Abstract
Objectives:
To analyze clinical spectrum, seriousness, outcome, causality, severity and preventability of ADRs in geriatrics and pediatric patients.
Materials and Methods:
All ADRs reported in geriatrics (≥ 65 years) and pediatrics (≤ 12 years) indoor as well outdoor patients from January, 2010 to April, 2016 at ADR monitoring centre, Department of Pharmacology, B. J. Medical College and Civil Hospital were identified. A retrospective analysis was carried out for clinical presentation, causality (as per WHO-UMC scale and Naranjo's algorithm), severity (Hatwig and Seigel scale) and preventability (Schaumock and Thornton criteria).
Results:
Out of 3690 ADRs, 160 were in geriatric patients (4.33%) while 231 in pediatric patients (6.26%). The most commonly affected body system was gastrointestinal (53, 33.13%) followed by neurological disorders (26, 16.25%) in geriatric patients. While in pediatric patients, the most commonly affected body system was skin and appendages (73, 31.60 %) followed by gastrointestinal disorders (58, 25.11%). The most common causal drugs in geriatric patients was cardiovascular (38, 23.75%) followed by antimicrobials (28, 13.25%). While in pediatric patients, the most common causal drug group was antimicrobials (85, 33.46%) followed by blood products (36, 14.12%). Total 17 ADRs reported following vaccination, 7 (41.17%) were injection site abscess and 11 (64.70%) were due to pentavalent vaccine. Polypharmacy was common in geriatrics (31, 19.37%). Causality assessment for majority of ADRs in geriatrics (83, 52.5%) and pediatrics (171, 67.32%) were probable.
Conclusion:
ADRs are common in geriatric and pediatric patients usually within four weeks of oral therapy. Active surveillance of drug safety monitoring in these vulnerable population is recommended.
Keywords: Adverse drug reaction, geriatric, pediatric, pharmacovigilance
INTRODUCTION
Adverse drug reactions (ADRs) are the one of the leading cause of repeated hospitalization and adversely affects the quality of life.[1] The prevalence of ADR is higher among geriatric (5%) and pediatric (9.5%) patients as compared to adults including 2.1% of hospital admissions.[2,3,4] The possible reasons for higher prevalence of ADRs in geriatric patients are other comorbidities, polypharmacy, and altered pharmacokinetic and altered pharmacodynamics.[5] In addition, infants and very young children are at high risk of ADRs because their capacity to metabolize the drug is not fully evaluated. Similarly, neonates are at high risk of ADRs due to immature hepatobiliary and renal tubular functions. Thus, elderly and pediatric patients are vulnerable to ADRs because of immature, altered, and unpredictable physiological changes that occur in extremes of age.
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.[6] The practice of PV requires special attention for geriatric and pediatric patients because diseases in these patients are qualitatively and quantitatively different from the adult patients.
Hence, an attempt has been made in this study to analyze the clinical spectrum and assess seriousness, outcome, causality, severity, and preventability of the ADRs in the geriatric and pediatric patients.
MATERIALS AND METHODS
Department of Pharmacology, B. J. Medical College is a recognized Adverse Reaction Monitoring Centre and regional training centre under pharmacovigilance program of India. The suspected ADRs from indoor as well as outdoor patients were diagnosed by treating consultants, and relevant details of each ADR were collected in spontaneous ADR reporting form. Each report was sent to the National coordinating centre through Vigiflow and simultaneously entered in the Microsoft Excel sheet. All ADRs reported in geriatric (≥65 years) and pediatric (≤12 years) patients from January 2010 to April 2016 were identified. Data were analyzed to find the time relationship between the event and the initiation of drug treatment, number of adverse events, causal drug groups and involved body system as per system organ classification (SOC) in both groups. An association of clinical presentation of geriatric and pediatric ADRs with the route of drug administration, other concomitant conditions and number of drugs prescribed, i.e., polypharmacy was also carried out. Causality assessment was done using WHO-UMC scale and Naranjo's algorithm.[7,8] Severity was assessed using modified Hartwig and Siegel [9] while preventability was assessed using modified Schumock and Thornton scale.[10]
RESULTS
Out of 3690 ADRs reported during the period, 160 were geriatric ADRs (4.33%) while 231 were pediatric ADRs (6.26%).
