Abstract
Abnormal regulation of long non-coding RNAs (lncRNAs) appears to be a primary feature of numerous types of human cancer. However, the association between the dysregulation of lncRNAs and functional alterations in gastric cancer (GC) remains unclear. In previous studies, we applied microarray and bioinformatics analyses to screen for key lncRNAs from the tumor tissues and matched adjacent non-tumor tissues of 10 patients with GC. There were seven key lncRNAs demonstrated to be significantly different between carcinoma tissues and adjacent non-tumor tissues. In the present study, the expression of these seven selected lncRNAs were validated in 82 patients with GC to further investigate the association between lncRNAs and GC clinical characterization. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) results demonstrated that RP5-919F19, MCPH1 antisense RNA 1 (CTD-2541M15) and urothelial carcinoma-associated 1 (UCA1) exhibited consistent upregulation in cancer compared with adjacent non-tumor tissues, whereas AP000459, LOC101928316, tumor suppressor candidate 8 (LINC01071) and maternally expressed 3 (MEG3) showed consistent downregulation. The results from the microarray and RT-qPCR experiments achieved 100% agreement. A correlation analysis indicated that RP5-919F19, LOC101928316 and MEG3 were significantly associated with tumor differentiation degree, RP5-919F19, UCA1 and MEG3 were significantly associated with lymph node metastasis, and RP5-919F19, CTD-2541M15 and UCA1 were significantly associated with tumor-node-metastasis stage (P<0.05). In addition, it was identified that the differential expression of LINC01071 and LOC101928316 significantly correlated with the age and gender of the GC patients, respectively (P<0.05). The results suggest that the lncRNAs RP5-919F19, LOC101928316, CTD-2541M15, UCA1 and MEG3 are closely associated with the invasion and metastasis of GC, which reveals these indicators as potential specificity biomarkers for the diagnosis, prognosis and classification of GC. Thus, these lncRNAs merit further study as novel candidate biomarkers for the clinical diagnosis of GC and as potential targets for therapy.
Keywords: gastric cancer, long non-coding RNA, function, biomarker
Introduction
Gastric cancer (GC) is one of the most lethal types of cancer and has been increasing in incidence and mortality over the last several decades. In China, it has been estimated that ~464,000 new cases were diagnosed in 2012, which accounts for over 40% of the total cases (~989,600) worldwide (1). According to evidence from clinical studies, the majority of patients with GC are diagnosed at an advanced stage and are thus not suitable for radical surgery (2). Previous studies have also reported that earlier diagnosis and treatment of GC could produce a 5-year survival rate of >90% (3).
Recent developments in the field of digestive system endoscopy have been remarkable due to their association with decreased trauma, accelerated recovery and fewer complications (4,5). However, endoscopic biopsy and observation of pathological morphology are unable to detect all precancerous lesions associated with early GC (6). Therefore, improvements in the early-stage diagnosis of GC and identification of sensitive and specific biomarkers for early detection are important research topics. These issues will be resolved by further investigation into the pathogenesis of GC as well as the identification of novel and reliable biomarkers for early diagnosis or molecular therapeutic targets for the treatment of this disease.
Over the past decade, various studies have indicated that the human transcriptome comprises not only of protein-coding mRNAs, but also a large number of non-protein-coding RNAs (7). Although a large number of studies focus on microRNAs (miRNAs; 18–200 nucleotides), a wide array of critical regulatory roles in biology have been associated with long non-coding RNAs (lncRNAs) (8). lncRNAs, which are tentatively defined as a series of RNA transcripts that are >200 nucleotides in length, have been confirmed as essential regulators in almost all aspects of biology (9). Accumulating evidence suggests that lncRNAs are important in tumorigenesis (10). As it is the functional end-product, the level of lncRNA expression correlates directly with the level of the active molecule (11). Thus, the use of lncRNAs in diagnostics has inherent advantages over the use of protein-coding RNAs. In addition, lncRNAs exhibit greater tissue specificity compared with protein-coding mRNAs and miRNAs, making them appealing in the search for novel diagnostic and prognostic cancer biomarkers (12). Therefore, further studies on GC tissues from endoscopic biopsy may aid in establishing the associations between lncRNAs and GC.
Previous studies have identified a subset of lncRNAs that are associated with GC, indicating their wide participation in the development and progression of GC (13,14). However, the biological functions and mechanisms of these lncRNAs remain unexplored (11). In the present study, differential expression profiles of lncRNAs and mRNAs were detected in advanced GC tissues and adjacent non-tumor tissues by microarray analysis. In addition, the present study aimed to identify the associations between significant differences in lncRNA levels and the clinicopathological characteristics of GC in order to elucidate the specific functions and mechanisms of these lncRNAs during GC development.
Materials and methods
Patients and tissue sample collection
Specimens from 10 patients with advanced GC, as well as their paired adjacent non-cancerous tissue specimens, were included in the lncRNA microarray analysis, and tissues from 82 patients, including 59 males and 23 females aged between 45 and 70 years, were collected for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis from the Gansu Wuwei Cancer Hospital (Wuwei, China) between September 2014 and May 2015. The present study was approved by the Ethics Committee of the Gansu Wuwei Cancer Hospital. All patients provided written informed consent to participate in the present study. All patients were assigned a diagnosis of GC based on histopathology and clinical history.
