Abstract
Objectives
To assess the recent prognostic trends in oesophageal adenocarcinoma and oesophageal squamous cell carcinoma undergoing resectional surgery and no such surgery. Additionally, risk factors for death were assessed in each of these patient groups.
Design
Cohort study.
Setting
A population-based, nationwide study in Sweden.
Participants
All patients diagnosed with oesophageal adenocarcinoma and oesophageal squamous cell carcinoma in Sweden from 1 January 1990 to 31 December 2013, with follow-up until 14 May 2017.
Outcome measures
Observed and relative (to the background population) 1-year, 3-year and 5-year survivals were analysed using life table method. Multivariable Cox regression provided HR with 95% CI for risk factors of death.
Results
Among 3794 patients with oesophageal adenocarcinoma and 4631 with oesophageal squamous cell carcinoma, 82% and 63% were men, respectively. From 1990–1994 to 2010–2013, the relative 5-year survival increased from 12% to 15% for oesophageal adenocarcinoma and from 9% to 12% for oesophageal squamous cell carcinoma. The corresponding survival following surgery increased from 27% to 45% in oesophageal adenocarcinoma and from 24% to 43% in oesophageal squamous cell carcinoma. In patients not undergoing surgery, the survival increased from 3% to 4% for oesophageal adenocarcinoma and from 3% to 6% for oesophageal squamous cell carcinoma. Women with oesophageal squamous cell carcinoma had better prognosis than men both following surgery (HR 0.71, 95% CI 0.61 to 0.83) and no surgery (HR 0.86, 95% CI 0.81 to 0.93).
Conclusions
The prognosis has improved over calendar time both in oesophageal adenocarcinoma and oesophageal squamous cell carcinoma in Sweden that did and did not undergo surgery. Women appear to have better prognosis in oesophageal squamous cell carcinoma than men, independent of treatment.
Keywords: gastrointestinal tumours, oesophageal disease, epidemiology, thoracic surgery
Strengths and limitations of this study.
The main strength of the study is the population-based design with complete and accurate ascertainment and follow-up of all patients diagnosed with oesophageal cancer in Sweden.
Valid estimation of disease-specific mortality was possible with highly accurate information on oesophageal cancer histology, surgical treatment and date of death and the calculation of relative survival rates.
The sample size was large enough to enable robust analyses of time trends in subgroups of patients, and to assess risk factors of mortality.
Limitations include the unavailability of some clinical variables, such as neoadjuvant or adjuvant treatment.
Tumour stage variable was unavailable before 2004, and complete for only surgically treated patients after 2004.
Introduction
Oesophageal cancer is the sixth most common cause of cancer death worldwide.1 The overall survival is poor; only 10%–22% of patients survive 5 years after diagnosis in Europe, the USA and China.2–4 Most studies have reported survival for oesophageal cancer in general, but despite that, oesophageal adenocarcinoma and squamous cell carcinoma are increasingly seen as separate diseases with different aetiologies, incidence trends and treatments.5–9
In the USA, the 5-year overall survival in patients with oesophageal cancer has increased from 18% in the 1990s to 22% in the 2000s,10 whereas in Europe, the survival has increased from 10% in 1999–2001 to 13% in 2005–2007.11 The surgical technique, neoadjuvant modalities and definitive chemoradiation therapies have seen much development over the last two decades.6 7 12 It has been acknowledged that surgeon and hospital volume are related to survival in oesophageal cancer, resulting in centralisation of oesophageal cancer treatment.13–15 However, it is not known whether and how survival in oesophageal adenocarcinoma or squamous cell carcinoma has changed during the last few years, and even more unclear is how this might have changed specifically in patients undergoing surgery and patients not undergoing surgery. Sweden provides an excellent setting to answer these questions because of its accurate and complete nationwide registries.16 17
This nationwide Swedish study was conducted with the aim of assessing survival in oesophageal adenocarcinoma and squamous cell carcinoma separately, and also specifically in patients undergoing resectional surgery (oesophagectomy) and those that do not undergo such surgery. Additionally, risk factors for death were assessed in each of these patient groups.
Methods
Design
This was a nationwide Swedish population-based cohort study, including all patients diagnosed with oesophageal adenocarcinoma or squamous cell carcinoma from 1 January 1990 through 31 December 2013, with follow-up for survival until 14 May 2017. All newly diagnosed patients were identified from the Swedish Cancer Registry, while information about surgery was retrieved from the Swedish Patient Registry, and mortality from the Swedish Causes of Death Registry. The reporting to these registries is required according to Swedish law. Patient data were linked by the unique personal identity numbers assigned to each resident in Sweden, which are ideal for registry-based research.16
Data collection
The Swedish Cancer Registry was used to identify the study patients. The nationwide completeness for recording of oesophageal cancer was 98% and the histological confirmation was 100%.17 The diagnosis codes 150.0, 150.8 and 150.9 in the seventh version of the International Classification of Diseases were used to identify patients with oesophageal cancer. The histology codes (WHO/HS/CANC/24.1 Histology Code) were used to separate patients with oesophageal adenocarcinoma (096) and squamous cell carcinoma (146). Tumour stage has been recorded in the Swedish Cancer Registry from June 2004 onwards. The tumour stage variable is >98% concordant with patient records for operated patients with oesophageal cancer.18 The tumour stage classification in the registry was completed according to the sixth edition of the Union Internationale Contre le Cancer.19
The Swedish Patient Registry was used to identify all patients who underwent resectional surgery and also to assess comorbidities at the time of diagnosis.20 The Swedish Classification of Operations and Major Procedures was used to identify the relevant operation codes (oesophagectomy codes 2820–2829 in 1990–1997 and codes JCC00-JCC97 in 1997–2014 and oesophagogastrectomy and gastrectomy codes 4411–4435 in 1990–1997 and codes JDC00-JDD96 in 1997–2014). Except for oesophagectomy codes, gastrectomy codes were included because some surgeons tend to combine oesophagectomy with gastrectomy in locally advanced cancer. Operation codes referring to oesophagogastrectomy or gastrectomy were found in a total of 78 (0.9%) patients. The positive predictive value for oesophageal cancer resection has been estimated at 99.6% in the Swedish Patient Registry.21
Comorbidities were defined according to the well-validated Charlson Comorbidity Index,22 not including the oesophageal cancer or metastatic solid tumours. The comorbidity information was retrieved from hospital admissions in the Patient Registry, which is at least 95% complete for most comorbidities,23 up to 3 years before the index admission or cancer diagnosis
The Swedish Causes of Death Registry was used to obtain mortality. This registry contains 100% complete information on date of death for all deceased Swedish residents from 1952 onwards.24
Statistical analysis
An experienced biostatistician (FM) conducted all data management and statistical analysis according to a detailed and predefined study protocol. All analyses were conducted using SAS (V.9.4, SAS Institute, Gary, North Carolina, USA). Observed and relative survivals were presented for survival at 1 and 5 years following a diagnosis date of oesophageal adenocarcinoma or squamous cell carcinoma. Observed survival with 95% CI was estimated using the life-table method,25 where the event was defined as death by any cause (all-cause mortality).