There were 115 men and 45 women with male:female ratio of 3.44:1 in geriatric patients. The mean age of geriatric patients with ADRs was 71.69 ± 0.45 years. While in pediatric group, there were 143 boys and 88 girls with boys: girls ratio of 1.63:1. The mean age of pediatric patients with ADRs was 2.98 ± 0.20 years.
Time for appearance of adverse drug reactions
Majority of the ADRs in geriatric (148, 92.5%) and pediatric (207, 86.61%) patients occurred within 4 weeks of drug therapy.
Clinical presentation of adverse drug reactions
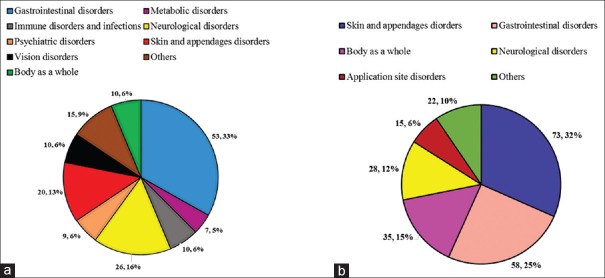
The most commonly affected body system (as per SOC) was gastrointestinal disorders (53, 33.13%) followed by neurological disorders (26, 16.25%), and skin and appendages (20, 12.5%) in geriatric patients [Table 1 and Figure 1a].
Table 1.
Detail analysis of adverse drug reactions in geriatrics and pediatric patients
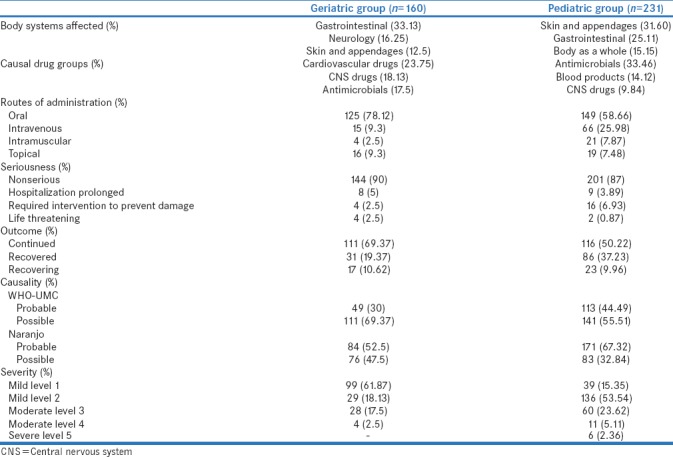
Figure 1.
(a) Body systems affected by adverse drug reactions in geriatrics patients (n = 160. (b) Body systems affected by adverse drug reactions in pediatric patients (n = 231)
While in pediatric patients, the most commonly affected body system was skin and appendages (73, 31.60%) followed by gastrointestinal disorders (58, 25.11%) and body as a whole (35, 15.15%) [Table 1 and Figure 1b].
Interestingly, total 17 (7.36%) adverse effect following vaccination were reported. The most common presentation was abscess at the site of injection (7, 41.17%) and pentavalent vaccine was the common causal agent (11, 64.70%).
Causal drug groups
The most common drug group causing ADRs in geriatric patients was cardiovascular drugs (38, 23.75%) followed by central nervous system (CNS) drugs (29, 18.13%), and antimicrobial agents (28, 17.5%). Among cardiovascular drug group, calcium channel blocker (9, 5.63%), diuretics (9, 5.63) beta blockers (6, 3.75%), and nitrates (5, 3.13%) were the most common causal drug groups. While in antimicrobials, fluoroquinolones (8, 5%), beta lactam antibiotics (7, 4.38%), antiamoebic agents (3, 1.88%), and macrolides (2, 1.25%) were the most common causal drug groups. Opioid analgesics were the most common agents among CNS drugs [Tables 1 and 2].
Table 2.