In addition, clinical information was recorded for each patient, including age, gender, tumor grade, tumor location, tumor stage, degree of differentiation, tumor-node-metastasis (TNM) stage, lymph node metastasis status and date of resection. The pathologist assessed the tumor by microscopic examination in every case, and the percentage of tumor tissue was estimated to be ≥80%. No patients had received preoperative radiotherapy or chemotherapy. Adjacent non-cancerous tissues were located ≥5 cm from the tumor edge. Tissue samples were immersed in RNAlater (Ambion; Thermo Fisher Scientific, Inc., Austin, TX, USA) and stored at −80°C until use.
Isolation of RNA
Total RNA was isolated from the GC tissues and adjacent non-tumor gastric mucosal epithelium using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) according to the manufacturer's instructions. The concentration and integrity of isolated RNA was assessed using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Finally, total RNA integrity was assessed by agarose gel electrophoresis.
lncRNA microarray analysis
RNA samples were isolated from 10 patients by pooling RNA from 2, 4 and 4 patients, respectively, into three groups as ‘one sample’. Thus, three pairs of pooled RNA samples were generated from GC specimens and their paired adjacent non-cancerous tissues. These three pairs of RNA samples were subjected to microarray analysis using a RiboArray Custom Array 1*90K (Guangzhou RiboBio Co., Ltd., Guangzhou, China), which could detect 32,987 lncRNAs from a number of authoritative data sources, including RefSeq (National Center for Biotechnology Information) (https://www.ncbi.nlm.nih.gov/gene/?term=), H-invDB (http://h-invitational.jp/hinv/ahg-db/index.jsp), UCSC (http://genome.ucsc.edu/) LncRNAdb (http://www.lncrnadb.org/#opennewwindow), and GENCODE LncRNA (https://www.gencodegenes.org). Signals were normalized using the median center tool for the genes. Analysis of variance (ANOVA) was used to compare differentially expressed lncRNAs and mRNAs.
In a previous study, we built an lncRNA-mRNA co-expression network that was based on the theory of competing endogenous RNAs, and the differentially expressed lncRNAs and mRNAs that were selected from GC specimens and their paired adjacent non-cancerous tissues (15). In the present study, standard selection criteria to identify differentially expressed lncRNAs and mRNAs were established at P<0.05 and fold change >2. The lncRNA-mRNA networks were constructed based on the associations between the differentially expressed lncRNAs and mRNAs in the previous study (15), and visualized using Cytoscape v3.0 (National Institute of General Medical Sciences, National Institutes of Health, Rockville, MD, USA).
RT-qPCR analysis
GAPDH was selected as the endogenous standard. RT reactions were conducted in two steps. First, the mixture containing 1 µg of RNA samples was incubated in a 96-well plate for 10 min at 70°C and held at 4°C. Subsequently, the 11.1-µl mixture, which comprised 4 µl MgCl2 (25 mM), 2 µl 10X RT buffer (Promega Corporation, Madison, WI, USA), 2 µl dNTPs (10 mM; Promega Corporation), 0.6 µl AMV reverse transcriptase (15 U/µl), 0.5 µl RNAsin, 2 µl random primers, and 6.9 µl ddH2O, was incubated in a 96-well plate at 25°C for 10 min, 42°C for 15 min, 95°C for 5 min, and subsequently held at 4°C.
qPCR was performed to detect the expression levels of candidate lncRNAs with the StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and GoTaq® qPCR Master Mix (Promega Corporation), according to the manufacturer's protocol. The total PCR reaction volume was 10 µl and included 1 µl cDNA, 5 µl GoTaq® qPCR Master Mix, 0.2 µM PCR primers (Shanghai Generay Biotech Co., Ltd., Shanghai, China) and RNase-free water. The reaction was performed at 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. A dissociation curve was analyzed from 60–95°C. Primers used for real-time RT-PCR as shown in Table I. RT-qPCR relative fold-change results were calculated using the 2−ΔΔCq method [where ΔCq=(CqRNAs-CqGAPDH); and ΔΔCq=ΔCqtumor tissues-ΔCqadjacent non-tumor tissues] (16). All experiments were repeated three times.
Table I.
Primer sequences used in the reverse transcription-quantitative polymerase chain reaction validation of long non-coding RNAs.
| Gene symbol | Primer sequences, 5′-3′ | Target size, bp | Tm, °C |
|---|---|---|---|
| RP5-919F19-F | TGGAGGAAGGAGAAGGTCAT | 88 | 59 |
| RP5-919F19-R | CGTGTCAGGTAGCCAAGG | ||
| CTD-2541M15-F | GATACTGCCTGTGACCTG | 120 | 59 |
| CTD-2541M15-R | GACTAAGCGTGACTCCTG | ||
| UCA1-F | TCCACACCCAAAACAAAA | 200 | 59 |
| UCA1-R | GCCCTCTAACAACAAACAAC | ||
| AP000459-F | CCATCTTTGAGGGCTTTT | 141 | 59 |
| AP000459-R | GGTGTGTCATTTTGTTTTCC | ||
| LOC101928316-F | AACAACGGGGACATTAGG | 119 | 59 |
| LOC101928316-R | AACTGGAAACATCACATAGCA | ||
| LINC01071-F | TTTCCATAAGGCACGATT | 220 | 59 |
| LINC01071-R | CCTAACCCACCACATTCA | ||
| MEG3-F | TGCCCATCTACACCTCAC | 112 | 59 |
| MEG3-R | TCCTCTTCATCCTTTGCC | ||
| GAPDH-F | GGGAGCCAAAAGGGTCATCA | 203 | 60 |
| GAPDH-R | TGATGGCATGGACTGTGGTC |
Statistical analysis
SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA) was used to perform the data analysis. Results are presented as mean ± standard error. Statistical analysis was performed using a Student's t-test for comparison of two groups in the microarray analysis, and one-way analysis of variance for multiple comparisons with the Student-Newman-Keuls post hoc test. For all comparisons, differences with P<0.05 were considered statistically significant. In addition, a conditional logistic regression analysis was used to evaluate any association between differentially expressed lncRNAs and the characteristics of patients with GC.
