
| No | 63 | 44 | 0.003 | N.S. | ||
| Yes | 1 | 9 | ||||
| Edmondson stage | ||||||
| I–II | 32 | 15 | 0.017 | N.S. | ||
| III–IV | 32 | 38 | ||||
| BCLC stage | ||||||
| 0+A | 53 | 30 | 0.002 | 2.91 | 1.16–7.26 | 0.022 |
| B+C | 11 | 23 | ||||
| CK19 | ||||||
| Negative | 42 | 30 | 0.318 | N.A. | ||
| Positive | 22 | 23 | ||||
| GPC3 | ||||||
| Negative | 24 | 12 | 0.083 | N.A. | ||
| Positive | 40 | 41 | ||||
| HSP70 | ||||||
| Negative | 12 | 15 | 0.222 | N.A. | ||
| Positive | 52 | 38 | ||||
| PIVKA-II | ||||||
| ≤40 | 24 | 6 | 0.001 | 3.77 | 1.31–10.88 | 0.014 |
| >40 | 40 | 47 | ||||
Bold values indicate P<0.05. Ki67 expressions were stratified according to median expression level of the study population. MVI, microvascular invasion; HR, hazard ratio; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CK19, cytokeratin 19; GPC3, glypican 3; HSP70, heat shock protein 70; PIVKA-II, prothrombin induced by vitamin K absence-II. N.S., not significant; N.A., not available.
Figure 3.
Predictive significance of PIVKA-II levels and other clinical parameters. (A) ROC analysis of different variables, including PIVKA-II, for predicting MVI (left) and AUC (right). (B) ROC analysis of different variables, including PIVKA-II, for predicting high Ki67 expression (left), and AUC (right). PIVKA-II, prothrombin induced by vitamin K absence-II; ROC, receiver operating characteristics; MVI, microvascular invasion; AUC, area under curve.
When we performed this two-step analysis, we found that macrovascular invasion, Edmondson stage, CK19, GPC3, and PIVKA-II levels correlated significantly with high Ki67 expression. Further, multivariate analysis identified PIVKA-II level (HR, 2.99; 95% CI, 1.19–7.52; P=0.020), Edmondson stage (HR, 2.61; 95% CI, 1.16–5.88; P=0.020), and GPC3 (HR, 2.65; 95% CI, 1.10–6.36; P=0.027) as independent predictors of high Ki67 expression, which reflect the high proliferative potential of tumor cells (Table III). The AUC of the PIVKA-II level used to predict Ki67 expression was 0.590 (95% CI, 0.487–0.694) with 83.60% sensitivity and 34.40% specificity (Fig. 3B). Taken together, these results showed PIVKA-II could serve as a promising indicator for predicting progressive HCC.
Table III.
Predictive factors of Ki67 expression in hepatocellular carcinomas.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinical characteristics | Ki67low | Ki67high | P-value | HR | 95% CI | P-value |
| Age, years | ||||||
| ≤50 | 15 | 16 | 0.549 | N.A. | ||
| >50 | 47 | 39 | ||||
| Sex | ||||||
| Female | 7 | 7 | 0.811 | N.A. | ||
| Male | 55 | 48 | ||||
| ALT, U/l | ||||||
| ≤40 | 48 | 39 | 0.421 | N.A. | ||
| >40 | 14 | 16 | ||||
| AST, Ul | ||||||
| ≤40 | 47 | 42 | 0.944 | N.A. | ||
| >40 | 15 | 13 | ||||
| AFP, ng/ml | ||||||
| ≤20 | 29 | 19 | 0.180 | N.A. | ||
| >20 | 33 | 36 | ||||
| No. of tumors | ||||||
| Single | 42 | 40 | 0.557 | N.A. | ||
| Multiple | 20 | 15 | ||||
| Tumor size, cm | ||||||
| ≤5 | 39 | 31 | 0.471 | N.A. | ||
| >5 | 23 | 24 | ||||
| Tumor border | ||||||
| Clear | 59 | 49 | 0.105 | N.A. | ||
| Unclear | 2 | 6 | ||||
| Satellite lesion | ||||||
| No | 52 | 43 | 0.432 | N.A. | ||
| Yes | 10 | 12 | ||||
| Macrovascular invasion | ||||||
| No | 60 | 47 | 0.029 | N.S. | ||
| Yes | 2 | 8 | ||||
| Microvascular invasion | ||||||
| No | 41 | 23 | 0.008 | N.S. | ||
| Yes | 21 | 32 | ||||
| Edmondson stage | ||||||
| I–II | 32 | 15 | 0.007 | 2.61 | 1.16–5.88 | 0.020 |
| III–IV | 30 | 40 | ||||
| BCLC stage | ||||||
| 0+A | 45 | 38 | 0.678 | N.A. | ||
