Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common pathological type of non-Hodgkin lymphoma (NHL). It is strongly correlated to the host immunity and infection status.
Aim: This study tested the hypothesis that hepatitis B virus (HBV) infection is also associated with DLBCL.
Methods: Clinical analysis of the correlation between DLBCL and HBV infection, detection of HBV in situ of DLBCL tissue, and biological experiments that determined whether HBV infects B lymphocytes were conducted.
Results: Our long-term clinical data showed that the positive rate of serum HBV was significantly increased in DLBCL patients (23.6%) compared to that in the general Chinese population (7.2%, P<0.001), especially in advanced stage lymphoma patients (P=0.003). In addition, HBV could infect B lymphocytes in vitro and the HBV antigen and nucleic acid could be detected intracellularly. Hepatitis B x protein (HBx) was also strongly expressed in tissues from DLBCL patients that were serum HBV surface antigen (HBsAg) positive. These patients responded less well to therapy with an odds ratio (OR) of 3.04.
Conclusions: HBV can infect B lymphocytes. It might be related to the development of DLBCL and may also impact the efficacy of treatment.
Keywords: Diffuse large B cell lymphoma, hepatitis B virus, infection
Introduction
Non-Hodgkin lymphoma (NHL) as one of the most common cancers accounts for 4-5% of all cancers 1. Diffuse large B-cell lymphoma (DLBCL) is the top pathological type occupying 30-60% of NHL 2. The cause of this disease is strongly correlated with the host immunity and infection status. All currently found tumor viruses can be related to the tumorigenesis and progression of NHL. For example, the Epstein-Barr virus, which has been shown to be associated with various cancers, including Burkitt's lymphoma, can infect B lymphocytes via the CD21 receptor on B-cell surface 3. Furthermore, the positive rate of hepatitis C virus antibody in B-cell NHL patients was significantly higher than that in healthy controls, suggesting a relationship between hepatitis C virus and B-cell NHL 4. Therefore, we speculated that hepatitis B virus (HBV) may also be associated with NHL.
HBV infection is a worldwide health problem with an estimated 257 million carriers of hepatitis B surface antigen (HBsAg) (WHO, 2017). HBV prevalence is highest in the endemic area of WHO Western Pacific Region, and the percentage of carriers of HBsAg is estimated to be 7.2% in China 5. Although there are few clinical trials to show a higher prevalence of HBsAg in DLBCL patients 6, there have been no large analyses of HBV that report the relationship to DLBCL severity, staging, or prognosis. At present, the effect of the HBV positivity rate of serum on the prognosis of DLBCL remains unknown 7, 8.
In patients with DLBCL that are positive for hepatitis B core antibody (HBcAb) and who are being treated with CD20-targeted antibodies, the ability of B-cells to secrete HBV-specific antibodies is impaired, which potentially activates replication of previously infected HBV and particularly increases the risk of serum HBsAg conversion 9, 10. However, a significantly higher proportion of HBV infection in patients before DLBCL treatment indicates that HBV infection in DLBCL patients is not correlated with the use of targeted chemotherapy drugs. In addition, human peripheral blood mononuclear cells (PBMCs) may habor HBV 11, suggesting that HBV infects antigen presenting lymphocytes 12, which indicates that lymphoma progression may be caused by the virus infection.
In order to test the hypothesis that HBV infection is correlated with DLBCL, interpretationally in part from HBV directly infecting B lymphocytes, a clinical analysis of the correlation between DLBCL and HBV infection was carried out, and whether HBV infects B lymphocytes in vitro was also determined. This project will shed light on the etiology of the novel interpretation to DLBCL by HBV infection, and facilitate the basis for new strategies to the treatment and prevention of this disease.
Materials and methods
Serum samples
Sera from NHL patients in the Shanghai Cancer Center from 2009 to 2017 January were included in this study. HBsAg, hepatitis B surface antibody (HBsAb), HBcAb, hepatitis B e antigen (HBeAg), and hepatitis B e antibody (HBeAb) in serum were tested by commercial electro-chemiluminescence assays (Abbott i2000, Abbott Laboratories, USA). HBV DNA was analyzed by quantitative polymerase chain reaction (PCR) using a commercial probe (Shanghai Kehua Bio-engineering, China) as per the manufacturer's instructions.