Results
Screening of candidate lncRNAs by microarray and bioinformatics analysis
Previous microarray analysis identified that 1,046 lncRNAs, including 427 upregulated and 619 downregulated lncRNAs, were significantly differentially expressed (fold-change ≥2.0) between advanced GC lesions and adjacent non-tumor tissues. The present study identified key lncRNAs, and a total of 6 key lncRNAs were revealed to be overexpressed in tumor tissues [RP5-919F19, RP11-54O7, RP11-20I23, MCPH1 antisense RNA 1 (CTD-2541M15), AC010731, and urothelial carcinoma-associated 1 (UCA1); Table II], in addition, there were 8 key lncRNAs were found to be downregulated in tumor tissues [AP000459, LOC101928316, RP11-167N4, tumor suppressor candidate 8 (LINC01071), RP11-111K18, TTC28 antisense RNA 1 (TTC28-AS1), MTOR antisense RNA 1 (MTOR-AS1), and maternally expressed 3 (MEG3); Table II]. The data also revealed that there were significant differences in the expression levels of RP5-919F19, CTD-2541M15, UCA1, AP000459, LOC101928316, LINC01071 and MEG3 in tumor tissues compared with adjacent non-tumor tissues (P<0.05). Clustering analysis was performed for all 14 abnormally expressed key lncRNAs (Fig. 1).
Table II.
Differential expression of key lncRNAs in gastric cancer.
| Name (lncRNA) | Transcript-ID | Regulation | Fold-change |
|---|---|---|---|
| RP5-919F19a | URS0000515CAC | Up | 36.92 |
| RP11-54O7 | URS00005B803E | Up | 32.52 |
| RP11-20I23 | URS00002B7786 | Up | 32.43 |
| CTD-2541M15a | URS0000359EF8 | Up | 28.03 |
| AC010731 | ENST00000543490 | Up | 25.33 |
| UCA1a | NR_015379 | Up | 11.51 |
| AP000459a | URS000048CBED | Down | −23.26 |
| LOC101928316a | XR_428890 | Down | −22.73 |
| RP11-167N4 | ENST00000537019 | Down | −22.73 |
| RP11-111K18 | URS00002FCA1A | Down | −18.52 |
| LINC01071a | NR_104174 | Down | −18.52 |
| TTC28-AS1 | ENST00000430525 | Down | −17.88 |
| MTOR-AS1 | NR_046600 | Down | −16.67 |
| MEG3a | NR_046473 | Down | −2.19 |
Statistically significant (P<0.05). lncRNA, long non-coding RNA; CTD-2541M15, MCPH1 antisense RNA 1; UCA1, urothelial carcinoma-associated 1; LINC01071, tumor suppressor candidate 8; TTC28-AS1, TTC28 antisense RNA 1; MTOR-AS1, MTOR antisense RNA 1; MEG3, maternally expressed 3.
Figure 1.
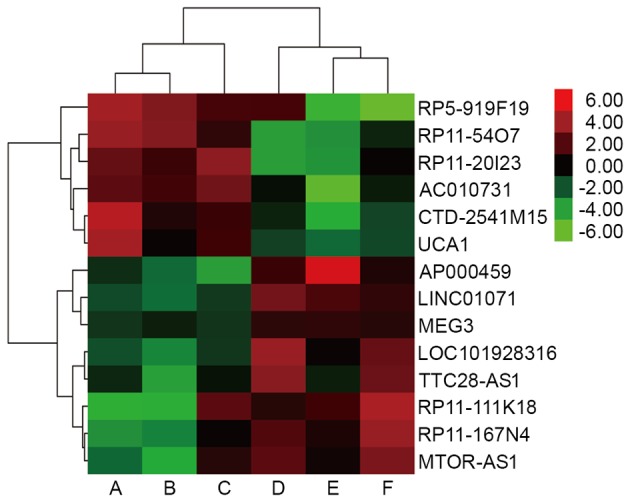
Clustering analysis diagram of the microarray results. Red indicates upregulated lncRNA expression and green indicates downregulated lncRNA expression. A-C are the groups of gastric cancer tissues, and D-F are the groups of adjacent non-tumor tissues. lncRNA, long non-coding RNA; CTD-2541M15, MCPH1 antisense RNA 1; UCA1, urothelial carcinoma-associated 1; LINC01071, tumor suppressor candidate 8; MEG3, maternally expressed 3; TTC28-AS1, TTC28 antisense RNA 1; MTOR-AS1, MTOR antisense RNA 1.
Verification of selected lncRNA expression in GC tissues
RT-qPCR was performed to confirm the reliability and validity of the detected expression levels of the selected lncRNAs in 82 GC tissues and adjacent non-tumor tissues. The results demonstrated that RP5-919F19, CTD-2541M15 and UCA1 showed consistent upregulation compared with adjacent non-tumor tissues, while AP000459, LOC101928316, LINC01071 and MEG3 showed consistent downregulation (Table III). A histogram (Fig. 2) shows the fold-changes detected by RT-qPCR (2−ΔΔCq) and lncRNA microarray data. The consistency between the microarray and RT-qPCR data confirms the reliability of the results.
Table III.