To assess disease-specific mortality, relative survival with 95% CIs was calculated as the observed to the expected survival ratio. The expected survival was derived from the survival in the general Swedish population of the same age (per year), sex and calendar year as the patients with oesophageal cancer. Both observed and relative survivals were presented as percentages (%). The survival in the general Swedish population was available from the start of the study period until the end of 2015; and for the calculation of relative survival rates for the years 2016 and 2017, the mortality rates from 2015 were used. The results were analysed for all patients independent of treatment and also stratified by resectional surgery (yes or no).
The observed survival was stratified by calendar periods (year 1990–1994, 1995–1999, 2000–2004, 2005–2009 or 2010–2013), age (<60, 60–69, 70–79 or ≥80 years), sex (male or female) and Charlson Comorbidity Index22 score (0, 1 or ≥2). Surgically treated patients were further stratified for tumour stage (0–I, II or III– IV) from the year 2005 onwards, when tumour stage data were available and of high completeness. Cox regression was used to calculate crude and adjusted HR with 95% CIs for each of the aforementioned stratification variables (calendar period, age, sex, Charlson Comorbidity Index score and tumour stage) with the same categorisation. The estimates were mutually adjusted for the risk factors where indicated. The missing data for tumour stage were assumed to be missing at random and were dealt with using complete case analysis.
Patient and public involvement
Patients or public were not involved in the development of the research question and study design or conducting the present study.
Results
Patients
A total of 3794 patients were diagnosed with oesophageal adenocarcinoma during the study period, including 1131 (30%) who had undergone oesophagectomy. Among all 4631 patients diagnosed with oesophageal squamous cell carcinoma, 1116 (24%) had undergone oesophagectomy. Men were over-represented in both oesophageal adenocarcinoma group (82%) and squamous cell carcinoma group (63%). The number of patients with oesophageal adenocarcinoma increased throughout the study period, while the number of patients with oesophageal squamous cell carcinoma decreased (table 1). The proportion of patients with adenocarcinoma who underwent oesophagectomy decreased from 38% in 1990–1994 to 27% in 2010–2013, and for patients with squamous cell carcinoma, this proportion decreased from 31% in 1990–1994 to 18% in 2010–2013 (table 1).
Table 1.
Observed and relative 1-year and 5-year survivals across calendar periods in oesophageal adenocarcinoma and squamous cell carcinoma, stratified by treatment strategy in 1990–2013, with follow-up until 2017
| Calendar period | Oesophageal adenocarcinoma | Oesophageal squamous cell carcinoma | ||||||||||
| Patients | Observed survival in % (95% CI) |
Relative survival in % (95% CI) |
Patients | Observed survival in % (95% CI) |
Relative survival in % (95% CI) |
|||||||
| Number (%) | Mean age | 1 year | 5 years | 1 year | 5 years | Number (%) | Mean age | 1 year | 5 years | 1 year | 5 years | |
| All patients | ||||||||||||
| 1990–1994 | 332 (9) | 71 | 33 (28 to 39) | 10 (7 to 14) | 35 (30 to 41) | 12 (8 to 16) | 1148 (25) | 70 | 31 (28 to 33) | 8 (6 to 9) | 32 (29 to 35) | 9 (7 to 11) |
| 1995–1999 | 557 (15) | 70 | 35 (31 to 39) | 11 (8 to 13) | 37 (33 to 41) | 13 (10 to 16) | 1014 (22) | 70 | 32 (29 to 35) | 10 (8 to 12) | 33 (30 to 36) | 12 (9 to 14) |
| 2000–2004 | 860 (23) | 70 | 39 (36 to 43) | 11 (9 to 13) | 41 (38 to 45) | 13 (11 to 16) | 931 (20) | 70 | 34 (30 to 37) | 7 (6 to 9) | 35 (32 to 38) | 9 (7 to 11) |