Cell culture
Pfeiffer cells, originally from the American Type Culture Collection, were cultured in RPMI 1640 (Gibco, USA) containing 10% FBS and 1% penicillin/streptomycin maintained at 37°C in a humidified 5% CO2 incubator. Cells were passaged every three days. PBMCs were separated from the uncoagulated blood by a density gradient centrifugation method using Ficoll Histopaque in a 15 mL centrifuge tube that was centrifuged for 20 min at 750×g in a swinging bucket without braking. The PBMCs in the interphase were aspirated and washed twice with sterile PBS by centrifugation at 300×g for 10 min. The pellet was resuspended by complete medium and cultured like the Pfeiffer cells.
HBV infection in vitro
Cells were infected with HBV at a multiplicity of infection (MOI) of 10. To synchronize the stage of infection, the plates were centrifuged at 300×g for 10 min and then co-incubated in a humidified 5% CO2 incubator for 24 h. Subsequently, the cells were washed extensively with pre-cooled phosphate-buffered solution to stop phagocytosis and extracellular HBV was removed by centrifugation at 300×g for 10 min three times.
To detect the HBV infection, a confocal method was used in which 2×105 cells per well were incubated on 0.1% polylysine-treated slides in a 24-well plate. After 1% paraformaldehyde fixation and 0.5% Triton X-100 permeabilization, cells were stained with a mouse anti-HBx primary antibody and TRITC-conjugated anti-mouse IgG was used as the secondary antibody. Cells were nuclear stained with DAPI and were detected by confocal microscopy (Leica SP5).
HBV DNA detection in infected cells
HBV-infected cells were lysed for 10 min at 100°C in lysis buffer supplemented with proteinase K (200 μg/mL), followed by centrifugal column extraction (Da'an, Zhong Shan University, China). DNA from DLBCL tissue was extracted by supporting magnetic kit (The EmerTher Company, China). It was detected by PCR using a protocol as previously described with modifications 13.
In particular, primer pairs for detection of full length transcripts (3.5kb-2270F: GAGTGTGGATTCGCACTCC and 3.5kb-2392R: GAGGCGAGGGAGTTCTTCT) and total transcripts (t-1805F: TCACCAGCACCATGCAAC and t-1896R: AAGCCACCCAAGGCACAG), were validated and used for PCR, which was performed with the Ex Taq kit (Takara Bio) on a BioRad T100 instrument. Amplification of the 123 bp 3.5kb-DNA and 92 bp total-DNA products was conducted by routine denaturation, annealing (60°C) and elongation. The products were separated by electrophoresis and sequenced for verification.
Immunohistochemistry
Primary antibodies against hepatitis B core (HBc), surface (HBs), and x (HBx) antigens, respectively, were used for immunohistochemistry (IHC), following the standard protocol recommended 14. Antigen expression was categorized by determining the immunoreactive score (IRS) as described previously 15. Each spot was assigned an intensity score from 0 to 3, and the proportion of tumor staining for that intensity was recorded in terms of 25% increments in the range 0-100 (P0, P1-4), while less than 5% was recorded as zero. A final IRS (range 0-12) was obtained by adding the products of scores obtained for each intensity multiplied by the proportion of the area stained.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 (IBM Corporation, USA), and figures were generated using GraphPad Prism software version 5.0. The data are expressed as the means ± standard deviation. The mean values obtained from the experiments were compared using one-way ANOVA analysis with post-hoc test. A P-value of less than 0.05 was considered significant.
Results
HBV infection is clinically related to the development of DLBCL
In this study, 1092 NHL patients were included in the statistical analysis. DLBCL was the dominant lymphoma responsible for 68.0% (508/747) of B-cell NHL and 46.5% (508/1092) of NHL. In total, there were 190 HBsAg-positive serum samples. The rate of HBsAg-positive serum was highest in DLBCL patients (23.6%, Table 1). In 97% (116/120) of the HBsAg-positive serum samples, serological markers against HBV of HBcAb were also detected. However, 222 of the 388 HBsAg-negative staff members also had HBcAb. The significantly higher rate (66.5%, 338/508), of HBcAb 16 in DLBCL patients suggests a basis of HBV infection in this kind of lymphoma and an association between HBV and DLBCL.
Table 1.
