Abstract
Sinomenine is a monomer extracted from the traditional Chinese medicine plant Sabia japonica, which possesses several pharmacological properties including prominent abirritation, mitigation, anti-inflammation, immune suppression, cough relief, stimulation of histamine release, decrease in blood pressure and antiarrhythmia. Sinomenine is clinically employed to treat rheumatic disease. To investigate the impact of combined sinomenine treatment with acupuncture on the progression of arthritis and explore the potential underlying molecular mechanisms, the present study analyzed a collagen-induced arthritis model. Results from the combined curative (CC) treatment group (combined treatment with sinomenine and acupuncture) demonstrated a decrease in volume changes and arthritis score changes within rat paws, and increased the overall body weight in arthritic rats. CC treatment significantly decreased tumor necrosis factor α, interleukin (IL)-6, IL-1β and IL-8 serum levels in arthritic rats. CC treatment significantly increased superoxide dismutase and inhibited malondialdehyde levels in arthritic rats. The protein expression of cyclooxygenase-2, inducible nitric oxide synthase, matrix metalloproteinase (MMP)2 and MMP9 in arthritic rats was suppressed owing to CC treatment. Finally, nuclear factor κB and phosphorylated p38 mitogen-activated protein kinase (MAPK) protein expression in arthritic rats were also suppressed following CC treatment. The results indicate that the combined treatment of sinomenine and acupuncture on collagen-induced arthritis takes effect through the nuclear factor κB and MAPK signaling pathway.
Keywords: sinomenine, acupuncture, arthritis, nuclear factor κB, mitogen-activated protein kinase
Introduction
Rheumatoid arthritis (RA) is an autoimmune condition characterized by chronic inflammation due to the host's own immune system attacking joints across the body (1). Inflammation or damage of peripheral tissues experienced in RA may lead to a series of alterations in the spinal cord (2). As RA progresses to an advanced stage, damaged articular tissue and arthromeningitis are able to activate peripheral silent nociceptors (2). This causes continuous and intense silent signals to be sent to the central nervous system for modulation and integration, leading to the stimulation of excitatory amino acids within the spinal cord, inducing the production and secretion of cytokines (3). Furthermore, the continuous excitability of spinal cord nociceptive neurons may be enhanced (3).
Nuclear factor-κB (NF-κB), a nuclear transcription factor in eukaryotes, is able to bind to nucleotide sequences in promoter regions to activate genetic transcription, which may serve a critical regulatory function in the genetic expression of a number of genes, particularly inflammatory or immunoreaction-related genes (4). Activated NF-κB is able to combine with specific κB sequences in gene promoter regions of inflammatory factors and participate in genetic transcription of tumor necrosis factor α (TNF-α), interleukin (IL)-1, IL-2, IL-6, IL-8, IL18, intercellular cell adhesion molecule-1 (ICAM-1), COX-2 and nitric oxide synthase (NOS). Within the nervous system, functional NF-κB exists in many types of neural cell, including neurons, astrocytes, microglial cells and oligodendrocytes, and participates in the regulation of nerve cell apoptosis and, on a symptomatic level, aching following nerve injury or an inflammatory response (5).
Mitogen-activated protein kinase (MAPK) is a key molecular signaling pathway in eukaryotes and serves an essential function in regulating cellular structure and functional activities (6). In eukaryotes, the MAPK signaling channel contains several subfamilies including p38, extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and ERK5 (6). A previous study demonstrated that the p38, ERK and JNK signal transduction pathways had a marked association with cartilage injury identified in RA (7). These signaling pathways are able to induce cartilage cells to produce matrix metalloproteinase (MMP), accelerate the pathological degradation of cartilago articularis and also mediate a series of responses, including the proliferation, apoptosis and differentiation of chondrocytes (8). Clarification of the underlying molecular mechanisms of the MAPK signaling pathway in the occurrence and progression of RA is required in future study.
Traditional Chinese medicine asserts that the majority of middle-aged and elderly individuals with knee osteoarthritis are caused by the deficiency of liver and kidney function, insufficiency of vital energy, and blood, tendon and vessel malnutrition (9). Sinomenine is one monomer of Chinese traditional herbs and may possess anti-inflammation, anti-oxidation and anti-apoptosis effects (10). Acupuncture is an important therapy used in complementary and alternative medicine, and may exert anti-inflammatory effects (11). Acupuncture targets specific acupoints to improve the body microenvironment and thus effectively treat certain diseases (12,13).