| 2005–2009 | 1054 (28) | 69 | 39 (36 to 42) | 12 (10 to 14) | 41 (38 to 44) | 14 (12 to 16) | 861 (19) | 70 | 34 (30 to 37) | 10 (8 to 12) | 35 (32 to 38) | 11 (9 to 14) |
| 2010–2013 | 991 (26) | 71 | 40 (37 to 43) | 13 (11 to 15) | 41 (38 to 45) | 15 (12 to 17) | 677 (15) | 70 | 34 (31 to 38) | 11 (9 to 13) | 36 (32 to 39) | 12 (10 to 15) |
| Surgery | ||||||||||||
| 1990–1994 | 126 (11) | 66 | 51 (42 to 60) | 22 (15 to 29) | 54 (44 to 63) | 27 (18 to 36) | 346 (31) | 65 | 57 (52 to 62) | 20 (15 to 24) | 60 (55 to 66) | 24 (19 to 29) |
| 1995–1999 | 191 (17) | 65 | 68 (61 to 75) | 29 (22 to 35) | 72 (65 to 79) | 35 (27 to 42) | 275 (25) | 64 | 64 (59 to 70) | 25 (20 to 31) | 68 (62 to 74) | 31 (24 to 37) |
| 2000–2004 | 275 (24) | 66 | 72 (67 to 78) | 27 (21 to 32) | 76 (71 to 82) | 31 (25 to 37) | 183 (16) | 65 | 69 (63 to 76) | 25 (18 to 31) | 73 (66 to 80) | 29 (22 to 36) |
| 2005–2009 | 277 (24) | 64 | 79 (75 to 84) | 38 (32 to 43) | 83 (78 to 88) | 43 (36 to 49) | 193 (17) | 64 | 70 (64 to 77) | 29 (23 to 35) | 74 (67 to 80) | 33 (26 to 41) |
| 2010–2013 | 262 (23) | 66 | 83 (78 to 87) | 40 (34 to 46) | 86 (82 to 91) | 45 (38 to 51) | 119 (11) | 66 | 83 (76 to 90) | 39 (30 to 48) | 87 (80 to 94) | 43 (33 to 54) |
| No surgery | ||||||||||||
| 1990–1994 | 206 (8) | 74 | 23 (17 to 29) | 3 (0 to 5) | 24 (18 to 30) | 3 (0 to 6) | 802 (23) | 72 | 19 (16 to 22) | 2 (1 to 3) | 20 (17 to 23) | 3 (1 to 4) |
| 1995–1999 | 366 (14) | 72 | 18 (14 to 22) | 1 (0 to 2) | 19 (15 to 23) | 1 (0 to 2) | 739 (21) | 72 | 19 (17 to 22) | 4 (2 to 5) | 21 (18 to 24) | 5 (3 to 6) |
| 2000–2004 | 585 (22) | 72 | 24 (20 to 27) | 4 (2 to 6) | 25 (21 to 29) | 5 (3 to 7) | 748 (21) | 72 | 25 (22 to 28) | 3 (2 to 5) | 26 (23 to 29) | 4 (2 to 5) |
| 2005–2009 | 777 (29) | 71 | 24 (21 to 27) | 3 (2 to 5) | 25 (22 to 29) | 4 (2 to 5) | 668 (19) | 71 | 23 (20 to 26) | 4 (3 to 6) | 24 (21 to 27) | 5 (3 to 7) |
| 2010–2013 | 729 (27) | 73 | 24 (21 to 27) | 3 (2 to 5) | 25 (22 to 29) | 4 (2 to 5) | 558 (16) | 71 | 24 (20 to 27) | 5 (3 to 7) | 25 (21 to 29) | 6 (4 to 8) |
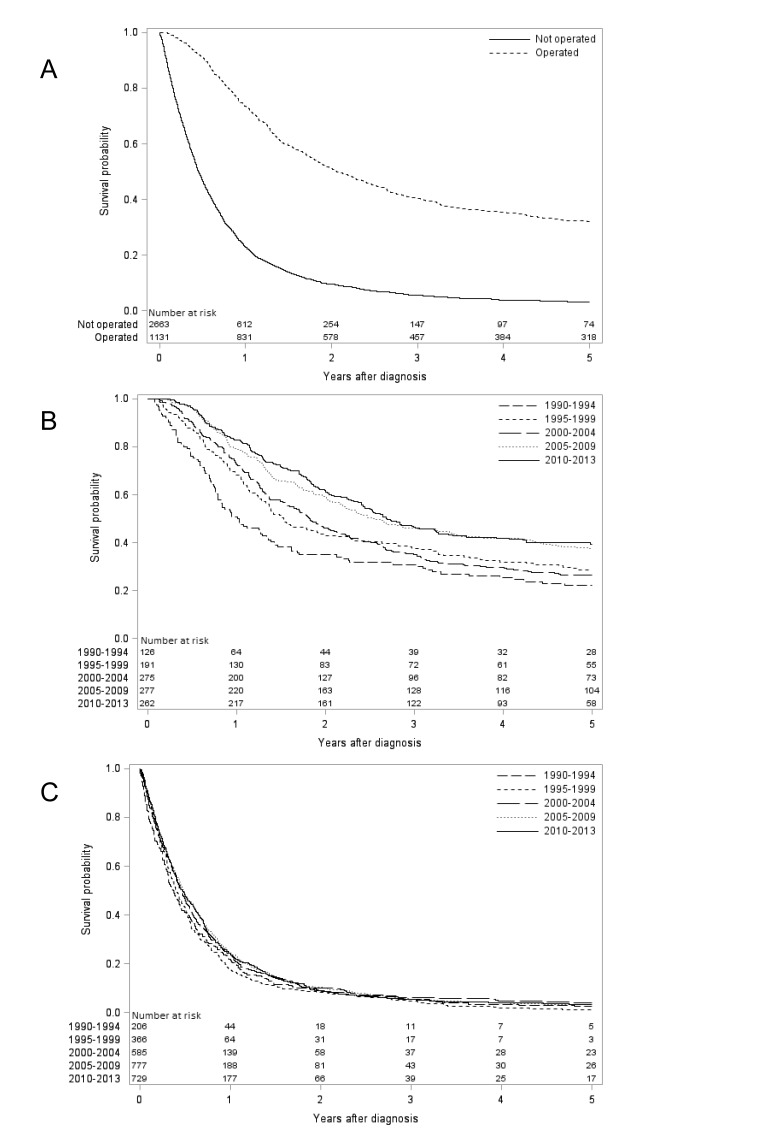
Survival trends in oesophageal adenocarcinoma
Because the observed survival closely mirrored the relative survival (table 1), only the results regarding relative survival are commented on here.
All patients
The relative survival in oesophageal adenocarcinoma improved during the study period; the 1-year survival increased from 35% in 1990–1994 to 41% in 2010–2013 (with follow-up to 2017), and the corresponding 5-year survival gradually increased from 12% to 15% (table 1). From the year 2000 onwards, the relative 5-year survival increased by 2% (table 1).
Surgically treated patients
The relative survival increased substantially in surgically treated patients. In the oesophagectomy group, the 1-year survival increased from 54% in 1990–1994 to 86% in 2010–2013 (with follow-up until 2017), and the corresponding 5-year survival increased from 27% to 45% (table 1, figure 1). From the year 2000 onwards, the relative 1-year survival increased by 11% and the 5 -ear survival by 12% (table 1).
Figure 1.
Kaplan-Meier survival curves showing observed 5-year survival oesophageal adenocarcinoma (A) stratified by surgical treatment (yes or no). Patients undergoing oesophageal resection for adenocarcinoma (B) and not undergoing oesophageal resection for adenocarcinoma (C) are further stratified by calendar periods.
Patients not undergoing surgery
In the patients not undergoing surgery, the 1-year survival increased from 24% in 1990–1994 to 25% in 2010–2013 (with follow-up to 2017), and the corresponding 5-year survival increased from 3% to 4% (table 1, figure 1). From 2000 onwards, the 1-year and 5-year survival estimates were stable (table 1).