Prevalence of serum HBsAg of lymphoma patients.
| Lymphoma type (Number of patients) | HBsAg prevalence % (n) | P value |
|---|---|---|
| All B-NHLs (n=747) | 21.6 (161) | <0.001 |
| DLBCL (n=508) | 23.6 (120) | <0.001 |
| FL (n=107) | 22.4 (24) | <0.001 |
| Other B-NHLs (n=132) | 12.9 (17) | 0.012 |
| All T-NHLs (n=338) | 8.6 (29) | 0.328 |
| NK/T (n=234) | 8.1 (19) | 0.587 |
| PTCL (n=72) | 9.7 (7) | 0.408 |
| Other T-NHLs (n=32) | 9.4 (3) | 0.500a |
| Unclassified NHL (n=7) | 0 (0) | 1.000a |
HBsAg prevalence of respective lymphoma type was compared with the prevalence (7.2%, 5888/81775) of Chinese population by χ2 tests.
a P values were given by Fisher's exact test for expected count less than 5.
FL: follicular lymphoma, T-NHL: T-cell non-Hodgkin lymphoma, PTCL: peripheral T-cell lymphoma, NK/T: NK/T cell lymphoma.
The HBsAg prevalence in NHL was compared with the prevalence (7.2%) 5, 16 in the Chinese population by χ2 tests. The rate of HBsAg-positive serum was only significantly increased in B-cell NHL, especially in DLBCL (Table 1). There was no definite difference in the rate of HBsAg positivity in other types of lymphoma, indicating that B-cell NHL in particular, DLBCL may be related to HBV infection.
By analyzing the retrospective data of DLBCL, it was found that there were differences in the positivity rate of HBsAg at different stages of DLBCL (Table 2). In addition, there was a positive correlation between serum HBsAg positivity rate and DLBCL stage with a higher positivity rate in DLBCL patients with advanced stage III and IV disease (P=0.041).
Table 2.
The positive rate of serum HBsAg in various DLBCL stage.
| DLBCL Stage (Number of patients) | Positive rate of HBsAg % (n) | P value |
|---|---|---|
| Early Stage, I+II (n=289) | 18.7 (54) | 0.003 |
| Advanced Stage, III+IV (n=219) | 30.1 (66) | |
| Stage I (n=117) | 21.4 (25) | 0.041 |
| Stage II (n=172) | 16.9 (29) | |
| Stage III (n=98) | 33.7 (33) | |
| Stage IV (n=121) | 27.3 (33) |
The difference of serum HBsAg positivity between early and advanced DLBCL stage was compared by χ2 test. Tendency difference among serum HBsAg and DLBCL stage was additionally compared by linear trend χ2 test.
HBV can directly infect lymphocytes in vitro
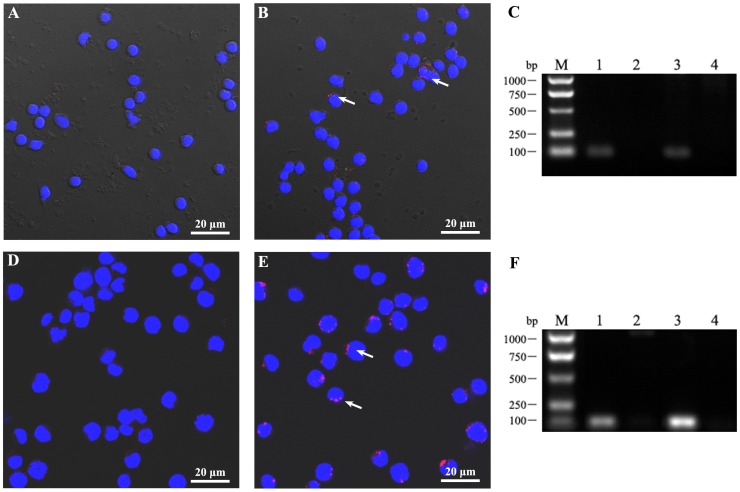
Pfeiffer cells and PBMCs from a cohort of cases were chosen for infection experiments in vitro. The criterion for defining “capable infection” is the successful detection of HBV antigen and nucleic acid in lymphocytes. After HBV infection of these cells for 24 h, intracellular HBV antigen was able to be detected by confocal microscopy of HBx protein staining in infected lymphocytes (Fig. 1). When nucleic acid was extracted from cells and amplified by PCR, intracellular HBV nucleic acid was also found (Fig. 1). This experiment indicates that HBV can infect B lymphocytes in vitro, intimating the possibility of HBV induced development of lymphoma genesis via directly infection.
Figure 1.