Therefore, the present study investigated the combined treatment of sinomenine and acupuncture on collagen-induced arthritis.
Materials and methods
Animal experimentation
Protocols followed in the present study were approved by the Animal Care and Use Committee [Chongqing TCM (No. 1) Hospital, Chongqing, China]. Male Sprague-Dawley rats (160±10 g) were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China) and maintained at 22±1°C and 55±5% humidity under a 12-h light/12-h dark cycle with free access to a standard pellet diet and tap water.
Induction of collagen-induced arthritis and grouping of data
All rats (n=50) were randomly assigned to 5 groups (10 per group): Control, model, acupuncture treatment group, sinomenine treatment group, and combined acupuncture and sinomenine treatment group. Collagen-induced arthritic rats were subcutaneously injected with 100 µl bovine type II collagen (Merck KGaA, Darmstadt, Germany). Rats which developed arthritis following collagen injection at 25 days were used in the present study. Within the acupuncture treatment group, acupuncture was performed on arthritic rats at the acupoints of Baihui and Yintang each day for 19 days. Within the sinomenine treatment group, 50 mg/kg sinomenine was gavaged into each arthritic rat every 2 days for 19 days. In the combined acupuncture and sinomenine group, acupuncture treatment and 50 mg/kg sinomenine was gavaged into each arthritic rat every 2 days for 19 days. In control and model groups, the normal rats or collagen-induced arthritis rats were treated with normal saline.
Evaluation of collagen-induced arthritis
Paw length and overall body weight for each rat were measured using vernier calipers every 2 days. Arthritis score was calculated as follows: 0, unaffected; 1, 1 type of joint affected; 2, 2 types of joint affected; 3, 3 types of joint affected; and 4, 3 types of joint affected plus maximal erythema and swelling (14).
Determination of serum cytokine levels
Following the 19-day treatment regime, rats were anesthetized with 40 mg/kg ketamine and 5 mg/kg xylazine, blood samples were collected using an orbital blood sampling protocol (14), and then rats were sacrificed. Serum samples were prepared following centrifugation at 1,800 × g for 15 min at 4°C. Serum levels of TNF-α (cat no. H052), IL-1β (cat no. H002), IL-6 (cat no. H007), IL-8 (cat no. H008), superoxide dismutase (cat no. A001-3), malondialdehyde (cat no. A003-1), MMP2 (cat no. H146-1) and MMP9 (cat no. H146-4) were determined using commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's protocol.
Western blot analysis
Following homogenization of each synovial tissue sample, total protein was extracted using radioimmunoprecipitation assay buffer on ice for 30–60 min and determined using a bicinchoninic acid assay kit. Total protein (50–100 µg) was separated by SDS-PAGE (8–10% gel) and then transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were blocked with 5% skimmed milk powder in tris-buffered saline with 0.1% Tween 20 for 1 h at 37 °C and incubated with primary antibodies against COX-2 (cat no., sc-7951, 1:500), inducible nitric oxide synthase (iNOS, cat no., sc-649, 1:500), NF-κB (cat no., sc-109, 1:500), phosphorylated p38 (cat no., sc-7975-R, 1:200) and GAPDH (cat no., sc-25778, 1:3,000) overnight at 4°C. All primary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodY (cat no., sc-2030, 1:5,000; Santa Cruz Biotechnology, Inc.) was incubated with the membranes for 1 h at 37°C. Protein blanks were visualized using an enhanced chemiluminescence detection reagent (Bio-Rad Laboratories, Inc.), determined using a ChemiDoc XRS gel imaging system (Bio-Rad Laboratories, Inc.), and analyzed using Quantity One software (version 3.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard deviation and evaluated using one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
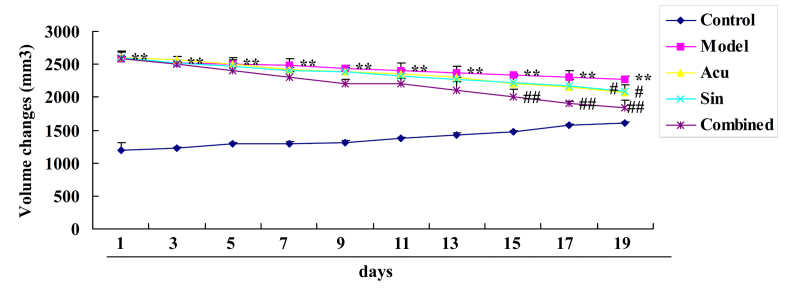
Combined curative (CC) treatment affects volume changes in collagen-induced arthritis
Results demonstrated that volume changes within the collagen-induced arthritis model group were greater compared with those of the control group (Fig. 1). Treatment with acupuncture or sinomenine significantly inhibited volume changes in collagen-induced arthritis model compared with the control group (Fig. 1); however, the CC treatment (acupuncture with sinomenine) significantly inhibited volume changes in collagen-induced arthritis compared with those of the acupuncture or sinomenine treatment groups (Fig. 1).