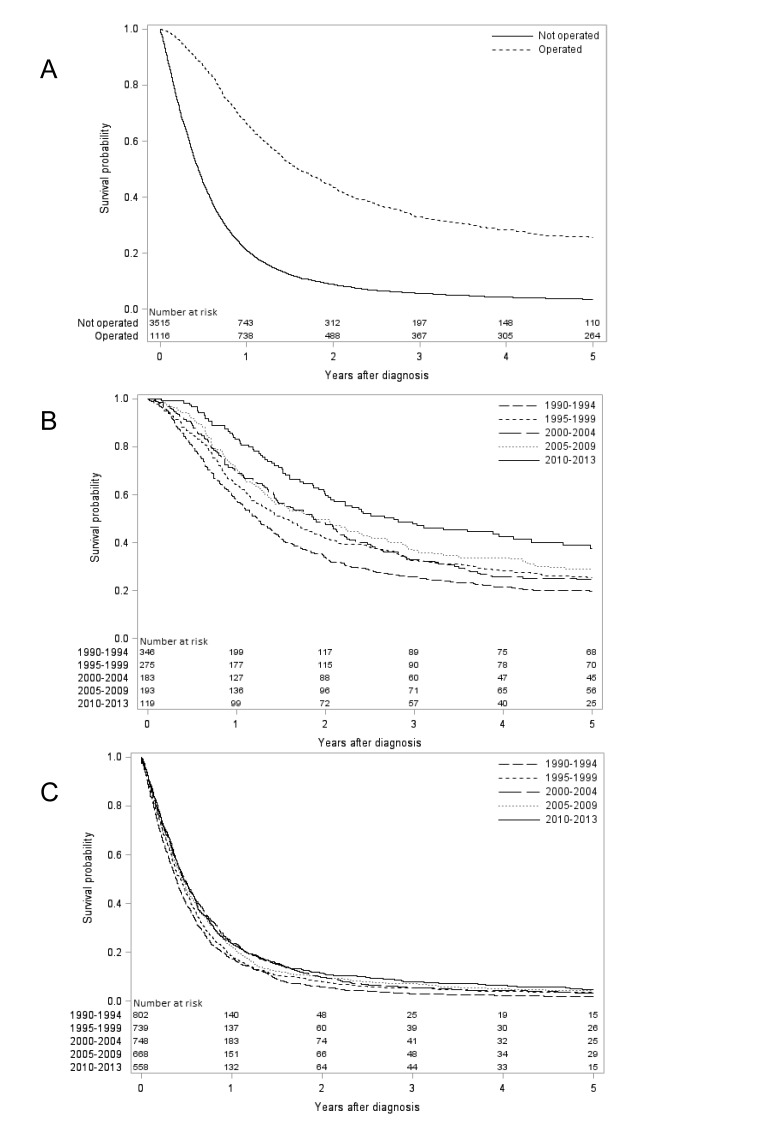
Survival trends in oesophageal squamous cell carcinoma
All patients
The relative survival in oesophageal squamous cell carcinoma improved over time. The relative 1-year survival increased from 32% in 1990–1994 to 36% in 2010–2013 (with follow-up until 2017), and the corresponding 5-year survival increased from 9% to 12% (table 1). From the year 2000 onwards, the relative 1-year survival increased by 1%, and the 5-year survival increased by 3% (table 1).
Surgically treated patients
The 1-year relative survival of surgically treated patients increased from 60% in 1990–1994 to 87% in 2010–2013 (with follow-up until 2017), and the corresponding 5-year survival increased from 24% to 43% (table 1, figure 2). From the year 2000 onwards, both 1-year and 5-year survival increased by 14% (table 1).
Figure 2.
Kaplan-Meier survival curves showing observed 5-year survival oesophageal squamous cell carcinoma (A) stratified by surgical treatment (yes or no). Patients undergoing oesophageal resection for squamous cell carcinoma (B) and not undergoing oesophageal resection for squamous cell carcinoma (C) are further stratified by calendar periods.
Patients not undergoing surgery
The relative 1-year survival in patients not undergoing surgery was 20% in 1990–1994 and 25% in 2010–2013 (with follow-up until 2017), and the corresponding 5-year survival doubled from 3% to 6% (table 1, figure 2). From the year 2000 onwards, both 1-year and 5-year survival increased by 2% (table 1).
Risk factors for 5-year mortality in oesophageal adenocarcinoma
All patients
In the multivariable analysis of all patients with oesophageal adenocarcinoma, the adjusted HR of mortality within 5 years of diagnosis was higher in earlier calendar periods (HR 1.17, 95% CI 1.02 to 1.33, first vs last calendar period), older age groups (HR 1.99, 95% CI 1.78 to 2.22, age ≥80 years vs <60 years) and in patients with more comorbidity (HR 1.27, 95% CI 1.15 to 1.40, ≥2 comorbidities vs no comorbidities), while sex did not influence the HR of mortality (table 2).
Table 2.