Relative expression levels of lncRNAs in 82 pairs of GC and non-tumor tissues.
| lncRNA expression, ΔCq (mean ± standard deviation) | |||||
|---|---|---|---|---|---|
| Gene symbol | GC tissues | Adjacent non-tumor tissues | 2−ΔΔCq | Change in expression in GC | P-value |
| RP5-919F19 | 11.448±4.136 | 12.393±3.613 | 3.778 | Up | 0.000a |
| CTD-2541M15 | 8.041±2.734 | 8.729±2.633 | 6.459 | Up | 0.001a |
| UCA1 | 9.666±3.459 | 10.764±4.011 | 8.521 | Up | 0.000a |
| AP000459 | 12.234±5.216 | 10.460±4.938 | −13.073 | Down | 0.000a |
| LOC101928316 | 10.198±3.749 | 8.789±3.677 | −10.878 | Down | 0.000a |
| LINC01071 | 13.757±4.260 | 12.152±4.809 | −13.062 | Down | 0.000a |
| MEG3 | 8.079±4.892 | 7.159±4.479 | −6.322 | Down | 0.003a |
Statistically significant (P<0.05). ΔCq=Cqtarget gene-CqGAPDH; ΔΔCq=ΔCqtumor tissues-ΔCqadjacent non-tumor tissues; ΔCq indicates relative lncRNA expression level (the higher the ΔCq value, the lower the lncRNA expression). lncRNA, long non-coding RNA; GC, gastric cancer; CTD-2541M15, MCPH1 antisense RNA 1; UCA1, urothelial carcinoma-associated 1; LINC01071, tumor suppressor candidate 8; MEG3, maternally expressed 3.
Figure 2.
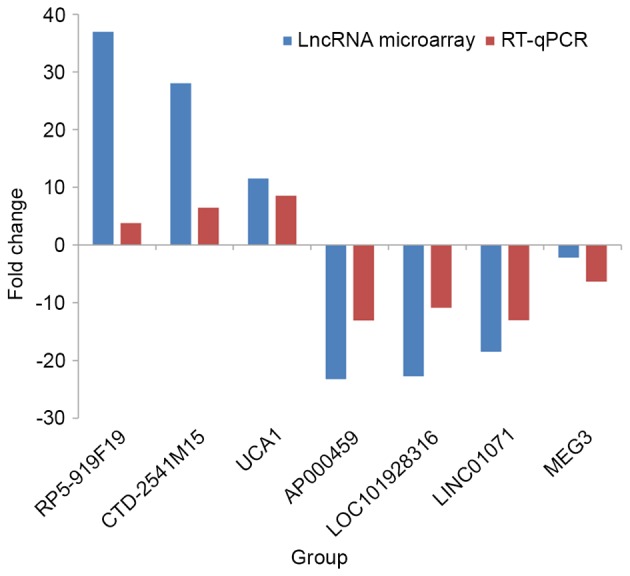
RT-qPCR validation of seven differentially expressed lncRNAs. Comparison of fold-change (2−ΔΔCq) of lncRNAs between tumor and adjacent non-tumor tissues, identified by microarray analysis and RT-qPCR results. lncRNA, long non-coding RNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CTD-2541M15, MCPH1 antisense RNA 1; UCA1, urothelial carcinoma-associated 1; LINC01071, tumor suppressor candidate 8; MEG3, maternally expressed 3.
Association between the identified lncRNAs and clinicopathological characteristics of GC
The associations between the expression levels of the seven selected lncRNAs in GC samples and the clinicopathological characteristics of the patients were analyzed. The results identified that one lncRNA, AP000459, had no statistically significant associations with the following clinicopathological characteristics: Patient gender or age; tumor size or differentiation degree; TNM stage; or lymph node metastasis status. However, the remaining six lncRNAs each demonstrated significant associations with certain characteristics: RP5-919F19, LOC101928316 and MEG3 were significantly associated with tumor differentiation degree; RP5-919F19, UCA1 and MEG3 were significantly associated with lymph node metastasis status; and RP5-919F19, CTD-2541M15 and UCA1 were significantly associated with TNM stage (all P<0.05). In addition, LINC01071 expression was associated with patient age; UCA1 was associated with tumor size; and LOC101928316 was associated with the sex of the patient (all P<0.05; Tables IV–X).
Table IV.
Association between the expression of RP5-919F19 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | Expression of RP5-919F19 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.672 | ||
| Male | 59 (72) | 4.284±11.597 | |
| Female | 23 (28) | 3.059±9.363 | |
| Age, years | 0.405 | ||
| ≤50 | 30 (37) | 2.446±12.746 | |
| >50 | 52 (63) | 4.617±9.843 | |
| Tumor size, cm | 0.238 | ||
| ≤5 | 43 (52) | 2.434±11.066 | |
| >5 | 39 (48) | 5.404±10.823 | |
| Degree of differentiation | 0.006a | ||
| Well/moderately | 28 (34) | −0.638±6.568 | |
| Poorly | 54 (66) | 6.452±12.135 | |
| TNM stage | 0.015a | ||
| I/II | 44 (54) | 1.170±7.709 | |
| III/IV | 38 (46) | 7.264±13.440 | |
| Lymph node status | 0.029a | ||
| No metastasis | 36 (44) | 0.890±7.236 | |
| Metastasis | 46 (56) | 6.337±12.893 |
Statistically significant (P<0.05). TNM, tumor-node-metastasis.
Table X.