Figure 1.
Combined (Acu and Sin) curative treatment affects volume changes in collagen-induced arthritis. **P<0.01 vs. control group, #P<0.01 vs. arthritis model group; ##P<0.01 vs. Acu treatment group. Acu, acupuncture; Sin, sinomenine.
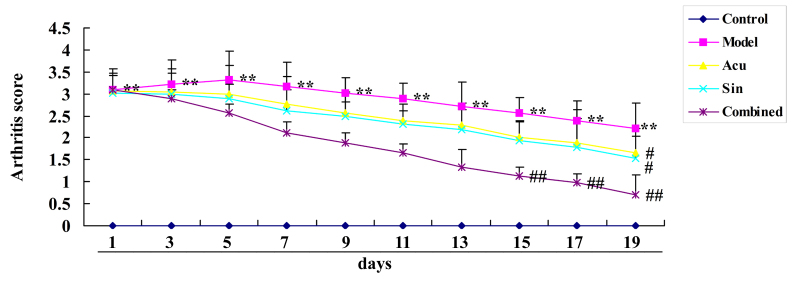
CC treatment affects arthritis score changes in collagen-induced arthritis
Results demonstrated that CC treatment affects the arthritis score in collagen-induced arthritis. Fig. 2 demonstrates that the arthritis score of the collagen-induced arthritis model group was increased compared with that of the control group. Treatment with acupuncture or sinomenine significantly decreased the arthritis score in arthritic rats compared with that of the collagen-induced arthritis model group (Fig. 2). Results demonstrated that the CC treatment decreased the arthritis score in collagen-induced arthritic rabbits compared with that of the collagen-induced arthritis model group (Fig. 2).
Figure 2.
Combined (Acu and Sin) curative treatment affects arthritis score changes in collagen-induced arthritis. **P<0.01 vs. control group; #P<0.01 vs. arthritis model group; ##P<0.01 vs. Acu treatment group. Acu, acupuncture; Sin, sinomenine.
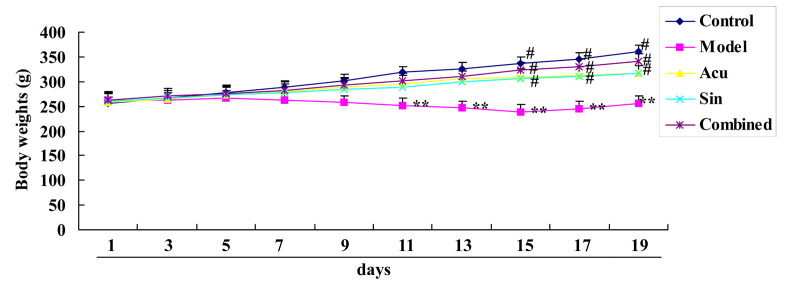
CC treatment affects body weights in collagen-induced arthritis
Results in Fig. 3 demonstrated that the overall body weight of collagen-induced arthritic rats was decreased compared with that of the control group. Treatment with acupuncture or sinomenine also led to a significant increase in body weight in collagen-induced arthritic rats compared with that in the collagen-induced arthritis model group (Fig. 3). Furthermore, overall body weights within the CC treatment group were increased compared with those of the acupuncture or sinomenine treatment groups; however, results were not statistically significant (Fig. 3).
Figure 3.
Combined (Acu and Sin) curative treatment affects body weight in collagen-induced arthritis. **P<0.01 vs. control group; #P<0.01 vs. arthritis model group. Acu, acupuncture; Sin, sinomenine.