Observed 5-year survival and adjusted HR with 95% CI for oesophageal adenocarcinoma and oesophageal squamous cell carcinoma in 1990–2013, with follow-up until 2017
| Covariate | Category | Oesophageal adenocarcinoma | Oesophageal squamous-cell carcinoma | ||||
| Patients Number (%) | 5-year survival (95% CI) | HR (95% CI)* | Patients Number (%) | 5-year survival (95% CI) | HR (95% CI)* | ||
| All patients | |||||||
| Calendar period | 1990–1994 | 332 (9) | 10 (7 to 14) | 1.17 (1.02 to 1.33) | 1148 (25) | 8 (6 to 9) | 1.19 (1.07 to 1.31) |
| 1995–1999 | 557 (15) | 11 (8 to 13) | 1.11 (0.99 to 1.24) | 1014 (22) | 10 (8 to 12) | 1.11 (1.00 to 1.23) | |
| 2000–2004 | 860 (23) | 11 (9 to 13) | 1.07 (0.97 to 1.18) | 931 (20) | 7 (6 to 9) | 1.13 (1.01 to 1.25) | |
| 2005–2009 | 1054 (28) | 12 (10 to 14) | 1.04 (0.94 to 1.16) | 861 (19) | 10 (8 to 12) | 1.07 (0.96 to 1.19) | |
| 2010–2013 | 991 (26) | 13 (11 to 15) | 1 (reference) | 677 (15) | 11 (9 to 13) | 1 (reference) | |
| Age (years) | <60 | 686 (18) | 18 (15 to 21) | 1 (reference) | 787 (17) | 14 (12 to 17) | 1 (reference) |
| 60–69 | 1079 (28) | 16 (13 to 18) | 1.05 (0.94 to 1.16) | 1344 (29) | 12 (10 to 14) | 1.11 (1.01–1.22) | |
| 70–79 | 1166 (31) | 12 (10 to 13) | 1.25 (1.13 to 1.39) | 1588 (34) | 8 (7 to 9) | 1.38 (1.26 to 1.51) | |
| ≥80 | 863 (23) | 2 (1 to 3) | 1.99 (1.78 to 2.23) | 912 (20) | 2 (1 to 2) | 2.06 (1.86 to 2.28) | |
| Sex | Male | 3098 (82) | 12 (11 to 13) | 1 (reference) | 2938 (63) | 8 (7 to 9) | 1 (reference) |
| Female | 696 (18) | 10 (8 to 12) | 1.04 (0.95 to 1.14) | 1693 (37) | 11 (9 to 12) | 0.83 (0.78 to 0.89) | |
| Comorbidity score | 0 | 2096 (55) | 14 (13 to 15) | 1 (reference) | 2367 (51) | 11 (10 to 13) | 1 (reference) |
| 1 | 1115 (29) | 11 (9 to 12) | 1.11 (1.03 to 1.20) | 1598 (35) | 7 (6 to 9) | 1.15 (1.08 to 1.23) | |
| ≥2 | 583 (15) | 6 (4 to 8) | 1.27 (1.15 to 1.40) | 666 (14) | 4 (2 to 5) | 1.45 (1.32 to 1.59) | |
| Surgery | |||||||
| Calendar period | 1990–1994 | 126 (11) | 22 (15 to 29) | 2.02 (1.56 to 2.61) | 346 (31) | 20 (15 to 24) | 2.03 (1.56 to 2.63) |
| 1995–1999 | 191 (17) | 29 (22 to 35) | 1.46 (1.16 to 1.83) | 275 (25) | 25 (20 to 31) | 1.62 (1.24 to 2.13) | |
| 2000–2004 | 275 (24) | 27 (21 to 32) | 1.45 (1.18 to 1.79) | 183 (16) | 25 (18 to 31) | 1.53 (1.15 to 2.04) | |
| 2005–2009 | 277 (24) | 38 (32 to 43) | 1.07 (0.86 to 1.33) | 193 (17) | 29 (23 to 35) | 1.41 (1.06 to 1.88) | |
| 2010–2013 | 262 (23) | 40 (34 to 46) | 1 (reference) | 119 (11) | 39 (30 to 48) | 1 (reference) | |
| Age (years) | <60 | 286 (25) | 40 (35 to 46) | 1 (reference) | 317 (28) | 25 (20 to 30) | 1 (reference) |
| 60–69 | 423 (37) | 31 (26 to 35) | 1.25 (1.03 to 1.51) | 443 (40) | 28 (24 to 32) | 1.00 (0.85 to 1.19) | |
| 70–79 | 370 (33) | 30 (25 to 34) | 1.28 (1.05 to 1.56) | 328 (29) | 24 (19 to 29) | 1.13 (0.94 to 1.35) | |
| ≥80 | 52 (5) | 15 (5 to 24) | 1.98 (1.41 to 2.77) | 28 (3) | 11 (−1 to 22) | 1.61 (1.07 to 2.45) | |
| Sex | Male | 987 (87) | 31 (28 to 34) | 1 (reference) | 729 (65) | 21 (18 to 24) | 1 (reference) |
| Female | 144 (13) | 37 (29 to 45) | 0.84 (0.68 to 1.05) | 387 (35) | 33 (29 to 38) | 0.71 (0.61 to 0.83) | |
| Comorbidity score | 0 | 733 (65) | 33 (29 to 36) | 1 (reference) | 698 (63) | 27 (24 to 31) | 1 (reference) |
| 1 | 299 (26) | 32 (27 to 38) | 1.05 (0.89 to 1.23) | 340 (30) | 23(18-27) | 1.13 (0.97 to 1.31) | |
| ≥2 | 99 (9) | 26 (18 to 36) | 1.19 (0.93 to 1.53) | 78 (7) | 22 (12 to 31) | 1.20 (0.92 to 1.57) | |
| No surgery | |||||||
| Calendar period | 1990–1994 | 206 (8) | 3 (0 to 5) | 1.10 (0.94 to 1.29) | 802 (23) | 2 (1 to 3) | 1.24 (1.11 to 1.39) |
| 1995–1999 | 366 (14) | 1 (0 to 2) | 1.14 (1.00 to 1.30) | 739 (21) | 4 (2 to 5) | 1.15 (1.02 to 1.28) | |
| 2000–2004 | 585 (22) | 4 (2 to 6) | 1.02 (0.91 to 1.13) | 748 (21) | 3 (2 to 5) | 1.06 (0.95 to 1.19) | |
| 2005–2009 | 777 (29) | 3 (2 to 5) | 1.00 (0.90 to 1.11) | 668 (19) | 4 (3 to 6) | 1.06 (0.95 to 1.19) | |
| 2010–2013 | 729 (27) | 3 (2 to 5) | 1 (reference) | 558 (16) | 5 (3 to 7) | 1 (reference) | |
| Age (years) | <60 | 400 (15) | 3 (1 to 4) | 1 (reference) | 470(13) | 7 (5 to 9) | 1 (reference) |
| 60–69 | 656 (25) | 6 (4 to 7) | 0.91 (0.80 to 1.04) | 901 (26) | 4 (3 to 5) | 1.07 (0.95 to 1.20) | |
| 70–79 | 796 (30) | 3 (2 to 4) | 1.05 (0.93 to 1.19) | 1260 (36) | 4 (3 to 5) | 1.19 (1.06 to 1.32) | |
| ≥80 | 811 (30) | 1 (0 to 2) | 1.21 (1.06 to 1.37) | 884 (25) | 1 (1 to 2) | 1.42 (1.26 to 1.60) | |
| Sex | Male | 2111 (79) | 3 (2 to 4) | 1 (reference) | 2209 (63) | 3 (2 to 4) | 1 (reference) |
| Female | 552 (21) | 3 (1 to 4) | 1.00 (0.90 to 1.10) | 1306 (37) | 4 (3 to 5) | 0.86 (0.81 to 0.93) | |
| Comorbidity score | 0 | 1363 (51) | 4 (3 to 5) | 1 (reference) | 1669 (47) | 5 (4 to 6) | 1 (reference) |
| 1 | 816 (31) | 3 (1 to 4) | 1.04 (0.95 to 1.14) | 1258 (36) | 3 (2 to 4) | 1.07 (0.99 to 1.15) | |
| ≥2 | 484 (18) | 2 (0 to 3) | 1.08 (0.97 to 1.20) | 588 (17) | 1 (0 to 2) | 1.30 (1.18 to 1.43) | |
*Adjusted for calendar period, age, sex and comorbidity.