HBV can infect lymphocytes in vitro. (A-C) PBMCs and (D-F) the DLBCL Pfeiffer cell line were infected by HBV (A, D) and a matched negative control (B, E) with MOI = 10. After infection for 24 h, the cells were collected and washed by centrifugation. (A, B, D, E) Following fixation and permeation, HBV anti-human HBx monoclonal antibody and a TRITC labeled secondary antibody was used to detect HBV by confocal microscopy and cells were stained with DAPI. (C, F) Cellular DNA was extracted and total HBV DNA was detected by PCR (fragment of 92bp). Lane 1, HBV-positive control; Lane 2, HBV-negative control; Lanes 3 and 4, cells infected for 24 h by HBV-positive (lane 3) or -negative (lane 4) serum. These data were expressed from at least three individual experiments.
HBV-infected cancer cells is found in situ
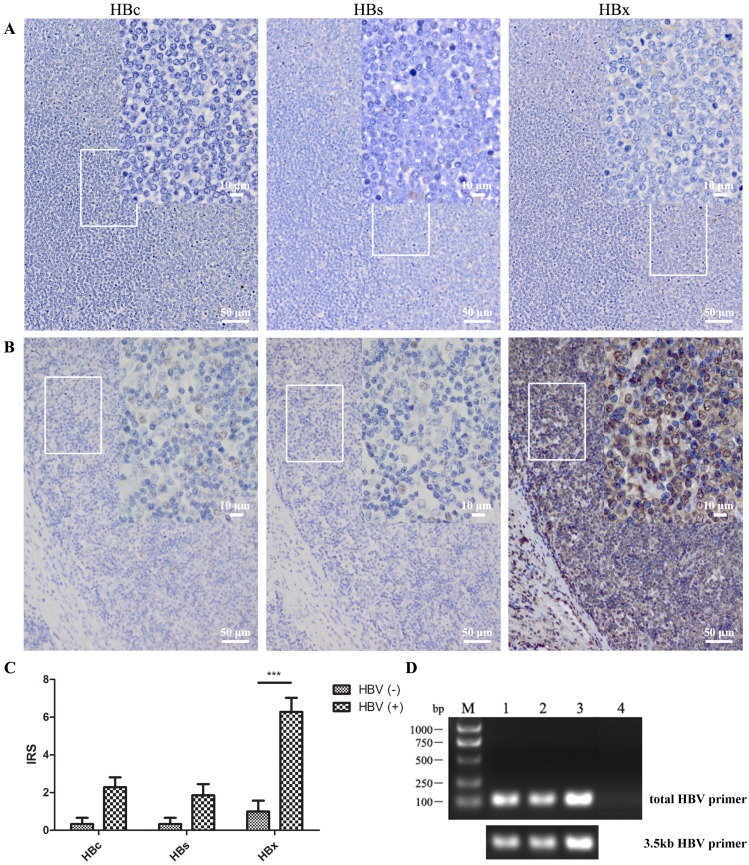
The results of immunohistochemistry illustrated that HBV antigen, especially HBx protein, was strongly expressed in DLBCL patients with serum HBsAg positivity (Fig. 2). There were fifteen tissue specimens from DLBCL patients with ten serum HBsAg positivity and five negativity. The HBx antigen could be detected in all HBsAg-positive samples, and was strongly increased compared to that in HBsAg-negative ones according to IRS (P<0.001). This finding of HBV infection in DLBCL tissue in situ shows that HBV was able to infect B lymphocytes thus indicating an association between HBV and lymphoma formation.
Figure 2.
HBV was detected in DLBCL in situ. Anti-HBc, -HBs, and -HBx antibodies were used to detect the HBV antigen in several lymphoma tissue sections by immunohistochemistry. (A) Lymph node tissue from a DLBCL patient with HBV antigen-negative serum; (B) Patient with HBsAg-positive serum (> 250 IU/mL) and HBV DNA-positive serum (5.2×103 IU/mL); (C) IRS of immunohistochemistry from five serum HBV antigen-negative and ten HBV antigen-positive patients. The scores are expressed as the mean ± standard deviation and statistics were carried out by one-way ANOVA, ***P<0.001; (D) DNA was extracted from DLBCL paraffin tissue, and HBV DNA was detected by PCR using total-HBV or 3.5kb-HBV DNA primers. DNA in lanes 1 to 3 were extracted from DLBCL tissues of HBV-positive serum and lane 4 was from that of HBV-negative serum.