Common adverse drug reactions and mapping to the common causal drug groups in geriatric and pediatric patients
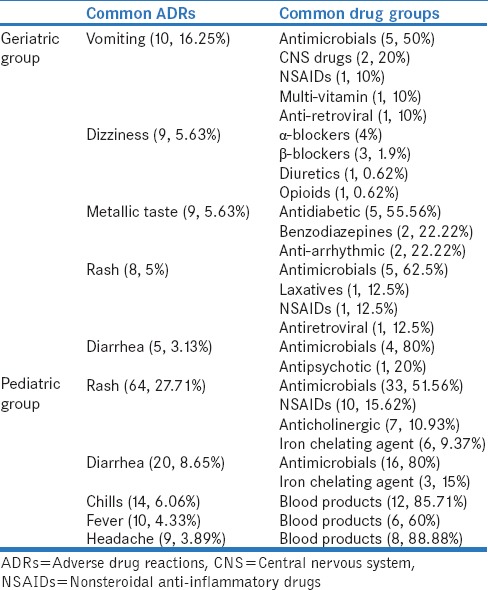
Among pediatric patients, most common drug group was antimicrobial agents (85, 33.46%) followed by blood products (36, 14.12%). Among antimicrobials, beta-lactum antibiotics (31, 36.47%) were the most common followed by macrolides (12, 14.12%), fluoroquinolones (6, 7.6%), antimalarial (6, 7.6%), and vancomycin (6, 7.6%) [Tables 1 and 2].
Routes of administration
Out of 160 geriatric patients, majority of the patients (125, 78.12%) received the causal drug orally followed by 15 (9.3%) intravenous and 4 (2.5%) intramuscular route.
Similarly, in pediatric patients, out of 254 suspected drugs, 149 (58.66%) were administered orally followed by 66 (25.98%) intravenous and 21 (7.87%) intramuscular route [Table 1].
Polypharmacy
Out of 160 ADRs in geriatric patients, polypharmacy (≥5 drugs per prescription) was observed in 31 (19.37%) patients. Among them, 8 (25.8%) were suffering from cardiovascular diseases, and 5 (16.12%) were on postoperative medications.
In pediatric group, only 14 patients (6%) were prescribed more than 5 drugs.
Seriousness of adverse drug reactions
Majority of ADRs were nonserious (144, 90%) in geriatric groups while the criteria for serious ADRs were initial or prolongation of hospitalization (8, 5%) followed by required interventions to prevent damage (4, 2.5%). Moreover, there were 4 (2.5%) life-threatening ADRs manifested as Steven–Johnson syndrome, hyperkalemia, severe anemia, and gastric erosions. Two cases of Steven–Johnson syndrome was reported in geriatric patients. The causal drugs for both the cases were fixed dose combination of zidovudine + lamivudine + nevirapine and diclofenac, respectively.
Similarly, in pediatric group, out of 231 ADRs, 201 (87%) were nonserious in nature. Among the serious ADRs, 16 (6.93%) required intervention to prevent damage followed by prolonged hospitalization (9, 3.89%). Moreover, two ADRs, laryngospasm and toxic epidermal necrolysis were life-threatening [Table 1]. One case of laryngospasm was reported in pediatric patients treated with intravenous contrast medium.
Outcome at the time of reporting
Majority of geriatric ADRs were continuing at the time of reporting (111, 69.37%). However, 31 (19.37%) recovered, and 17 (10.62%) were recovering. While the majority of ADRs in pediatric patients were continuing (116, 50.22%), 86 (37.23%) were recovered, and 23 (9.96%) were recovering at the time reporting [Table 1].
Causality assessment
According to the WHO-UMC scale, majority of the geriatric and pediatric ADRs were categorized as possible (111, 69.37%; 141, 55.51%) followed by probable (49, 30%; 113, 44.49%) in nature. Whereas according to the Naranjo Algorithm, 84 (52.5%) and 171 (67.32%) were probable while 76 (47.5%) and 83 (32.68%) were possible in pediatric and geriatric patients, respectively [Table 1].
Severity and preventability
In geriatric group, s everity of the ADRs were mild level 1 (99, 61.87), mild level 2 (29, 18.13%), moderate level 3 (28, 17.5%), and moderate level 4 (4, 2.5%) while severity of pediatric ADRs were mild level 2 (136, 53.54%), mild level 3 (60, 23.62%), moderate level 4 (11, 5.11%), and severe level 5 (6, 2.36%) according to the Hartwig and Siegel scale. Preventability according to Schaumock and Toronto scale were not preventable for both pediatric and geriatric patients [Table 1].