Association between the expression of MEG3 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | Expression of MEG3 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.674 | ||
| Male | 59 (72) | −4.960±15.210 | |
| Female | 23 (28) | −3.492±7.248 | |
| Age, years | 0.841 | ||
| ≤50 | 30 (37) | −5.008±11.633 | |
| >50 | 52 (63) | −4.342±14.338 | |
| Tumor size, cm | 0.791 | ||
| ≤5 | 43 (52) | −4.067±13.301 | |
| >5 | 39 (48) | −4.911±13.640 | |
| Degree of differentiation | 0.036a | ||
| Well/moderately | 28 (34) | −1.220±12.479 | |
| Poorly | 54 (66) | −8.317±13.822 | |
| TNM stage | 0.778 | ||
| I/II | 44 (54) | −4.064±13.291 | |
| III/IV | 38 (46) | −4.968±13.672 | |
| Lymph node status | 0.024a | ||
| No metastasis | 36 (44) | 0.822±11.519 | |
| Metastasis | 46 (56) | −6.447±14.730 |
Statistically significant (P<0.05). MEG3, maternally expressed 3; TNM, tumor-node-metastasis.
Association between candidate lncRNAs and the lymph node metastasis of GC
Conditional logistic regression analysis was used to evaluate the association between differentially expressed lncRNAs and the lymph node metastasis status of GC. As shown in Table XI, a significantly increased risk of lymph node metastasis was associated with the increased expression of RP5-919F19 [odds ratio (OR), 1.199] and reduced expression of MEG3 (OR, 0.924). This suggested that RP5-919F19 and MEG3 may participate in the lymphatic metastasis of GC.
Table XI.
Associations between aberrant expression of long non-coding RNAs and lymph node metastasis status of gastric cancer by logistic regression analysis.
| Gene symbol | β | Standard error | Wald | P-value | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|---|
| RP5-919F19 | 0.181 | 0.070 | 6.639 | 0.010a | 1.199 | 1.044–1.376 |
| UCA1 | 0.071 | 0.045 | 2.476 | 0.116 | 1.073 | 0.983–1.172 |
| MEG3 | −0.079 | 0.033 | 5.689 | 0.017a | 0.924 | 0.866–0.986 |
Statistically significant (P<0.05). UCA1, urothelial carcinoma-associated 1; MEG3, maternally expressed 3.
Discussion
Although there appears to have been a steady global decline in the incidence of GC and associated mortality over several decades (17), it is still a disease of substantial incidence and mortality in China, with a large number of patients diagnosed at an advanced stage and with a poor prognosis (18). Furthermore, endoscopic biopsy and pathological morphological observation cannot detect all precancerous lesions and early stages of GC (19). Therefore, in order to improve this situation, the identification of the genes and regulatory mechanisms involved in lymph node metastasis has become a research area of increasing interest. In recent years, a large number of lncRNAs have been identified in genetic studies, and have been found to be associated with various diseases (20). The mechanisms by which lncRNAs may participate in cancer development are currently being studied (21–23). However, lncRNAs in GC have predominantly been reported in Western countries and in Japan, whereas few studies have been performed on Chinese populations (24). Although the mechanism of GC has been widely studied, the exact pathogenesis of this disease remains unclear (25). The molecular pathology of GC also varies among populations, mainly due to differential exposures to disease risk factors, including customs and habits, Helicobacter pylori variants, and medical conditions (26). In the present study, the aim was to establish lncRNA expression profiles for GC in a known high-risk population in Wuwei, north-west China, and to investigate the association between significant differential expression of various lncRNAs and the clinicopathological characteristics of GC.
To the best of our knowledge, the present study is the first report on differential lncRNA expression in a population of patients with GC from Wuwei. The results revealed that certain lncRNA expression levels in GC samples differed from those in adjacent non-tumor tissues. Prior to this research, we had detected mRNA expression profiles with a lncRNA-mRNA combined microarray (15). Through constructing the lncRNA-mRNA co-expression network and with bioinformatics analysis, 14 key lncRNAs were identified, of which the majority were reported for the first time. Our previous research results also revealed that there were significant differences in the levels of RP5-919F19, CTD-2541M15, UCA1, AP000459, LOC101928316, LINC01071 and MEG3 in tumor tissues compared with adjacent non-tumor tissues (P<0.05). In the present study, these seven lncRNAs were selected for further validation by RT-qPCR in 82 pairs of human primary GC tissues and their adjacent non-tumor tissues. Subsequently, the associations between the expression levels of the seven selected lncRNAs in the GC samples and various clinicopathological characteristics were analyzed. Finally, logistic regression analysis was used to evaluate the association between differentially expressed lncRNAs and the lymph node metastasis status of GC.
In the present study, the results from the microarray and RT-qPCR experiments were in 100% agreement. Correlation analyses of the expression levels of the seven differentially expressed lncRNAs and the associated clinicopathological characteristics were performed. The results identified that six lncRNAs (RP5-919F19, CTD-2541M15, UCA1, LOC101928316, LINC01071 and MEG3) were associated with some of the following clinicopathological parameters: Patient gender, patient age, tumor size, tumor differentiation degree, TNM stage and lymph node metastasis status. Statistics revealed that RP5-919F19, LOC101928316 and MEG3 were associated with tumor differentiation degree, and RP5-919F19, UCA1 and MEG3 were significantly associated with lymph node metastasis (P<0.05), indicating that these lncRNAs are possibly involved in the invasion and metastasis of GC. In addition, logistic regression analysis suggested that RP5-919F19 and MEG3 may participate in the lymphatic metastasis of GC. Thus, the present findings may provide a novel method of exploration that will improve the prediction of lymphatic metastatic status in patients with GC post-surgery. Notably, the abnormal expression levels of LINC01071 and LOC101928316 were significantly associated with the age and gender of the patients, respectively (P<0.05).