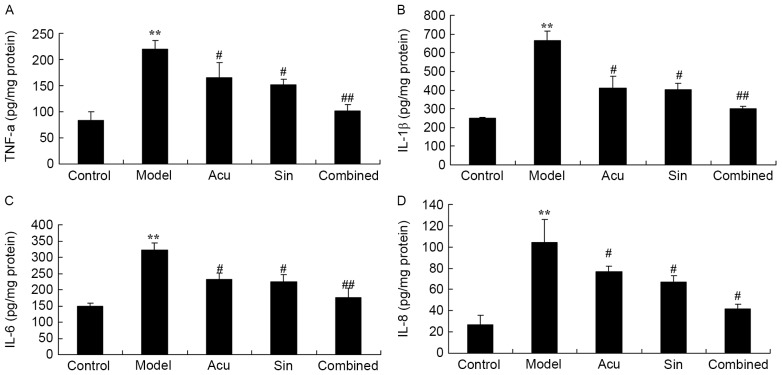
CC treatment affects TNF-α, IL-6, IL-1β and IL-8 serum levels in collagen-induced arthritis
Anti-inflammatory effects of CC treatment in collagen-induced arthritis were determined. Fig. 4 presents the significant increase in TNF-α, IL-6, IL-1β and IL-8 serum levels in collagen-induced arthritic rabbits compared with those of the control group. However, TNF-α, IL-6, IL-1β and IL-8 serum levels increases were lessened by acupuncture or sinomenine treatment compared with the model group (Fig. 4). The CC treatment markedly reduced TNF-α, IL-6, IL-1β and IL-8 serum levels in collagen-induced arthritic rabbits compared with those in the acupuncture or sinomenine group (Fig. 4).
Figure 4.
Combined (Acu and Sin) curative treatment affects TNF-α, IL-6, IL-1β and IL-8 serum levels in collagen-induced arthritis. Combined curative treatment effects on (A) TNF-α, (B) IL-6, (C) IL-1β and (D) IL-8 serum levels in collagen-induced arthritis. **P<0.01 vs. control group; #P<0.01 vs. arthritis model group, ##P<0.01 vs. arthritis model group. TNF-α, tumor necrosis factor α; IL, interleukin; Acu, acupuncture; Sin, sinomenine.
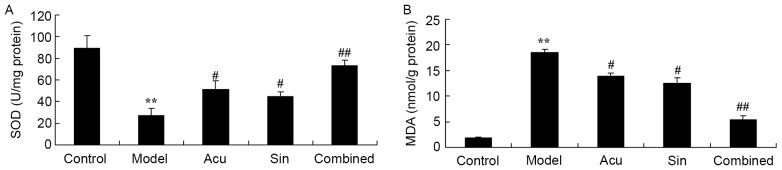
CC treatment affects SOD and MDA serum levels in collagen-induced arthritis
Anti-inflammatory effects of the CC treatment on oxidative stress in collagen-induced arthritic rabbits was investigated. Compared with the control group, SOD serum level inhibition and MDA serum level induction were observed in collagen-induced arthritic rabbits (Fig. 5). Treatment with acupuncture or sinomenine significantly increased SOD serum level inhibition and reduced MDA serum level induction in collagen-induced arthritic rabbits compared with that in the model group (Fig. 5). The CC treatment significantly reversed SOD serum level inhibition and MDA serum level induction in collagen-induced arthritic rabbits compared with that in the acupuncture or sinomenine group (Fig. 5).
Figure 5.
Combined (Acu and Sin) curative treatment affects SOD and MDA serum levels in collagen-induced arthritis. Combined curative treatment effects on (A) SOD and (B) MDA serum levels in collagen-induced arthritis. **P<0.01 vs. control group, #P<0.01 vs. arthritis model group, ##P<0.01 vs. arthritis model group. SOD, superoxide dismutase; MDA, malondialdehyde; Acu, acupuncture; Sin, sinomenine.