DISCUSSION
This retrospective study is an attempt to analyze ADRs occurred in geriatric and pediatric patients at tertiary care teaching hospital. In our study, the reporting rate of geriatric ADR was 4.33% which is lower than Mandavi et al.[11] While in pediatric group, the reporting rate of ADR was high (6.26%) compared to Digra et al.(0.3%).[12] Men were more commonly affected than women. Our study observed that most of the geriatric patients belonged to 65–70 years while study done by Pauldurai et al. showed that common age was 60–65 years.[13] The common pediatric age affected in our study was 5-10 years while Priyadarshini et al. reported more number of ADRs in 1-6 years.[3]
The most common body system affected was gastrointestinal followed by neurological and skin and appendageal disorders in geriatric patients. Our observation was similar to Pauldurai et al. [Table 3]. This may be because most of the suspected drugs were administred orally in present study. In addition, most common causal drug groups were cardiovascular and antimicrobial agents which are known to cause gastrointestinal and skin reactions. In pediatric group, skin and appendages were commonly affected body system followed by gastrointestinal and body as a whole, which is similar to Digra et al. and Priyadharsini et al. [Table 3]. A study by Ghataliya et al. also reported that cutaneous ADRs are common with blood transfusion in pediatric patients.[14] Probably dermatological reactions are easy and immediately identified, and antimicrobials agents as well as blood products are common causal drug groups known to cause skin reactions. The most common causal drug group for geriatric patients was cardiovascular groups followed by antimicrobial agents which are supported by other Indian studies too [Table 3].[15,16] This may be attributed to common cardiovascular morbidities and prevalence of infectious diseases in geriatric age group. Thus, cardiovascular and antimicrobials were figured as two of the most common drug groups causing ADRs in elderly. While in pediatric patients, most common causal drug group was antimicrobial agents followed by blood products which are similar to Indian study by Mandha et al.[16] Another study by Smyth et al. performed at United Kingdom (UK) has shown that nonsteroidal anti-inflammatory drugs (NSAIDs) were the most common causal drug group. This indicates that the prevalence of infectious diseases is less in the UK [17] whereas infections and malnutrition are the common problems in India. Antimicrobial agents and blood products are most commonly associated with hypersensitivity reactions. Thus, it further supports our previous finding that dermatological ADRs are common in pediatric patients. While injection site abscess following, vaccination denotes medication administration error. A study by Patel et al. has reported medication administration error is common in pediatric patients.[18] This calls for need of training of health-care workers and monitoring following vaccination.
Table 3.
Comparison of geriatric and pediatric adverse drug reactions with available literature
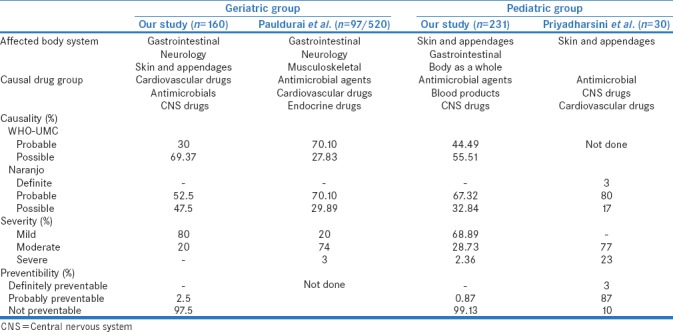
Our study shows that 19% of geriatric patients were prescribed more than 5 drugs while it was 86% as reported by Pauldurai et al. It also substantiates that polypharmacy is very common in India. The chance of drug–drug interactions and risk of ADRs are more with polypharmacy. Thus, polypharmacy is one of the risk factors for developing the ADRs in geriatrics. Although majority of the ADRs were nonserious in nature, the common criteria for serious ADRs were initiation or prolongation of hospitalization and required intervention to prevent damage in geriatric and pediatric group, respectively. Similiar observation has been reported by Prajapati et al.[19]
The common causality association with suspected drug was 'possible' or 'probable' in majority of cases. Similar observations have been reported by Pauldurai et al. and Priyadharsini et al. [Table 3]. The absence of other alternative causes and positive dechallange justifies the probable criteria. Frequently causality assessment has been a challenge due to lack of information on dechallange and rechallange, simultaneous starting of multiple drugs and existence of comorbidites with similar symptoms. Thus, causality association comes down to lower 'possible' grade. However, this does not undermine the importance of causal association with suspected drug and causality assessment per se.