An increasing number of studies have also identified a biological link between aberrant expression of lncRNAs and GC (27). Differential expression of certain lncRNAs, including H19, UCA1, HOTAIR, PVTI, CCAT1 and MEG3, have been hypothesized to be important features of GC (28). In combining the results of our studies, only UCA1 and MEG3 have been reported previously, and there was limited information available regarding the other lncRNAs.
The LncRNAdb and LncRNA Diseases databases indicated that UCA1, which is located on chromosome 19, comprises three exons. UCA1 was initially found and established in bladder transitional cell carcinoma (29). A recent study also reported that its expression was markedly increased in GC tissues and cell lines compared with that in the normal control tissues, and that high UCA1 expression correlated with poorer differentiation, tumor size, invasion depth and TNM stage in GC; furthermore, increased UCA1 expression was associated with decreased overall and disease-free survival times of the patients (30).
The present study identified downregulated levels of MEG3 (which is located on the chromosome 14q32, and acts as a tumor suppressor gene) in GC tissues compared with healthy tissues; MEG3 downregulation is associated with poor prognosis and promotes cell proliferation in GC (31). These results indicate that UCA1 and MEG3 lncRNAs are important factors in the development of GC, as well as in the invasion and lymphatic metastasis of this cancer type.
The present study found that RP5-919F19, LOC101928316 and MEG3 were significantly associated with the degree of tumor differentiation; RP5-919F19, UCA1 and MEG3 were significantly associated with lymph node metastasis status; and RP5-919F19, CTD-2541M15 and UCA1 were significantly associated with TNM stage of GC patients These lncRNAs may prove useful for further study as novel candidate biomarkers in the diagnosis and classification of GC.
The current results indicate that RP5-919F19, CTD-2541M15, UCA1, LOC101928316 and MEG3 are potential novel molecular biomarkers that may be involved in the infiltration and metastasis of GC. In addition, according to epidemiological reports, the incidence of GC significantly increases with age, with a peak age of 50–65 years (32), and men are 2–3-fold more likely to develop GC compared with females (33). Thus, the significant associations identified between the differential expression of LINC01071 and LOC101928316 in GC tissues and patient age and gender may be of importance for diagnosis.
In conclusion, the present study revealed that RP5-919F19, CTD-2541M15, UCA1, LOC101928316, LINC01071 and MEG3 are involved in the development of GC. This provides preliminary data that may aid in increasing the understanding of the potential functions of these lncRNAs. The results also suggest that RP5-919F19, LOC101928316, CTD-2541M15, UCA1 and MEG3 are closely associated with the invasion and metastasis of GC, which suggests that these lncRNAs may have potential as biomarkers for the diagnosis, prognosis and classification of GC. Further studies of these targets are required to assess their potential clinical uses.
Table V.
Association between the expression of CTD-2541M15 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | Expression of CTD-2541M15 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.376 | ||
| Male | 59 (72) | 2.643±7.687 | |
| Female | 23 (28) | 1.108±3.997 | |
| Age, years | 0.176 | ||
| ≤50 | 30 (37) | 0.618±6.310 | |
| >50 | 52 (63) | 2.858±7.537 | |
| Tumor size, cm | 0.449 | ||
| ≤5 | 43 (52) | 1.724±7.374 | |
| >5 | 39 (48) | 2.902±6.368 | |
| Degree of differentiation | 0.488 | ||
| Well/moderately | 28 (34) | 1.487±6.306 | |
| Poorly | 54 (66) | 2.615±7.201 | |
| TNM stage | 0.023a | ||
| I/II | 44 (54) | 0.704±4.116 | |
| III/IV | 38 (46) | 4.214±8.920 | |
| Lymph node status | 0.111 | ||
| No metastasis | 36 (44) | 0.922±3.961 | |
| Metastasis | 46 (56) | 3.398±8.473 |
Statistically significant (P<0.05). CTD-2541M15, MCPH1 antisense RNA 1; TNM, tumor-node-metastasis.
Table VI.
Association between the expression of UCA1 and clinicopathological characteristics of gastric cancer.
| Variable | Cases, n (%) | Expression of UCA1 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.080 | ||
| Male | 59 (72) | 2.968±6.813 | |
| Female | 23 (28) | −0.064±4.803 | |
| Age, years | 0.143 | ||
| ≤50 | 30 (37) | 3.040±10.778 | |
| >50 | 52 (63) | 6.848±19.462 | |
| Tumor size, cm | 0.020a | ||
| ≤5 | 43 (52) | 1.712±4.265 | |
| >5 | 39 (48) | 13.028±29.713 | |
| Degree of differentiation | 0.531 | ||
| Well/moderately | 28 (34) | 2.968±3.459 | |
| Poorly | 54 (66) | 4.880±14.617 | |
| TNM stage | 0.028a | ||
| I/II | 44 (54) | 2.135±7.055 | |
| III/IV | 38 (46) | 12.856±29.643 | |
| Lymph node status | 0.031a | ||
| No metastasis | 36 (44) | 1.258±4.306 | |
| Metastasis | 46 (56) | 11.779±27.650 |
Statistically significant (P<0.05). UCA1, urothelial carcinoma-associated 1; TNM, tumor-node-metastasis.
Table VII.
