Aging is well known to be the main risk factor for the neurodegenerative pathologies, in particular, Parkinson's disease (PD) and Alzheimer's disease (AD). In aging and in the diseases, similar changes in various hallmarks of neurodegeneration (lipofuscin accumulation, autophagia weakening, and disturbances in functions of mitochondria and lysosomes) were shown (Tan et al., 2014). Furthermore, dopaminergic system (DAS) involvement in mechanisms of aging, PD, and AD were revealed (Martorana and Koch, 2014). Dopamine-producing neurons are predominantly concentrated in the substantia nigra (SN) and the ventral tegmental area (VTA). Dopamine (DA) terminals are widespread in the brain, dominating in the hippocampus, prefrontal cortex, striatum, and olfactory bulbs (Martorana and Koch, 2014). DA oxidization is well known to be accompanied by generation of various highly toxic compounds, in particular, reactive oxygen species (ROS). This is aggravated by auto-oxidization of DA with consequent accumulation of toxic DA-quinones (DA-Q) and other DA derivatives (Dauer and Przedborski, 2003). Uncombined DA-Q is quickly neutralized by nucleophilic glutathione resulting in accumulation of 5-S-glutathionyl-DA, which, in turn, is transformed by enzymatic degradation into 5-S-cystenil-DA, one of the components of neuromelanin (NM). NM, predominantly accumulated in the SN, is able to bind free iron and, thus, to block both oxidative transformation of DA into its toxic forms and the ROS generation. These mechanisms allow the protection of neurons from the oxidative stress (Zucca et al., 2017), thus, supporting normal functioning of DAS, in particular.
Aging seems to be associated with disturbances in regulation of multiple protective mechanisms in the brain, which are expressed in the mitochondrial malfunctioning, the lowering of antioxidants level and, in turn, in ROS rising. These seem to initiate the neurodegenerative processes development, whereas the brain phenotype is biased to a form, which is atypical for normal aging (Dauer and Przedborski, 2003). Among factors shifting the DAS balance and, consequently, affecting the interrelations between the mechanisms of aging and neurodegeneration, the senescent cells (SC) involvement is thought to have priority. Indeed, the senescence-associated secretory phenotype (SASP), arising from senescence-associated growth arrest (SAGA), has been shown either to activate or inhibit the brain adaptive mechanisms in relation to the disease progression (Acosta et al., 2013). In early stage, SASP machinery, involving cytokines, enzymes, growth factors, and extracellular matrix ingredients, is able to activate the repairing and remodeling mechanisms through the cytokines secretion and the release of growth factors and proteases. In later stage, SC negatively affect these mechanisms by such SASP components as interleukins (IL) through autocrine regulation of SAGA and paracrine mediation of surrounding cells. The latter is accompanied by the senescent phenotype transformation resulting in elimination of adaptive/regenerative abilities of the cells (Tan et al., 2014; Chinta et al., 2015). SASP expression has been shown to be under control of an inflammatory signal IL-1 and, evoking local tissue inflammation and ROS rise in normal cells, to be able to induce both the aging of these cells and their transformation into SC (Passos et al., 2010; Acosta et al., 2013). SC populations have been revealed to rise intensively in PD and AD even at higher level than in aging (Chinta et al., 2015). In DAS, SC, originated predominantly from glial cells (Tan et al., 2014), are able to produce an inflammatory center accumulating ROS and other oxidizers. However, aging has been shown to be characterized by the lowering of anti-oxidative activities in DAS (Zucca et al., 2017), and the SC population rising is expected to aggravate this deleterious process. Thus, DA-containing neurons in the SN and VTA seem to be suffered with both the oxidative stress and consecutive accumulation of neurotoxic derivates of the DA oxidation in the brain areas with highly concentrated DA terminals. Generated here ROS can be transient centers of DNA damage that is developed into chronic damage DNA response (DDR), needed and sufficient for the steady stopping of both the cell growing and SASP production in the affected brain areas (Passos et al., 2010). In aging, PD, and AD, populations