Majority of ADRs were mild in nature in geriatric patients contrary to Pauldurai et al. study. This is because the most common affected body system was gastrointestinal system that includes mild reactions such as nausea, vomiting, metallic taste, and diarrhea for which stoppage of drugs and hospitalization are generally not required. Interestingly, the majority of ADRs were nonpreventable because skin was the common target in both the age groups and most of the cutaneous reactions are idiosyncratic in nature [20] [Table 3].
This was a retrospective study, wherein all ADRs were reported spontaneously. Underreporting, inability to find incidence rate, lack of follow-up data till recovery, lack of information about substituted drugs or treatment of ADRs, lack of information on recently introduced drugs, and single center are the major limitations. Inspite of these limitations, the data provides an opportunity for devising strategies to closely monitor geriatric and pediatric age group patients for ADRs.
CONCLUSION
ADRs are common in geriatric and pediatric patients usually within first 4 weeks of oral therapy. Reactions, mostly mild, nonserious, are common with cardiovascular and CNS drugs, antimicrobials and blood products and frequently target gastrointestinal, skin, and CNSs. Polypharmacy and extremes of age increase the risk of ADRs. This calls for the need for active surveillance of drug safety monitoring in these vulnerable populations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: A prospective analysis of 3695 patient-episodes. PLoS One. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.8. Occurrence of Adverse Drug Reactions in Elderly Population (PvPI Newsletter) 2014. Apr, [Last accessed on 2016 Sep 10]. Available from: http://www.ipc.gov.in/PvPI1/Newsletter_April_issue.pdf .
- 3.Priyadharsini R, Surendiran A, Adithan C, Sreenivasan S, Sahoo FK. A study of adverse drug reactions in pediatric patients. J Pharmacol Pharmacother. 2011;2:277–80. doi: 10.4103/0976-500X.85957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoleone E. Children and ADRs (Adverse Drug Reactions) Ital J Pediatr. 2010;36:4. doi: 10.1186/1824-7288-36-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alomar MJ. Factors affecting the development of adverse drug reactions (Review article) Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharmacovigilance, WHO 2014. [Last accessed on 2016 Sep 10]. Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/
- 7.The use of the WHO–UMC system for standardised case causality assessment. [Last accessed on 2016 Sep 10]. Available from: http://www.WHO-UMC.org/graphics/4409.pdf .
- 8.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 9.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 10.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 11.Mandavi, D'cruz S, Sachdev A. Adverse drug reactions and their risk factors among Indian ambulatory elderly patients. Indian J Med Res. 2012;136:404–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Digra KK, Pandita A, Saini GS, Bharti R. Pattern of adverse drug reactions in children attending the department of pediatrics in a tertiary care center: A prospective observational study. Clin Med Insights Pediatr. 2015;9:73–8. doi: 10.4137/CMPed.S29493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauldurai M, Kannaaiyan D, Rao R. Adverse drug reaction monitoring in geriatric patients of rural teaching hospital. Der Pharm Lett. 2015;7:187–93. [Google Scholar]
- 14.Ghataliya KJ, Kapadia JD, Desai MK, Mehariya KM, Rathod GH, Bhatnagar N, et al. Transfusion related adverse drug reactions in pediatric and surgical patients at a tertiary care teaching hospital in India. Asian J TransfusSci. 2017;11:180–87. doi: 10.4103/0973-6247.214348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah R, Gajjar B, Desai S. A profile of adverse drug reactions with risk factors among geriatric patients in a tertiary care teaching rural hospital in India. Natl J Physiol Pharm Pharmacol. 2012;2:113–22. [Google Scholar]
- 16.Mandha M, Reddy KP, Reddy KR. Evaluation of adverse drug reaction in pediatric patients. Indian J Pharm Pract. 2013;3:32–25. [Google Scholar]
- 17.Smyth RM, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children – A systematic review. PLoS One. 2012;7:e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel N, Desai M, Shah S, Patel P, Gandhi A. A study of medication errors in a tertiary care hospital. Perspect Clin Res. 2016;7:168–73. doi: 10.4103/2229-3485.192039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prajapati K, Desai M, Shah S, Panchal J, Kapadia J, Dikshit R. An analysis of serious adverse drug reactions at a tertiary care teaching hospital. Perspect Clin Res. 2016;7:181–6. doi: 10.4103/2229-3485.192044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SP, Desai MK, Dikshit RK. Analysis of cutaneous adverse drug reaction sat tertiary care hospital - A prospective study. Trop J Pharm Res. 2011;10:517–22. [Google Scholar]