HBV DNA was also found in three of ten tissue samples from HBsAg-positive DLBCL patients suggesting the possibility of long-term HBV retention or integration (Fig. 2D).
HBV infection status influences the therapeutic efficacy in DLBCL patients
Since HBV could successfully infect lymphocytes, we next explored the clinical significance of this infection. Of the DLBCL patients in this study, 92.9% (472/508) underwent standard chemotherapy based on the first-line regimen of CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone). Of these patients, 81.8% (386/472) were also treated with rituximab within the standard chemotherapy, but there was no difference between the HBsAg groups (76.1% (86/113) HBsAg positive vs. 83.6% (300/359) HBsAg negative, P>0.05). Based on these similar chemotherapy regimens, ordinal logistic regression exhibited a significant difference in therapeutic effect on individual factors of DLBCL stage or HBsAg status (Table 3). HBsAg-positive DLBCL patients had a worse therapeutic outcome of at least one grade than HBsAg-negative patients with an odds ratio (OR) of 3.04 (95% confidence interval, 2.00-4.62). As the effect of staging grade on the prognosis of DLBCL was removed, the sensitivity to chemotherapy of HBsAg-positive patients was significantly lower in each stage, especially in patients with early-stage DLBCL (Table 3). Therefore, HBV positivity is an independent factor that is not conducive to DLBCL chemotherapy. This retrospective study suggests our important role of HBV in DLBCL, which requires close clinical monitoring and active intervention.
Table 3.
Effect of serum HBsAg on the therapeutic efficacy in various DLBCL stage.
| Factors | Therapeutic Efficacy | P value | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ||||
| HBsAg Status | |||||||
| Negative (n=359) | 220 | 96 | 17 | 26 | <0.001 | 1 | |
| Positive (n=113) | 31 | 57 | 12 | 13 | 3.04 (2.00~4.62) | ||
| DLBCL Stage | HBsAg Status |
||||||
| I | Negative (n=88) | 73 | 14 | 0 | 1 | 0.003 | 1 |
| Positive (n=25) | 13 | 12 | 0 | 0 | 4.28 (1.64~11.1) | ||
| II | Negative (n=135) | 88 | 39 | 4 | 4 | 0.007 | 1 |
| Positive (n=29) | 10 | 17 | 0 | 2 | 2.97 (1.36~6.53) | ||
| III | Negative (n=55) | 24 | 22 | 5 | 4 | 0.011 | 1 |
| Positive (n=30) | 5 | 15 | 6 | 4 | 3.05 (1.29~7.19) | ||
| IV | Negative (n=81) | 35 | 21 | 8 | 17 | 0.028 | 1 |
| Positive (n=29) | 3 | 13 | 6 | 7 | 2.39 (1.10~5.22) | ||
Ordinal logistic regression was introduced to access the factors in therapeutic efficacy of DLBCL. Effect of serum HBsAg positivity on the evaluation of therapeutic efficacy was also compared in separated DLBCL stage. All patients undergone CHOP regimen were evaluated in statistics.
Therapeutic efficacy was divided into 4 grade, such as CR (complete remission), PR (partial remission), SD (stable disease), and PD (progression disease).
OR: odds ratio, CI: confidence interval.
Discussion
In this research, we performed a retrospective study to analyze the clinical characteristics of DLBCL patients with HBV infection. Almost all HBV related lymphomas are of B-cell origin, comprising a heterogeneous group including an aggressive clinical course with a poor therapeutic effect of traditionally tolerated chemotherapy in DLBCL (Tables 1-3). Our clinical studies support a correlation between HBV infection and DLBCL progression, indicating that HBV may play a key role in the development of B-cell NHL. Dalia et al also demonstrated that there was an increased odds ratio of 2.24 for developing DLBCL in high HBV prevalence countries 17. These clinical findings highlight the further study on latent biological mechanisms responsible for lymphomagenesis and progression in patients with HBV infection.