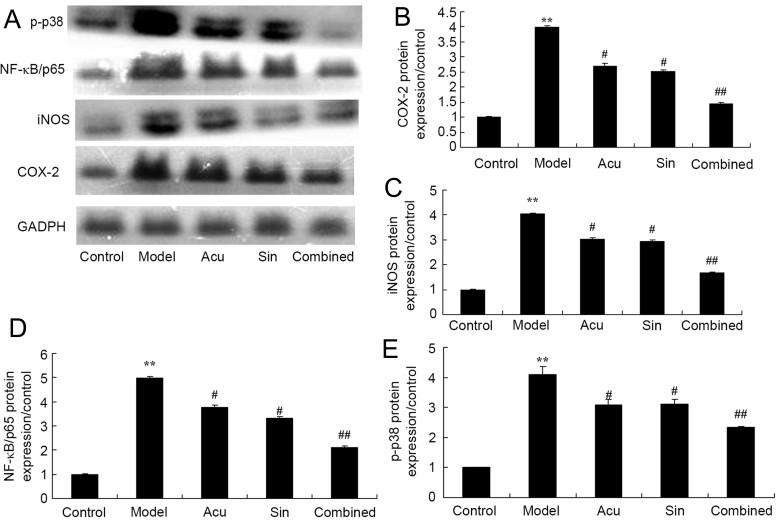
CC treatment affects COX-2, iNOS, NF-κB and p-p38 protein expression in collagen-induced arthritis
The CC treatment affected COX-2, iNOS, NF-κB and p-p38 protein expression in collagen-induced arthritis. The results demonstrated in Fig. 6 demonstrated that COX-2, iNOS, NF-κB and p-p38 protein expression was significantly increased in the collagen-induced arthritis model group compared with that in the control group. Acupuncture or sinomenine treatment significantly decreased COX-2, iNOS, NF-κB and p-p38 protein expression in collagen-induced arthritic rabbits compared with that in the collagen-induced arthritis model group (Fig. 6). CC treatment significantly decreased COX-2 protein expression in collagen-induced arthritic rabbits compared with that in the acupuncture or sinomenine treatment group (Fig. 6).
Figure 6.
Combined (Acu and Sin) curative treatment affects COX-2, iNOS, NF-κB and p-p38 protein expression in collagen-induced arthritis. (A) Western blot analysis demonstrating the effect of combined curative treatment on COX-2, iNOS, NF-κB and p-p38 protein expression. (B-E) Quantification of COX-2, iNOS, NF-κB and p-p38 protein expression in collagen-induced arthritis. **P<0.01 compared with control group; #P<0.01 compared with arthritis model group, ##P<0.01 compared with arthritis model group.
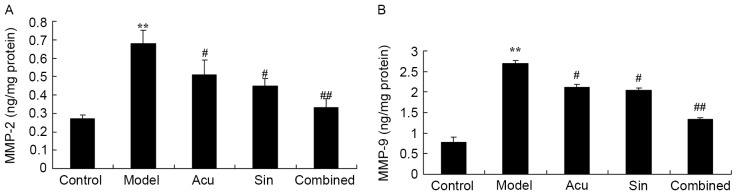
CC treatment affects MMP2 and MMP9 contents in collagen-induced arthritis
ELISA was utilized in order to unambiguously investigate the effects of the CC treatment on MMP2 and MMP9 levels in collagen-induced arthritis. Acupuncture or sinomenine treatment significantly decreased MMP2 and MMP9 contents in collagen-induced arthritis rats compared with those in the collagen-induced arthritis model group (Fig. 7). CC treatment significantly decreased MMP2 and MMP9 levels in collagen-induced arthritic rats compared with those in the acupuncture or the sinomenine treatment group (Fig. 7). Results in Fig. 7 demonstrate that MMP2 and MMP9 levels in the collagen-induced arthritis model group were increased compared with those in the control group.
Figure 7.
Combined (Acu and Sin) curative treatment affects MMP2 and MMP9 contents in collagen-induced arthritis. (A) Combined curative treatment affects MMP2 and (B) MMP9 contents in collagen-induced arthritis. **P<0.01 vs. control group; #P<0.01 vs. arthritis model group, ##P<0.01 compared with arthritis model group.
Discussion
RA is characterized by fibrosis, rhagades, anabrosis and the progressive destruction of the cartilago articularis, formation of osteophytes within the joint margin and synovial inflammation (15). RA is the primary cause of joint disability in middle-aged and aged individuals. As the aging population within China increases, the morbidity of RA also increases annually (15,16). As a result, RA has attracted extensive attention. Currently, RA therapy is insufficient in preventing disease progression, eventually resulting in severe malformation in the functioning of the joints (16). In order to produce effective RA treatment, the underlying molecular mechanisms require elucidation. Despite previous studies outlining potential RA risk factors, including senility, obesity, inflammation, trauma and genetic factors, the underlying molecular pathogenesis of RA remains unclear (16). Results of the present study demonstrated that CC treatment inhibited volume change decreases and decreased the arthritis score in the paws of rats, and also increased overall body weight in arthritic rats.