| B+C | 17 | 17 | ||||
| CK19 | ||||||
| Negative | 44 | 28 | 0.026 | N.S. | ||
| Positive | 18 | 27 | ||||
| GPC3 | ||||||
| Negative | 25 | 11 | 0.017 | 2.65 | 1.10–6.36 | 0.027 |
| Positive | 37 | 44 | ||||
| HSP70 | ||||||
| Negative | 15 | 12 | 0.761 | N.A. | ||
| Positive | 47 | 43 | ||||
| PIVKA-II | ||||||
| ≤40 | 21 | 9 | 0.030 | 2.99 | 1.19–7.52 | 0.020 |
| >40 | 41 | 46 | ||||
Bold values indicate P<0.05. Ki67 expressions were stratified according to median expression level of the study population. HR, hazard ratio; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CK19, cytokeratin 19; GPC3, glypican 3; HSP70, heat shock protein 70. PIVKA-II, prothrombin induced by vitamin K absence-II; N.S., not significant; N.A., not available.
Discussion
We conducted a retrospective study of Chinese patients with HBV-associated HCC to assess the value of determining PIVKA-II levels as a biomarker of HCC, in particular, for early-stage disease. We found that the overlap between PIVKA-II and AFP levels in patients with early HCC was low and that evaluating their levels in combination may serve as an effective component of strategies for the management of early-stage HCC. Further, we show here that high PIVKA-II levels were significantly associated with MVI and high Ki67 expression, indicating an increased probability of tumors to progress. Together, our data strengthen the prognostic value of PIVKA-II levels. Moreover, multivariate analysis revealed that PIVKA-II levels were an independent predictor of MVI and high Ki67 expression.
Although the impact of HBV infection is declining because of the recent implementation of China's nationwide HBV vaccination program, liver cirrhosis caused by chronic HBV infection is the major cause of HCC (3), which differs from the causes of HCC in Japanese, Europeans, or Americans (23). Hence, we investigated the clinical significance of PIVKA-II on this cohort of patients. PIVKA-II is a promising biomarker for the diagnosis and prognosis of HCC (14–18). For example, our previous study demonstrates that PIVKA-II is an independent indicator of early recurrence in patients with early HCC (35). PIVKA-II exhibited greater diagnostic performance compared with AFP because of its higher sensitivity and specificity (18). Moreover, the combination of PIVKA-II and AFP was not more advantageous compared with PIVKA-II (16,18,19), and this combination impaired the diagnostic efficiency of PIVKA-II in some subgroups because of the poor performance of AFP. However, in the present study, we observed low overlap between positive PIVKA-II and AFP data for patients with early HCC, and combining the data greatly increased the rates of positive detection of patients with early HCC.
We speculated that this discordance is attributed to factors such as patient selection and different staging systems. We therefore strongly recommend using these two biomarkers together for HCC screening, in particular, for more patients with early-stage tumors. These patients should undergo high-resolution imaging scans to establish a definite diagnosis. Such efforts will likely increase the number of patients suitable for curative resection to improve prognosis. Further, our results suggest combining PIVKA-II and AFP levels when evaluating the response to treatment of patients with early HCC to compensate for the disadvantages of using a single marker and to provide comprehensive information that will improve the accuracy of clinical assessments.