Surgically treated patients
Among the patients with oesophageal adenocarcinoma who underwent oesophagectomy, earlier calendar period (HR 2.02, 95% CI 1.56 to 2.61, first vs last calendar period) and older age (HR 1.98, 95% CI 1.41 to 2.77, age ≥80 years vs <60 years) were associated with an increased risk of mortality, while comorbidity and sex did not statistically significantly influence this risk (table 2).
In a subanalysis of patients diagnosed between 2005 and 2013, that is, when tumour stage data were available and adjusted for, higher tumour stage and older age were statistically significant poor prognostic factors (table 3).
Table 3.
HR with 95% CI of 5-year mortality after surgery for oesophageal adenocarcinoma and oesophageal squamous cell carcinoma in 2005–2013, with follow-up until 2017
| Covariates | Oesophageal adenocarcinoma | Oesophageal squamous-cell carcinoma | ||||
| Patients Number (%) | Crude HR (95% CI) |
Adjusted HR (95% CI)* |
Patients Number (%) | Crude HR (95% CI) |
Adjusted HR (95% CI)* |
|
| Tumour stage† | ||||||
| 0–I | 62 (12) | 1 (Reference) | 1 (Reference) | 34 (11) | 1 (Reference) | 1 (Reference) |
| II | 221 (41) | 2.53 (1.54 to 4.14) | 2.37 (1.44 to 3.90) | 143 (46) | 2.19 (1.25 to 3.83) | 2.20 (1.25 to 3.87) |
| III–IV | 186 (35) | 4.14 (2.53 to 6.76) | 4.04 (2.46 to 6.63) | 98 (31) | 2.71 (1.53 to 4.79) | 2.64 (1.48 to 4.71) |
| Calendar | ||||||
| 2005–2009 | 277 (51) | 1.12 (0.88 to 1.41) | 1.04 (0.82 to 1.32) | 193 (62) | 1.42 (1.05 to 1.93) | 1.48 (1.08 to 2.02) |
| 2010–2013 | 262 (49) | 1 (Reference) | 1 (Reference) | 119 (38) | 1 (Reference) | 1 (Reference) |
| Age (years) | ||||||
| <60 | 138 (26) | 1 (Reference) | 1 (Reference) | 79 (25) | 1 (Reference) | 1 (Reference) |
| 60–69 | 227 (42) | 1.52 (1.11 to 2.08) | 1.41 (1.02 to 1.94) | 137 (44) | 0.88 (0.61 to 1.28) | 0.81 (0.55 to 1.18) |
| 70–79 | 148 (27) | 1.52 (1.08 to 2.14) | 1.47 (1.03–2.08) | 86 (28) | 1.23 (0.83 to 1.81) | 1.21 (0.81 to 1.81) |
| ≥80 | 26 (5) | 3.39 (2.05 to 5.60) | 3.58 (2.14 to 5.98) | 10 (3) | 1.57 (0.71 to 3.48) | 1.58 (0.71 to 3.53) |
| Sex | ||||||
| Male | 470 (87) | 1 (Reference) | 1 (Reference) | 210 (67) | 1 (Reference) | 1 (Reference) |
| Female | 69 (13) | 0.84 (0.59 to 1.19) | 0.82 (0.57 to 1.18) | 102 (33) | 0.67 (0.49 to 0.93) | 0.66 (0.47 to 0.92) |
| Comorbidity score | ||||||
| 0 | 341 (63) | 1 (Reference) | 1 (Reference) | 195 (63) | 1 (Reference) | 1 (Reference) |
| 1 | 144 (27) | 1.04 (0.80 to 1.36) | 0.95 (0.73 to 1.25) | 86 (28) | 1.35 (0.98 to 1.87) | 1.40 (1.01 to 1.95) |
| ≥2 | 54 (10) | 1.07 (0.72 to 1.59) | 1.02 (0.68 to 1.52) | 31 (10) | 1.31 (0.82 to 2.12) | 1.60 (0.98 to 2.62) |
*Adjusted for calendar period, age, sex, Charlson Comorbidity Index and tumour stage.
†70 (13%) patients with oesophageal adenocarcinoma and 37 (12%) patients with oesophageal squamous-cell carcinoma had missing tumour stage.
Patients not undergoing surgery
Among the patients with oesophageal adenocarcinoma not undergoing surgery, older age (HR 1.21, 95% CI 1.06 to 1.37, age ≥80 years vs <60 years) was the only factor associated with an increased risk of mortality (table 2).
Risk factors for 5-year mortality in oesophageal squamous cell carcinoma
All patients
The multivariable analysis in all patients with oesophageal squamous cell carcinoma showed that risk factors for 5-year mortality were earlier calendar period (HR 1.19, 95% CI 1.07 to 1.31, first vs last calendar period), older age (HR 2.06, 95% CI 1.86 to 2.28, age ≥80 years vs <60 years), male sex (HR 0.83, 95% CI 0.78 to 0.89, women vs men) and comorbidity (HR 1.45, 95% CI 1.32 to 1.59,≥2 comorbidities vs no comorbidities) (table 2).
Surgically treated patients
Among the patients with oesophageal squamous cell carcinoma who underwent oesophagectomy, earlier calendar period (HR 2.03, 95% CI 1.56 to 2.63, first vs last calendar period), older age (HR 2.06, 95% CI 1.86 to 2.28, age ≥80 years vs <60 years) and male sex (HR 0.71, 95% CI 0.61 to 0.83, women vs men) were associated with an increased risk of mortality, while comorbidity did not statistically significantly influence this risk (table 2). In a subanalysis of patients diagnosed between 2005 to 2013, that is, when tumour stage data were available and adjusted for, more advanced tumour stage, earlier calendar period, older age, male sex and more comorbidity were poor prognostic factors in oesophageal squamous cell carcinoma (table 3).