Evidence suggests that synovial inflammation serves an important function in RA disease progression (17). Early-stage RA exhibits hypertrophic synovium and fibrosis of the joints, secretion of inflammatory cytokines and the presence of cartilage matrix-degrading enzymes (17). In a previous study carried out on patients with RA, arthroscopy was utilized following a 1-year follow-up visit and demonstrated that hypertrophic synovium and changes in inflammation were increased by 50%, with an increase in articular cartilage injury also evident (18). Therefore, synovial inflammation is considered to be a predictive factor of the exacerbation of the degeneration of articular cartilage in RA.
The stimulation of IL-1β was able to induce the increase in MMP-2 expression in fibroblast-like synovial cells of rheumatoid arthritis (19). Evidence suggests that the synthesis and secretion of IL-15 in synovial cells of RA is able to activate and stimulate T-lymphocytes and therefore secrete pro-inflammatory cytokines in vitro. These pro-inflammatory cytokines are able to stimulate synoviocytes to express IL-15, IL-8 and IL-6 and induce a positive feedback regulatory pathway (20). Activated T-lymphocytes are also able to act on synoviocytes through the secretion of IL-17, and stimulation of IL-8 and IL-6 expression (21). The present study demosntrates that CC treatment markedly inhibited TNF-α, IL-6, IL-1β and IL-8 serum levels, and COX-2 and iNOS protein expression in collagen-induced arthritis rats compared with acupuncture or sinomenine treatment alone.
NF-κB is a key molecule in the Toll-like receptor 4 (TLR4) signal transduction pathway (22). Several programmed cells that serve a function in inflammation, immune response, cell apoptosis, tumorigenesis and tumor metastasis are regulated by NF-κB (23). Therefore, developing therapies that specifically target NF-κB in RA is of primary importance due to the hypothesis that the destruction associated with RA may be decreased through the suppression of NF-κB activity (23).
The TLR4 signaling pathway may also promote the expression of synovioblasts in knee osteoarthritis (22). NF-κB has been associated with a regulatory effect on osteopontin in arthritis (22). Results of the present study demonstrate that CC treatment also inhibited NF-κB protein expression in collagen-induced arthritic rats. Results of the present study demonstrate that CC treatment also inhibited NF-κB protein expression in collagen-induced arthritic rats. Zhao et al (24) demonstrated that sinomenine inhibits the maturation of monocyte-derived dendritic cells through NF-κB expression. Furthermore, Zhang et al (25) demonstrated that acupuncture decreased NF-κB p65 expression in chronic atrophic rats with gastritis.
Chondrocytes synthesize cartilage matrix and serve an essential function in sustaining the normal structure and function of the cartilago articularis. When chondrocytes are stimulated by inflammatory (including TNF-α, IL-6, IL-1β and IL-8) and mechanical stress, they transmit signals to transcription factors through signal transduction pathways and regulate pathophysiological processes including the production, reconstruction, stability and repair of bone (26). The MAPK signaling pathway is the most important signal transduction system in mediating cartilage injury observed in RA (7). A recent study suggested that a key pathological change in RA was the degenerative degradation of the extracellular matrix (ECM), resulting in the progressive loss of cartilage elements, and degeneration of the structure and function of chondrocytes (27). In RA inflammatory and growth factors combine with receptors on the cytomembrane to activate the MAPK signal transduction pathway, leading to an increase in MMP expression, apoptosis of chondrocytes and cartilage destruction (28). Furthermore, MMPs are essential in RA disease progression as these molecules degrade the vast majority of cartilage ECM within the cartilago articularis (29). In particular, MMP2, MMP3 and MMP13 are the primary proteinases responsible for the acceleration of ECM degradation in RA (30). Therefore, repression of MMP2 and MMP9 expression may be a potential therapeutic strategy for RA in the future (30). Results of the present study indicated that CC treatment significantly suppressed p-p38 protein expression, and MMP2/MMP9 levels in collagen-induced arthritic rabbits when compared with acupuncture or sinomenine treatment alone. Fu et al (30) demonstrated that acupuncture promotes angiogenesis following myocardial ischemia through p38-MAPK expression.