Association between the expression of AP000459 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | Expression of AP000459 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.556 | ||
| Male | 59 (72) | −14.210±28.921 | |
| Female | 23 (28) | −9.352±36.174 | |
| Age, years | 0.497 | ||
| ≤50 | 30 (37) | −10.731±27.139 | |
| >50 | 52 (63) | −6.491±22.496 | |
| Tumor size, cm | 0.531 | ||
| ≤5 | 43 (52) | −15.297±30.456 | |
| >5 | 39 (48) | −10.649±31.745 | |
| Degree of differentiation | 0.861 | ||
| Well/moderately | 28 (34) | −11.958±27.352 | |
| Poorly | 54 (66) | −13.322±33.011 | |
| TNM stage | 0.924 | ||
| I/II | 44 (54) | −13.403±27.846 | |
| III/IV | 38 (46) | −12.689±36.611 | |
| Lymph node status | 0.662 | ||
| No metastasis | 36 (44) | −14.864±30.097 | |
| Metastasis | 46 (56) | −11.601±31.939 |
TNM, tumor-node-metastasis.
Table VIII.
Association between the expression of LOC101928316 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | Expression of LOC101928316 2−ΔΔCq (mean ± standard deviation) | P-value |
|---|---|---|---|
| Gender | 0.030a | ||
| Male | 59 (72) | −5.877±18.569 | |
| Female | 23 (28) | −23.165±28.777 | |
| Age, years | 0.464 | ||
| ≤50 | 30 (37) | −8.078±20.457 | |
| >50 | 52 (63) | −5.235±13.043 | |
| Tumor size, cm | 0.684 | ||
| ≤5 | 43 (52) | −4.714±12.656 | |
| >5 | 39 (48) | −6.018±14.965 | |
| Degree of differentiation | 0.048a | ||
| Well/moderately | 28 (34) | −0.853±7.533 | |
| Poorly | 54 (66) | −8.436±20.044 | |
| TNM stage | 0.913 | ||
| I/II | 44 (54) | −5.443±14.873 | |
| III/IV | 38 (46) | −5.091±12.109 | |
| Lymph node status | 0.796 | ||
| No metastasis | 36 (44) | −7.206±21.983 | |
| Metastasis | 46 (56) | −6.129±13.627 |
Statistically significant (P<0.05). TNM, tumor-node-metastasis.
Table IX.
Association between the expression of LINC01071 and clinicopathological characteristics of gastric cancer patients.
| Variable | Cases, n (%) | 2−ΔΔCq (mean ± standard deviation) Expression of LINC01071 | P-value |
|---|---|---|---|
| Gender | 0.988 | ||
| Male | 59 (72) | −12.848±37.204 | |
| Female | 23 (28) | −12.984±28.666 | |
| Age, years | 0.039a | ||
| ≤50 | 30 (37) | −12.208±21.206 | |
| >50 | 52 (63) | −3.312±13.601 | |
| Tumor size, cm | 0.679 | ||
| ≤5 | 43 (52) | −9.411±19.770 | |
| >5 | 39 (48) | −7.330±21.682 | |
| Degree of differentiation | 0.613 | ||
| Well/moderately | 28 (34) | −11.744±22.416 | |
| Poorly | 54 (66) | −8.719±25.297 | |
| TNM stage | 0.557 | ||
| I/II | 44 (54) | −9.716±19.924 | |
| III/IV | 38 (46) | −6.733±21.545 | |
| Lymph node status | 0.908 | ||
| No metastasis | 36 (44) | −9.720±20.630 | |
| Metastasis | 46 (56) | −10.392±27.488 |
Statistically significant (P<0.05). LINC01071, tumor suppressor candidate 8; TNM, tumor-node-metastasis.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the National Natural Science Foundation of China (grant nos. 81673132, 81472939 and 8117261), the Qing Lan Project (grant no. 2012), the 333 Project of Jiangsu Province (grant no. 2012), the Liu Da Ren Cai Gao Feng Project of Jiangsu Province (grant no. 2013-WSW-053) and the Fundamental Research Funds for the Central Universities.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
CYL and GYL conceived and designed the study. CYL, WZY, JS and SY performed the experiments. YQZ and SMM analyzed and interpreted the results. YCY, ZYZ and WHZ performed the gastric cancer patients' tissue sample collection and quality control. LHY and YPP assisted with study design and provided advice throughout. CYL performed analysis and quality control, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Gansu Wuwei Tumor Hospital. All patients provided written informed consent to participate in the present study.