DLBCL is HBV-defining conditions including HBx strongly expression and HBV DNA stable intracellular existence (Fig. 2). These findings in HBsAg-positive DLBCL patients were consistent with the significant increase of HBx in hepatocellular carcinoma (HCC) induced by stable HBV integration 18, 19. The integrated HBx controls the level of HBV replication and plays a critical role in HCC 20, 21. It interacts with CREBBP, BCL2, and other molecules, causing changes in cell cycle, apoptosis, DNA damage repair, and other phenotypes with a significant difference compared to non-HBV induced liver cancer 22-24. In the pathogenesis of DLBCL, about 32% of the DLBCL patients also have CREBBP inactivation and in 35% of DLBCL-GCB subtypes exist BCL2 ectopic expression 25, 26. The pathogenesis of DLBCL appears consistent with the HCC pathogenesis by HBx, and so HBV-infected lymphocytes may also lead to the development of lymphoma through the corresponding pathway. However, this hypothesis requires further studies on the function of HBx, in particular on the effect on chemotherapy efficacy, which was different in distinct HBV infection status of DLBCL patients.
Therefore, HBV infection may be one of the risk factors for developing DLBCL. That HBV can infect B lymphocytes highlights the mechanisms involved in their pathogenesis (Fig. 1). HBV has been found in PBMC in vivo 11, and our HBV can infect PBMC in vitro, which suggests that HBV infection of B lymphocytes before tumorigenesis. However, the traditional HBV receptor is not expressed on B lymphocytes 13. Appropriate protein and nucleic acid technology may be used to further screen and verify the related receptors for HBV infection 27.
In summary, HBV can infect B lymphocytes and may be related to the development of DLBCL. However, there is still disputed in the clinical need for DLBCL susceptible population to use anti-HBV preventive treatment. Discovery of the phagocytic pathway and HBx function inducing DLBCL development remains our further study for persuasive treatment strategies.
Acknowledgments
This work was supported in part by the Research Project of Shanghai Municipal Commission of Health and Family Planning (grant no. 20164Y0053), and the National Natural Science Foundation of China (grant no. 81572552). We gratefully acknowledge the Department of Pathogen Biology, Shanghai Medical College, Fudan University for experimental virus preparation and for providing antibodies.
Abbreviations
- HBV
hepatitis B virus
- DLBCL
Diffuse large B-cell lymphoma
- NHL
non-Hodgkin lymphoma
- HBsAg
hepatitis B surface antigen
- HBsAb
hepatitis B surface antibody
- HBcAb
hepatitis B core antibody
- HBeAg
hepatitis B e antigen
- HBeAb
hepatitis B e antibody
- HBx
hepatitis B x protein
- PBMCs
peripheral blood mononuclear cells
- IHC
immunohistochemistry
- IRS
immunoreactive score
- MOI
multiplicity of infection
- PCR
polymerase chain reaction
- OR
odds ratio
- HCC
hepatocellular carcinoma.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Perry AM, Diebold J, Nathwani BN, MacLennan KA, Muller-Hermelink HK, Bast M. et al. Non-Hodgkin lymphoma in the Far East: review of 730 cases from the international non-Hodgkin lymphoma classification project. Annals of hematology. 2016;95:245–51. doi: 10.1007/s00277-015-2543-4. [DOI] [PubMed] [Google Scholar]
- 3.Arredouani MS, Bhasin MK, Sage DR, Dunn LK, Gill MB, Agnani D. et al. Analysis of host gene expression changes reveals distinct roles for the cytoplasmic domain of the Epstein-Barr virus receptor/CD21 in B-cell maturation, activation, and initiation of virus infection. Journal of virology. 2014;88:5559–77. doi: 10.1128/JVI.03099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasleem S, Sood GK. Hepatitis C Associated B-cell Non-Hodgkin Lymphoma: Clinical Features and the Role of Antiviral Therapy. Journal of clinical and translational hepatology. 2015;3:134–9. doi: 10.14218/JCTH.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F. et al. Reprint of: Epidemiological serosurvey of Hepatitis B in China-declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31(Suppl 9):J21–8. doi: 10.1016/j.vaccine.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Deng L, Song Y, Young KH, Hu S, Ding N, Song W. et al. Hepatitis B virus-associated diffuse large B-cell lymphoma: unique clinical features, poor outcome, and hepatitis B surface antigen-driven origin. Oncotarget. 2015;6:25061–75. doi: 10.18632/oncotarget.