In conclusion, in the present study, CC treatment inhibited volume changes and arthritis score changes within rat paws, increased overall body weight, and suppressed inflammation, oxidative stress, and COX-2, iNOS, MMP2 and MMP9 expression in arthritic rats through the NF-κB and MAPK signaling pathways. Further studies are required to validate the results generated from the present study, and therefore provide an evidence base for the future use of combined therapy (sinomenine and acupuncture) in the treatment of RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MX and SL conceived and designed the experiments; MX, RW and YC performed the experiments; MX and YC analyzed the data; SL wrote the paper.
Ethics approval and consent to participate
Protocols followed in the present study were approved by the Animal Care and Use Committee of Chongqing TCM No. 1 Hospital (Chongqing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kuncewitch M, Yang WL, Jacob A, Khader A, Giangola M, Nicastro J, Coppa GF, Wang P. Stimulation of Wnt/β-catenin signaling pathway with Wnt agonist reduces organ injury after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78:793–800. doi: 10.1097/TA.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B, Zhang H, Zeng M, He W, Li M, Huang X, Deng DY, Wu J. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biol Int. 2015;39:192–200. doi: 10.1002/cbin.10359. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb J, Zamora MR, Hodges T, Musk AW, Sommerwerk U, Dilling D, Arcasoy S, DeVincenzo J, Karsten V, Shah S, et al. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant. 2016;35:213–221. doi: 10.1016/j.healun.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: Results of a pilot study. Am J Respir Crit Care Med. 2003;168:121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira MFC, Rodrigues JC, Leone C, Adde FV. Acute bronchodilator responsiveness to tiotropium in postinfectious bronchiolitis obliterans in children. Chest. 2013;144:974–980. doi: 10.1378/chest.12-2280. [DOI] [PubMed] [Google Scholar]
- 6.Celik S, Doesch AO, Konstandin MH, Kristen AV, Ammon K, Sack FU, Schnabel P, Katus HA, Dengler TJ. Increased incidence of acute graft rejection on calcineurin inhibitor-free immunosuppression after heart transplantation. Transplant Proc. 2011;43:1862–1867. doi: 10.1016/j.transproceed.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 7.Cao XP, Han DM, Zhao L, Guo ZK, Xiao FJ, Zhang YK, Zhang XY, Wang LS, Wang HX, Wang H. Hepatocyte growth factor enhances the inflammation-alleviating effect of umbilical cord-derived mesenchymal stromal cells in a bronchiolitis obliterans model. Cytotherapy. 2016;18:402–412. doi: 10.1016/j.jcyt.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z, Liu JX, Zheng YQ. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin. 2015;36:421–428. doi: 10.1038/aps.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TT, Lu J, Zheng PQ, Liu SL, Wu J, Sun W, Sun QM, Ma NX, Ding XL, Chen M, Zou X. Yiqi Huayu Jiedu decoction inhibits the invasion and metastasis of gastric cancer cells through TGF-β/Smad pathway. Evid Based Complement Alternat Med. 2017;2017:1871298. doi: 10.1155/2017/1871298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang M, Li J, Zhu D, Luo C, Li C, Zhu C, Fan M, Yung KK, Mo Z. Effect of sinomenine on the morphine-dependence and related neural mechanisms in mice. Neurochem Res. 2017;42:3587–3596. doi: 10.1007/s11064-017-2407-5. [DOI] [PubMed] [Google Scholar]
- 11.Mi J, Chen X, Lin X, Guo J, Chen H, Wei L, Hong H. Treatment of persistent allergic rhinitis via acupuncture at the sphenopalatine acupoint: A randomized controlled trial. Trials. 2018;19:28. doi: 10.1186/s13063-017-2339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MS, Chen KH, Chen IF, et al. The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0150367. doi: 10.1371/journal.pone.0150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X, Zhou J, Qin Z, Liu Z. Acupuncture for Erectile Dysfunction: A Systematic Review. Biomed Res Int. 2016;2016:2171923. doi: 10.1155/2016/2171923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Q, Chen M, Fang X, Lau WB, Xue L, Zhao L, Zhang H, Liang YH, Bai X, Niu HY, et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age (Dordr) 2013;35:1017–1026. doi: 10.1007/s11357-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, de Latour Peffault R, Dominique S, de Revel T, Juvin K, Maillard N, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. doi: 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 16.Kapila A, Baz MA, Valentine VG, Bhorade SM. AIRSAC investigators: Reliability of diagnostic criteria for bronchiolitis obliterans syndrome after lung transplantation: A survey. J Heart Lung Transplant. 