Consent for publication
All participants confirmed that the data can be published.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lan H, Zhu N, Lan Y, Jin K, Teng L. Laparoscopic gastrectomy for gastric cancer in China: An overview. Hepatogastroenterology. 2015;62:234–239. [PubMed] [Google Scholar]
- 2.Yajima H, Omura N, Takahashi N, Yoshida K, Yanaga K. Additional gastrectomy after endoscopic mucosal resection for early gastric cancer. Int Surg. 2015;100:169–172. doi: 10.9738/INTSURG-D-14-00144.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruyama K, Katai H. Surgical treatment of gastric cancer in Japan, trend from standardization to individualization. Chirurgia (Bucur) 2014;109:722–730. [PubMed] [Google Scholar]
- 4.Kwon KA. Is the double channel gastroscope useful in endoscopic mucosal resection for large sessile colon polyps? Clin Endosc. 2015;48:89–90. doi: 10.5946/ce.2015.48.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Y, Huang J, Zhang Y, Ma LM, Fan ZN. Using a gastroscope to accomplish ERCP: A forward-viewing endoscope for cannulation of the intradiverticular papilla. Endoscopy. 2014;46(Suppl 1) doi: 10.1055/s-0033-1359194. UCTN: E139. [DOI] [PubMed] [Google Scholar]
- 6.Ju H, Ma Y, Liang K, Zhang C, Tian Z. Function of high-resolution manometry in the analysis of peroral endoscopic myotomy for achalasia. Surg Endosc. 2016;30:1094–1099. doi: 10.1007/s00464-015-4304-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 8.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhu YP, Bian XJ, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ. Long noncoding RNA expression signatures of bladder cancer revealed by microarray. Oncol Lett. 2014;7:1197–1202. doi: 10.3892/ol.2014.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng WS, Tao H, Hu EP, Liu S, Cai HR, Tao XL, Zhang L, Mao JJ, Yan DL. Both genes and lncRNAs can be used as biomarkers of prostate cancer by using high throughput sequencing data. Eur Rev Med Pharmacol Sci. 2014;18:3504–3510. [PubMed] [Google Scholar]
- 11.Wang S, Tran EJ. Unexpected functions of lncRNAs in gene regulation. Commun Integr Biol. 2013;6:e27610. doi: 10.4161/cib.27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung T, Chang HY. Long noncoding RNA in genome regulation: Prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin XC, Zhu Y, Chen WB, Lin LW, Chen DH, Huang JR, Pan K, Lin Y, Wu BT, Dai Y, Tu ZG. Integrated analysis of long non-coding RNAs and mRNA expression profiles reveals the potential role of lncRNAs in gastric cancer pathogenesis. Int J Oncol. 2014;45:619–628. doi: 10.3892/ijo.2014.2431. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, Teng L. LncRNAs: Emerging biomarkers in gastric cancer. Future Oncol. 2015;11:2427–2441. doi: 10.2217/fon.15.175. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Liang G, Yao W, Sui J, Shen X, Zhang Y, Ma S, Ye Y, Zhang Z, Zhang W, et al. Differential expression profiles of long non-coding RNAs reveal potential biomarkers for identification of human gastric cancer. Oncol Rep. 2016;35:1529–1540. doi: 10.3892/or.2015.4531. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Patru CL, Surlin V, Georgescu I, Patru E. Current issues in gastric cancer epidemiology. Rev Med Chir Soc Med Nat Iasi. 2013;117:199–204. [PubMed] [Google Scholar]
- 18.Li G, Hu Y, Liu H. Current status of randomized controlled trials for laparoscopic gastric surgery for gastric cancer in China. Asian J Endosc Surg. 2015;8:263–267. doi: 10.1111/ases.12198. [DOI] [PubMed] [Google Scholar]
- 19.Rajan E, Gostout CJ, Aimore BE, Moran EA, Locke RG, Szarka LA, Talley NJ, Deters JL, Miller CA, Knipschield MA, et al. Endoscopic full-thickness biopsy of the gastric wall with defect closure by using an endoscopic suturing device: Survival porcine study. Gastrointest Endosc. 2012;76:1014–1019. doi: 10.1016/j.gie.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, Zhang Q, Yan G, Cui Q. LncRNADisease: A database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:D983–D986. doi: 10.1093/nar/gks1099. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 23.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: New links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YY, Ye ZY, Zhao ZS, Tao HQ, Li SG. Systems biology approach to identification of biomarkers for metastatic progression in gastric cancer. J Cancer Res Clin Oncol. 2010;136:135–141. doi: 10.1007/s00432-009-0644-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Song Y, Piao D, Liu T, Zhao L. Identification of genes and long non-coding RNAs associated with the pathogenesis of gastric cancer. Oncol Rep. 2015;34:1301–1310. doi: 10.3892/or.2015.4129. [DOI] [PubMed] [Google Scholar]
- 26.Pizzi M, Saraggi D, Fassan M, Megraud F, Di Mario F, Rugge M. Secondary prevention of epidemic gastric cancer in the model of Helicobacter pylori-associated gastritis. Dig Dis. 2014;32:265–274. doi: 10.1159/000357857. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Sun J, Song Y, Gao P, Zhao J, Huang X, Liu B, Xu H, Wang Z. The novel long noncoding RNA AC138128.1 may be a predictive biomarker in gastric cancer. Med Oncol. 2014;31:262. doi: 10.1007/s12032-014-0262-7. [DOI] [PubMed] [Google Scholar]
- 28.Li PF, Chen SC, Xia T, Jiang XM, Shao YF, Xiao BX, Guo JM. Non-coding RNAs and gastric cancer. World J Gastroenterol. 2014;20:5411–5419. doi: 10.3748/wjg.v20.i18.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie XJ, Li X, Wang F, Chen W. Cellular localization and tissue expression pattern of UCA1, a non-coding RNA. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:57–60. (In Chinese) [PubMed] [Google Scholar]
- 30.Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C, Ye H, Zhou B, Chen JJ, Chen P. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol. 2015;17:640–646. doi: 10.1007/s12094-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 32.Oh S, Kim N, Yoon H, Choi YJ, Lee JY, Park KJ, Kim HJ, Kang KK, Oh DH, Seo AY, et al. Risk factors of atrophic gastritis and intestinal metaplasia in first-degree relatives of gastric cancer patients compared with age-sex matched controls. J Cancer Prev. 2013;18:149–160. doi: 10.15430/JCP.2013.18.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Zhao Q. The demographic characteristics of histological types of gastric cancer with gender, age, and tumor location. J Gastrointest Cancer. 2009;40:98–100. doi: 10.1007/s12029-009-9107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