Patients not undergoing surgery
Among the patients with oesophageal squamous cell carcinoma not undergoing surgery, earlier calendar period (HR 1.24, 95% CI 1.11 to 1.39, first vs last calendar period), older age (HR 1.42, 95% CI 1.26 to 1.60, age ≥80 years vs <60 years), male sex (HR 0.86, 95% CI 0.81 to 0.93, women vs men) and comorbidity (HR 1.30, 95% CI 1.18 to 1.43,≥2 comorbidities vs no comorbidities) were associated with an increased risk of mortality (table 2).
Discussion
This study indicates that the overall prognosis of both oesophageal adenocarcinoma and oesophageal squamous cell carcinoma is improving over time, especially in the groups of patients that underwent surgery. Female patients with oesophageal squamous cell carcinoma had better prognosis than male patients.
Among the strengths of the study is the population-based design with complete and accurate ascertainment and follow-up of all patients diagnosed with oesophageal cancer in Sweden. The assessment of the oesophageal cancer histology, surgical treatment and date of death was highly accurate and the calculation of relative survival rates allowed valid estimation of disease-specific mortality. The sample size was large enough to enable robust analyses of time trends in subgroups of patients, and to assess risk factors of mortality. Limitations include the unavailability of some clinical variables, such as use of neoadjuvant or adjuvant treatment. In patients not undergoing surgery, it was not possible to assess the treatment modalities used, which adds clinical heterogeneity to this group of patients. Tumour stage data were available only after 2004 and complete only in patients who had undergone surgery. However, the main purpose of the study was to evaluate time trends in survival and to separate these trends into patients who had undergone surgery or not, which was fully possible to achieve.
Previously, a registry-based study from the USA showed an increase in 5-year overall survival in patients with oesophageal cancer from 18% in 1990s to 22% in 2000s.10 A European registry-based study (EUROCARE-5) also showed improvement in 5-year overall age-standardised survival rates in oesophageal cancer from 10% in 1999–2001 to 13% in 2005–2007.11 In China, the age-standardised 5-year relative survival rate for oesophageal cancer was 21% in 2003–2005.26 Taken together, earlier studies have shown improving prognosis in oesophageal cancer over time. However, they reported only on earlier calendar periods, and information on histology-specific or treatment-specific survival were lacking.
The findings of increasing survival over time in both oesophageal adenocarcinoma and oesophageal squamous cell carcinoma despite a decreasing utilisation of oesophagectomy are encouraging. Increased awareness and diagnostic developments might explain the improved prognosis. The treatment might have been improved by centralisation of surgery,14 15 use of neoadjuvant chemoradiotherapy27 28 or definitive chemoradiotherapy.29 The more clearly improved 5-year survival in patients with non-surgically managed oesophageal squamous cell carcinoma might be due to a higher sensitivity to chemoradiotherapy in oesophageal squamous cell carcinoma, resulting in a higher success rate of definite oncological therapy.30 31 Additionally, careful selection of treatment for the elderly, comorbid and frail patients by the multidisciplinary teams, preoperative optimisation of patients and improved perioperative and postoperative care are likely reasons for improved survival in the patients undergoing surgery.
The better prognosis in women with oesophageal squamous cell carcinoma was unexpected. However, some earlier population-based studies have associated female sex with favourable prognosis in oesophageal cancer,32–34 although these studies did not separate the histological subtypes, tumour stage or treatment strategies.35 In the present study, female sex was a strong positive predictor of survival in both surgically and non-surgically treated patients with oesophageal squamous cell carcinoma, also after adjustment. A possible biological mechanism for a sex difference is oestrogenic influence, which could inhibit cancer cell growth.36–38 Additionally, hormone replacement therapy associates to lower risk of oesophageal squamous cell carcinoma.39 40 Healthcare-seeking patterns might also differ between the sexes, with women more readily and more often using health resources available to them, compared with men.41 42 It is however unclear why these patterns would differ between patients with oesophageal adenocarcinoma and squamous cell carcinoma, or patients with squamous cell carcinoma undergoing surgery and not undergoing surgery. The sex differences in survival after oesophageal squamous cell carcinoma might also be due to differences in socioeconomic and lifestyle factors, which could not be adjusted for in the present study, and should be specifically examined in future studies.
The treatment of oesophageal cancer in the Swedish publicly funded healthcare system follows clinical guidelines, including routine neoadjuvant therapy and centralisation to fewer hospitals during the last years. Thus, the findings of the present study should be generalisable to many other countries with a similar healthcare system as in Sweden. These findings suggest that the recent changes in the Swedish healthcare system, that is, careful selection of patients undergoing surgery, multidisciplinary management, centralisation of services, and use of neoadjuvant treatment in surgical candidates, and definitive chemoradiotherapy in patients ineligible for surgery, might result in improved prognosis of patients with oesophageal cancer.
In conclusion, this nationwide Swedish study with complete ascertainment and follow-up of patients with oesophageal cancer shows that the prognosis in both oesophageal adenocarcinoma and oesophageal squamous cell carcinoma is improving. The improved prognosis is stronger in surgically managed patients, but is also indicated in non-operated patients. Non-operated patients still have a poor prognosis. The favourable prognosis in women with oesophageal squamous cell carcinoma warrants further research.
Supplementary Material
Footnotes
Contributors: Design of the study: JHK, FM, NB, JL; data collection and preparation for analyses: FM and JL; data analysis: FM; data interpretation: JHK, FM, NB, JL; writing of first draft: JHK. Revised and approved by JHK, FM, NB and JL.
Funding: This work was supported by the Swedish Research Council (Vetenskapsrådet) (JL), the Swedish Cancer Society (Cancerfonden) (JL), Sigrid Jusélius Foundation (Sigrid Juséliuksen Säätiö) (JHK) and Orion Research Foundation (Orionin Tutkimussäätiö) (JHK).
Disclaimer: The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Regional Ethical Review Board in Stockholm, Sweden (2015/1916-31/1)
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We are willing to share data upon request after ethical approval has been approved by the relevant committee and the governmental agencies that maintain the data.