2015;34:65–74. doi: 10.1016/j.healun.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Xia T, Jiang K, Qiao X, Zhang X, Li J, Wang J, Nie J. Apoptosis of the tracheal epithelium can increase the number of recipient bone marrow-derived myofibroblasts in allografts and exacerbate obliterative bronchiolitis after tracheal transplantation in mice. Transplantation. 2016;100:1880–1888. doi: 10.1097/TP.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 18.Walker NM, Belloli EA, Stuckey L, Chan KM, Lin J, Lynch W, Chang A, Mazzoni SM, Fingar DC, Lama VN. Mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2 as key signaling intermediates in mesenchymal cell activation. J Biol Chem. 2016;291:6262–6271. doi: 10.1074/jbc.M115.672170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: Tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6:2080–2088. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 20.Königshoff M, Kneidinger N, Eickelberg O. Tgf-beta signaling in COPD: Deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly. 2009;139:554–563. doi: 10.4414/smw.2009.12528. [DOI] [PubMed] [Google Scholar]
- 21.Zarin AA, Behmanesh M, Tavallaei M, Shohrati M, Ghanei M. Overexpression of transforming growth factor (TGF)-beta1 and TGF-beta3 genes in lung of toxic-inhaled patients. Exp Lung Res. 2010;36:284–291. doi: 10.3109/01902140903578868. [DOI] [PubMed] [Google Scholar]
- 22.Ladak SS, Ward C, Ali S. The potential role of microRNAs in lung allograft rejection. J Heart Lung Transplant. 2016;35:550–559. doi: 10.1016/j.healun.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Nayak D, Yang W, Baskaran G, Ramachandran S, Sarma N, Aloush A, Trulock E, Hachem R, Patterson GA, Mohanakumar T. Dysregulated microRNA expression and chronic lung allograft rejection in recipients with antibodies to donor HLA. Am J Transplant. 2015;15:1933–1947. doi: 10.1111/ajt.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Li J, Yu K, Liu Y, Chen X. Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int Immunopharmacol. 2007;7:637–645. doi: 10.1016/j.intimp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Huang K, Zhong G, et al. Acupuncture Decreases NF-kappaB p65, miR-155, and miR-21 and Increases miR-146a Expression in Chronic Atrophic Gastritis Rats. Evid Based Complement Alternat Med. 2016;2016:9404629. doi: 10.1155/2016/9404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Yang W, Steward N, Sweet SC, Danziger-Isakov L, Heeger PS, Mohanakumar T. Role of circulating microRNAs in the immunopathogenesis of rejection following after pediatric lung transplantation. Transplantation. 2017;101:2461–2468. doi: 10.1097/TP.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong M, Wang X, Zhao HL, Chen XL, Yuan JH, Guo JY, Li KQ, Li G. Integrated analysis of transcription factor, microRNA and lncRNA in an animal model of obliterative bronchiolitis. Int J Clin Exp Pathol. 2015;8:7050–7058. [PMC free article] [PubMed] [Google Scholar]
- 28.Mauad T, van Schadewijk A, Schrumpf J, Hack CE, Fernezlian S, Garippo AL, Ejzenberg B, Hiemstra PS, Rabe KF, Dolhnikoff M. São Paulo BO Study Group: Lymphocytic inflammation in childhood bronchiolitis obliterans. Pediatr Pulmonol. 2004;38:233–239. doi: 10.1002/ppul.20064. [DOI] [PubMed] [Google Scholar]
- 29.Moeser A, Pletz MW, Hagel S, Kroegel C, Stallmach A. Lung disease and ulcerative colitis-mesalazine-induced bronchiolitis obliterans with organizing pneumonia or pulmonary manifestation of inflammatory bowel disease? Z Gastroenterol. 2015;53:1091–1098. doi: 10.1055/s-0041-103377. [DOI] [PubMed] [Google Scholar]
- 30.Fu SP, He SY, Xu B, Hu CJ, Lu SF, Shen WX, Huang Y, Hong H, Li Q, Wang N, et al. Acupuncture promotes angiogenesis after myocardial ischemia through H3K9 acetylation regulation at VEGF gene. PLoS One. 2014;9:e94604. doi: 10.1371/journal.pone.0094604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.