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law MF, Lai HK, Chan HN, Ha CY, Ng C, Yeung YM. et al. The impact of hepatitis B virus (HBV) infection on clinical outcomes of patients with diffuse large B-cell lymphoma. European journal of cancer care. 2015;24:117–24. doi: 10.1111/ecc.12166. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Yang H, He W, Xia Y, Xia Z, Li S. et al. Association between extranodal natural killer/T-cell lymphoma and hepatitis B viral infection: a case-control study. Journal of Cancer. 2017;8:2676–83. doi: 10.7150/jca.19665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM. et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2011;22:1170–80. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalia S, Suleiman Y, Croy DW, Sokol L. Association of Lymphomagenesis and the Reactivation of Hepatitis B Virus in Non-Hodgkin Lymphoma. Cancer control: journal of the Moffitt Cancer Center. 2015;22:360–5. doi: 10.1177/107327481502200315. [DOI] [PubMed] [Google Scholar]
- 11.Cabrerizo M, Bartolome J, Caramelo C, Barril G, Carreno V. Molecular analysis of hepatitis B virus DNA in serum and peripheral blood mononuclear cells from hepatitis B surface antigen-negative cases. Hepatology. 2000;32:116–23. doi: 10.1053/jhep.2000.8541. [DOI] [PubMed] [Google Scholar]
- 12.Loggi E, Gamal N, Bihl F, Bernardi M, Andreone P. Adaptive response in hepatitis B virus infection. Journal of viral hepatitis. 2014;21:305–13. doi: 10.1111/jvh.12255. [DOI] [PubMed] [Google Scholar]
- 13.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei L, Shen Z, Zhao X, Wu Y, Liu W, Zhang J. et al. A broadly reactive monoclonal antibody detects multiple genotypes of hepatitis B virus X protein. Archives of Virology. 2014;159:2731–5. doi: 10.1007/s00705-014-2111-6. [DOI] [PubMed] [Google Scholar]
- 15.Halon A, Donizy P, Surowiak P, Matkowski R. ERM/Rho protein expression in ductal breast cancer: a 15 year follow-up. Cellular Oncology. 2013;36:181–90. doi: 10.1007/s13402-013-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong Hao G, Da Xing F, Jin X, Xiu Hong F, Pu Mei D, Jun L. et al. The prevalence of hepatitis B infection in central China: An adult population-based serological survey of a large sample size. Journal of medical virology. 2017;89:450–7. doi: 10.1002/jmv.24649. [DOI] [PubMed] [Google Scholar]
- 17.Dalia S, Chavez J, Castillo JJ, Sokol L. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leukemia research. 2013;37:1107–15. doi: 10.1016/j.leukres.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y. et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nature genetics. 2012;44:765–9. doi: 10.1038/ng.2295. [DOI] [PubMed] [Google Scholar]
- 19.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World journal of gastroenterology. 2014;20:11630–40. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slagle BL, Andrisani OM, Bouchard MJ, Lee CG, Ou JH, Siddiqui A. Technical standards for hepatitis B virus X protein (HBx) research. Hepatology. 2015;61:1416–24. doi: 10.1002/hep.27360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhenda S, Cougot D, Buendia M-A, Neuveut C. Chapter 4 Hepatitis B Virus X Protein. 2009; 103: 75-109. [DOI] [PubMed]
- 22.Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Seminars in liver disease. 2013;33:147–56. doi: 10.1055/s-0033-1345721. [DOI] [PubMed] [Google Scholar]
- 23.Geng X, Harry BL, Zhou Q, Skeen-Gaar RR, Ge X, Lee ES. et al. Hepatitis B virus X protein targets the Bcl-2 protein CED-9 to induce intracellular Ca2+ increase and cell death in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18465–70. doi: 10.1073/pnas.1204652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringelhan M, O'Connor T, Protzer U, Heikenwalder M. The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets. The Journal of pathology. 2015;235:355–67. doi: 10.1002/path.4434. [DOI] [PubMed] [Google Scholar]
- 25.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nature reviews Immunology. 2015;15:172–84. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 26.Pasqualucci L, Dalla-Favera R. SnapShot: diffuse large B cell lymphoma. Cancer cell. 2014;25:132–e1. doi: 10.1016/j.ccr.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Psathas JN, Doonan PJ, Raman P, Freedman BD, Minn AJ, Thomas-Tikhonenko A. The Myc-miR-17-92 axis amplifies B-cell receptor signaling via inhibition of ITIM proteins: a novel lymphomagenic feed-forward loop. Blood. 2013;122:4220–9. doi: 10.1182/blood-2012-12-473090. [DOI] [PMC free article] [PubMed] [Google Scholar]