Here we evaluated the correlation between PIVKA-II with the pathological parameters tumor cell differentiation, MVI, CK19, GPC3, and HSP70, because they are significantly associated with the prognosis of HCC (5,29,31,34) and are routinely determined by the Department of Pathology, Fudan University. We found further that preoperative PIVKA-II was an independent predictor for MVI (HR, 3.77; 95% CI, 1.31–10.88; P=0.014), which is consistent with findings of studies of Japanese (36) and Western European cohorts (19). Together, these findings strongly suggest an association between PIVKA-II expression and tumor cell invasion. Moreover, for the first time to our knowledge, we report that PIVKA-II levels served as an independent predictor for high Ki67 expression (HR, 2.99; 95% CI, 1.19–7.52; P=0.020). Previous studies established the correlation between the PIVKA-II level and tumor size (19,36), and PIVKA-II maintains the growth of HCC (37). The present study provides direct clinical evidence of the correlation between PIVKA-II and cell proliferation. Taken together, our findings indicate that high PIVKA-II levels may reflect a more aggressive tumor phenotype and will therefore significantly improve the evaluation of tumor prognosis to facilitate the delivery of individualized treatment strategies.
When we performed ROC analysis, we found that the sensitivity and specificity of PIVKA-II for predicting MVI and high Ki67 expression were approximately 90.00 and 30.00%, respectively. These findings indicate that a high true-positive rate of PIVKA-II may nevertheless serve as an effective screening tool, although the true-negative rate was insufficient for this purpose. We suggest that the reason for low specificity may be the cutoff value of 40 mAU/ml, which is considered optimal for diagnosing Chinese patients with HCC (16). We did not use a different another cutoff value because of the small number of subjects (n=117) studied here. Nevertheless, the correlation between PIVKA-II and pathogenesis was significant. Establishing a panel containing all independent predictors might overcome this limitation. In fact, based on present cohort of patients, we established a model comprising AFP, BCLC stage, and PIVKA-II, which showed an AUC of 0.755 with 71.80% sensitivity and 68.70% specificity for predicting MVI (data not shown). Moreover, we are conducting a large cohort, multicenter study to systematically evaluate the predictive performance of PIVKA-II.
There are limitations to our study other than the small number of subjects. First, our study was retrospective. Second, we did not perform next-generation PIVKA-II detection, because it is not routinely preformed in clinical laboratories (38), and we will further investigate its clinical significance in the near future. Third, the mechanism of the regulation of PIVKA-II of tumor cell invasion (e.g., via modulating the EMT) was not investigated in detail.
In conclusion, the present study shows low overlap between PIVKA-II and AFP levels in Chinese patients with HBV-associated early HCC, which required implementing a combination strategy. Further, we found for the first time, that the PIVKA-II level served as an independent predictor of MVI and high Ki67 expression, indicating the clinical significance of PIVKA-II levels and their value for enhancing the management of patients with HCC. Moreover, we contribute new insights into the mechanism of metastasis of HCC cells that may facilitate future studies aimed to develop more effective treatment strategies.
Acknowledgements
This study was supported by grants from the National High Technology Research and Development Program (863 Program) of China (2015AA020401), the State Key Program of National Natural Science of China (81530077), the National Natural Science Foundation of China (81472676, 81572823, 81372317 and 81572064), the Projects from the Shanghai Science and Technology Commission (13140901900, 134119a1201, 14DZ1940300, 14411970200 and 14140902301), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12010202), Specialized Research Fund for the Doctoral Program of Higher Education and Research Grants Council Earmarked Research Grants Joint Research Scheme (20130071140008), Key Developing Disciplines of Shanghai Municipal Commission of Health and Family Planning (2015ZB0201), Research Project of Shanghai Municipal Commission of Health and Family Planning (201540052), the Funding Plan for Outstanding Youth Doctors Training of Shanghai (2016-01), the National Natural Science Foundation of China (81572064), and the Research Funding of Shanghai Municipal Commission of Health and Family Planning (201440389).