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524–48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin AT, Francisci S, Foschi R, et al. Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol 2012;36:505–12. 10.1016/j.canep.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Dubecz A, Gall I, Solymosi N, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol 2012;7:443–7. 10.1097/JTO.0b013e3182397751 [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Mattsson F, Mattson F. Diverging trends in recent population-based survival rates in oesophageal and gastric cancer. PLoS One 2012;7:e41352 10.1371/journal.pone.0041352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112–20. 10.4251/wjgo.v6.i5.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383–96. 10.1016/S0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 7.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin 2013;63:232–48. 10.3322/caac.21185 [DOI] [PubMed] [Google Scholar]
- 8.Brown LM, Hoover RN, Greenberg RS, et al. Are racial differences in squamous cell esophageal cancer explained by alcohol and tobacco use? J Natl Cancer Inst 1994;86:1340–5. 10.1093/jnci/86.17.1340 [DOI] [PubMed] [Google Scholar]
- 9.Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev 2017;26:107–18. 10.1097/CEJ.0000000000000249 [DOI] [PubMed] [Google Scholar]
- 10.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol 2016;31:1141–6. 10.1111/jgh.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson LA, Tavilla A, Brenner H, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999-2007: Results from EUROCARE-5. Eur J Cancer 2015;51:2144–57. 10.1016/j.ejca.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauppila JH, Helminen O, Kytö V, et al. Short-Term Outcomes Following Minimally Invasive and Open Esophagectomy: A Population-Based Study from Finland and Sweden. Ann Surg Oncol 2018;25:326–32. 10.1245/s10434-017-6212-9 [DOI] [PubMed] [Google Scholar]
- 13.Markar SR, Mackenzie H, Lagergren P, et al. Surgical Proficiency Gain and Survival After Esophagectomy for Cancer. J Clin Oncol 2016;34:1528–36. 10.1200/JCO.2015.65.2875 [DOI] [PubMed] [Google Scholar]
- 14.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut 2014;63:1393–400. 10.1136/gutjnl-2013-306074 [DOI] [PubMed] [Google Scholar]
- 15.Derogar M, Sadr-Azodi O, Johar A, et al. Hospital and surgeon volume in relation to survival after esophageal cancer surgery in a population-based study. J Clin Oncol 2013;31:551–7. 10.1200/JCO.2012.46.1517 [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–67. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindblad M, Ye W, Lindgren A, et al. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg 2006;243:479–85. 10.1097/01.sla.0000205825.34452.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brusselaers N, Vall A, Mattsson F, et al. Tumour staging of oesophageal cancer in the Swedish Cancer Registry: A nationwide validation study. Acta Oncol 2015;54:903–8. 10.3109/0284186X.2015.1020968 [DOI] [PubMed] [Google Scholar]
- 19.Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol 2002;80:119–20. 10.1002/jso.10114 [DOI] [PubMed] [Google Scholar]
- 20.Naessén T, Parker R, Persson I, et al. Time trends in incidence rates of first hip fracture in the Uppsala Health Care Region, Sweden, 1965-1983. Am J Epidemiol 1989;130:289–99. 10.1093/oxfordjournals.aje.a115335 [DOI] [PubMed] [Google Scholar]
- 21.Lagergren K, Derogar M. Validation of oesophageal cancer surgery data in the Swedish Patient Registry. Acta Oncol 2012;51:65–8. 10.3109/0284186X.2011.633932 [DOI] [PubMed] [Google Scholar]
- 22.Armitage JN, van der Meulen JH. Royal College of Surgeons Co-morbidity Consensus G. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg 2010;97:772–81. [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol 2017;32:765–73. 10.1007/s10654-017-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler SJ, Ederer F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis 1958;8:699–712. 10.1016/0021-9681(58)90126-7 [DOI] [PubMed] [Google Scholar]
- 26.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921–30. 10.1002/ijc.29227 [DOI] [PubMed] [Google Scholar]
- 27.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681–92. 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 28.Little AG, Lerut AE, Harpole DH, et al. The Society of Thoracic Surgeons practice guidelines on the role of multimodality treatment for cancer of the esophagus and gastroesophageal junction. Ann Thorac Surg 2014;98:1880–5. 10.1016/j.athoracsur.2014.07.069 [DOI] [PubMed] [Google Scholar]
- 29.Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer--a meta-analysis of the randomized trials. Cancer Treat Rev 2012;38:599–604. 10.1016/j.ctrv.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310–7. 10.1200/JCO.2005.00.034 [DOI] [PubMed] [Google Scholar]
- 31.Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007;25:4110–7. 10.1200/JCO.2007.12.0881 [DOI] [PubMed] [Google Scholar]
- 32.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012;30:2265–72. 10.1200/JCO.2011.38.8751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellison LF. Differences in cancer survival in Canada by sex. Health Rep 2016;27:19–27. [PubMed] [Google Scholar]
- 34.Bus P, Lemmens VE, van Oijen MG, et al. Prognostic factors for medium- and long-term survival of esophageal cancer patients in the Netherlands. J Surg Oncol 2014;109:465–71. 10.1002/jso.23513 [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Garfield D, Jiang Y, et al. Does sex affect survival of patients with squamous cell esophageal cancer? J Clin Oncol 2013;31:815–6. 10.1200/JCO.2012.45.5071 [DOI] [PubMed] [Google Scholar]
- 36.Ueo H, Matsuoka H, Sugimachi K, et al. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res 1990;50:7212–5. [PubMed] [Google Scholar]
- 37.Utsumi Y, Nakamura T, Nagasue N, et al. Role of estrogen receptors in the growth of human esophageal carcinoma. Cancer 1989;64:88–93. [DOI] [PubMed] [Google Scholar]
- 38.Nozoe T, Oyama T, Takenoyama M, et al. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res 2007;13:4046–50. 10.1158/1078-0432.CCR-07-0449 [DOI] [PubMed] [Google Scholar]
- 39.Wang BJ, Zhang B, Yan SS, et al. Hormonal and reproductive factors and risk of esophageal cancer in women: a meta-analysis. Dis Esophagus 2016;29:448–54. 10.1111/dote.12349 [DOI] [PubMed] [Google Scholar]
- 40.Brusselaers N, Maret-Ouda J, Konings P, et al. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer 2017;140:1693–9. 10.1002/ijc.30588 [DOI] [PubMed] [Google Scholar]
- 41.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs 2005;49:616–23. 10.1111/j.1365-2648.2004.03331.x [DOI] [PubMed] [Google Scholar]
- 42.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health 1984;5:433–58. 10.1146/annurev.pu.05.050184.002245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.