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- AFP
α-fetoprotein
- PIVKA-II
prothrombin induced by vitamin K absence-II
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- MVI
microvascular invasion
- BCLC
Barcelona clinic liver cancer
- GPC3
glypican 3
- HSP70
heat shock protein 70
- CK19
cytokeratin 19
- ROC
receiver operating characteristics
- AUC
area under curve
- HR
hazard ratio
- CI
confidence interval
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(Suppl 1):S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran R, Venkatesh SK, Torbenson MS, Roberts LR. Clinical implications of basic research in hepatocellular carcinoma. J Hepatol. 2016;64:736–745. doi: 10.1016/j.jhep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 8.Zhu GQ, Shi KQ, Yu HJ, He SY, Braddock M, Zhou MT, Chen YP, Zheng MH. Optimal adjuvant therapy for resected hepatocellular carcinoma: A systematic review with network meta-analysis. Oncotarget. 2015;6:18151–18161. doi: 10.18632/oncotarget.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Yang XR, Sun YF, Shen MN, Ma XL, Wu J, Zhang CY, Zhou Y, Xu Y, Hu B, et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform. Clin Cancer Res. 2014;20:4794–4805. doi: 10.1158/1078-0432.CCR-14-0251. [DOI] [PubMed] [Google Scholar]
- 10.Yang XR, Xu Y, Shi GM, Fan J, Zhou J, Ji Y, Sun HC, Qiu SJ, Yu B, Gao Q, et al. Cytokeratin 10 and cytokeratin 19: Predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res. 2008;14:3850–3859. doi: 10.1158/1078-0432.CCR-07-4338. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: A large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res. 2014;44:E11–E25. doi: 10.1111/hepr.12201. [DOI] [PubMed] [Google Scholar]
- 13.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meguro M, Mizuguchi T, Nishidate T, Okita K, Ishii M, Ota S, Ueki T, Akizuki E, Hirata K. Prognostic roles of preoperative α-fetoprotein and des-γ-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:4933–4945. doi: 10.3748/wjg.v21.i16.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JM, Hyuck C, Kwon D, Joh JW, Lee JH, Paik SW, Park CK. Protein induced by vitamin K antagonist-II (PIVKA-II) is a reliable prognostic factor in small hepatocellular carcinoma. World J Surg. 2013;37:1371–1378. doi: 10.1007/s00268-013-1966-0. [DOI] [PubMed] [Google Scholar]
- 16.Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, Zhou F, Zhou W, Shen F, Gao C. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: A Large-Scale, Multicentre study. PLoS One. 2016;11:e0153227. doi: 10.1371/journal.pone.0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: A critical review. Hepatology. 1993;18:990–997. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]
- 18.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Biscegile AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–854. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Kamiyama T, Yokoo H, Kakisaka T, Orimo T, Wakayama K, Kamachi H, Tsuruga Y, Yanmashita K, Shimamura T, Todo S, Taketomi A. Multiplication of alpha-fetoprotein and protein induced by vitamin K absence-II is a powerful predictor of prognosis and recurrence in hepatocellular carcinoma patients after a hepatectomy. Hepatol Res. 2015;45:E21–E31. doi: 10.1111/hepr.12451. [DOI] [PubMed] [Google Scholar]
- 21.Masuda T, Beppu T, Okabe H, Nitta H, Imai K, Hayashi H, Chikamoto A, Yamamoto K, Ikeshima S, Kuramoto M, et al. Predictive factors of pathological vascular invasion in hepatocellular carcinoma within 3 cm and three nodules without radiological vascular invasion. Hepatol Res. 2016;46:985–991. doi: 10.1111/hepr.12637. [DOI] [PubMed] [Google Scholar]
- 22.Kondo Y, Kimura O, Shimosegawa T. Significant biomarkers for the management of hepatocellular carcinoma. Clin J Gastroenterol. 2015;8:109–115. doi: 10.1007/s12328-015-0568-9. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 24.Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: A pilot study. J Hepatol. 2010;52:880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HM, Li SP, Yu Y, Wang Z, He JD, Xu YJ, Zhang RX, Zhang JJ, Zhu ZJ, Shen ZY. Bi-directional roles of IRF-1 on autophagy diminish its prognostic value as compared with Ki67 in liver transplantation for hepatocellular carcinoma. Oncotarget. 2016;7:37979–37992. doi: 10.18632/oncotarget.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, Aishima S, Taketomi A, Shirabe K, Ishida T, Maehara Y. Predictors for microinvasion of small hepatocellular carcinoma ≤2 cm. Ann Surg Oncol. 2012;19:2027–2034. doi: 10.1245/s10434-011-2195-0. [DOI] [PubMed] [Google Scholar]
- 29.Schmilovitz-Weiss H, Tobar A, Halpern M, Levy I, Shabtai E, Ben-Ari Z. Tissue expression of squamous cellular carcinoma antigen and Ki67 in hepatocellular carcinoma-correlation with prognosis: A historical prospective study. Diagn Pathol. 2011;6:121. doi: 10.1186/1746-1596-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haruyama Y, Yorita K, Yamaguchi T, Kitajima S, Amano J, Ohtomo T, Ohno A, Kondo K, Kataoka T. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer. 2015;137:1643–1651. doi: 10.1002/ijc.29518. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Zhang Y, Guo K, Wang N, Jin H, Liu Y, Qin W. Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential. Int J Cancer. 2016;138:1824–1834. doi: 10.1002/ijc.29723. [DOI] [PubMed] [Google Scholar]
- 32.Kang GH, Lee BS, Lee ES, Kim SH, Lee HY, Kang DY. Prognostic significance of p53, mTOR, c-Met, IGF-1R, and HSP70 overexpression after the resection of hepatocellular carcinoma. Gut Liver. 2014;8:79–87. doi: 10.5009/gnl.2014.8.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun DW, Zhang YY, Sun XD, Chen YG, Qiu W, Ji M, Lv GY. Prognostic value of cytokeratin 19 in hepatocellular carcinoma: A meta-analysis. Clin Chim Acta. 2015;448:161–169. doi: 10.1016/j.cca.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Feng J, Zhu R, Chang C, Yu L, Cao F, Zhu G, Chen F, Xia H, Lv F, Zhang S, Sun L. CK19 and Glypican 3 expression profiling in the prognostic indication for patients with HCC after surgical resection. PLoS One. 2016;11:e0151501. doi: 10.1371/journal.pone.0151501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang BL, Tan QW, Gao XH, Wu J, Guo W. Elevated PIVKA-II is associated with early recurrence and poor prognosis in BCLC 0-A hepatocellular carcinomas. Asian Pac J Cancer Prev. 2014;15:6673–6678. doi: 10.7314/APJCP.2014.15.16.6673. [DOI] [PubMed] [Google Scholar]
- 36.Shirabe K, Toshima T, Kimura K, Yamashita Y, Ikeda T, Ikegami T, Yoshizumi T, Abe K, Aishima S, Maehara Y. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014;34:937–941. doi: 10.1111/liv.12459. [DOI] [PubMed] [Google Scholar]
- 37.Cui SX, Zhang YS, Chu JH, Song ZY, Qu XJ. Des-gamma-carboxy prothrombin (DCP) antagonizes the effects of gefitinib on human hepatocellular carcinoma cells. Cell Physiol Biochem. 2015;35:201–212. doi: 10.1159/000369688. [DOI] [PubMed] [Google Scholar]
- 38.Kurokawa T, Yamazaki S, Mitsuka Y, Moriguchi M, Sugitani M, Takayama T. Prediction of vascular invasion in hepatocellular carcinoma by next-generation des-r-carboxy prothrombin. Br J Cancer. 2016;114:53–58. doi: 10.1038/bjc.2015.423. [DOI] [PMC free article] [PubMed] [Google Scholar]