of DA neurons have been shown to shrink, whereas those of SC to enlarge (Dauer and Przedborski, 2003; Burns et al., 2005; Chinta et al., 2015). This negative correlation seemingly highlights the role of SC in disintegration of DA neurons in SN followed by the releasing of encapsulated NM into the extracellular space. The NM is able to be active for a long time and to initiate chronic inflammation by neurotoxic compounds, which were previously adsorbed by NM's molecules (Zucca et al., 2017). Furthermore, the NM-containing granules seem to be targeted by activated microglia with release of iron ions from their binding with NM. These ions have been revealed to prevent the amyloid-beta (Aβ) plaque generation, thus, supporting the toxicity of Aβ oligomers (Liu et al., 2011). Furthermore, iron, in its free form, has been shown to induce α-synuclein aggregation that is duplicated by 3,4-dihydroxyphenylaldehyde (DOPAL), a derivate of DA distraction by the monoamine oxidase B (MAO-B). Oxidized form of DOPAL, 3,4-dihydroxyphenylacetaldehyde-quinone (DOPAL-Q), binds covalently to lysine residues on alpha-synuclein molecules, converting them to toxic synuclein oligomers. In particular, proto-fibrillar α-synuclein has been shown in vitro to be able to perforate the vesicle membranes and, thus, to stimulate the leakage of endogenous DA into the cytosol (Caudle et al., 2008; Zucca et al., 2017). A role of α-synuclein in the senescence mechanisms deserves to be analyzed in details in further studies.
Senescence in neurons seems to have peculiarities associated with their post-mitotic nature. Indeed, the transformation of neurons is thought to be modulated by glial cells (astrocytes, oligodendrocytes, and microglia) whose structural, metabolic, and trophic support for neurons is well known. Thus, ROS, generated in glial and/or SC surrounding the neurons, are thought to affect those (Chinta et al., 2015). Disruption of normal calcium homeostasis, with increased intracellular resting levels of Ca2+ and impaired ability to remove excessive Ca2+ in response to glutamate stimulation is one of extensively studied hallmarks of aged neurons. The impaired calcium homeostasis may be the result of an age-related reduction in glutathione, a major antioxidant that was also found to contribute to the oxidative stress-dependent SС (Tan et al., 2014). However, in DAS, the age-dependent transformation of DA neurons into their senescent form seems to be dependent on MAO-B activity as well. Decomposition of DA molecules is associated with intensive formation of hydrogen peroxide, one of the main sources of ROS. In contrast to DA neurons, MAO-B is predominantly stored in the glial cells and protected from hydrogen peroxide by highly expressed glutathione and glutathione peroxidase, which together transform hydrogen peroxide into water (Kumar and Andersen, 2004). In aging, SC proportion within population of glial cells surrounding DA neurons is raised (Chinta et al., 2015). The malfunctioning of the glial cells is expected to be accompanied by increased DA concentration in the extracellular space and in DA neurons because of increased DA reuptake. Given the age-dependent increase of MAO-B level in the brain (Kumar and Andersen, 2004), the fates of individual neurons suppose to be determined by corresponding balance between activities of the reuptake and MAO mechanisms. Additional factors initiating and/or supporting the aging and development of PD and AD can be associated with toxic DA derivatives, in particular, DA-Q (Dauer and Przedborski, 2003; Zucca et al., 2017) and hydrogen peroxide (Kumar and Andersen, 2004). Regardless of unusual mechanisms, the main attention in further studies should be directed to specific differences between senescence-associated transformations in glial and neuronal cells.
The spreading of senescent-associated processes from their sources to the brain areas with enhanced neurogenesis has a peculiar interest for studies of the interrelations between aging and neurodegeneration. The senescence has been shown to be involved, through SASP, in mechanisms of neuronal stem cells (NSC) elimination, resulting in both narrowing of their pool and proliferative and neurogenesis disturbances (Chinta et al., 2015). SC are able to induce the senescence of adjacent cells through the autocrine and paracrine signaling that allows the targeting of various brain structures. One of the initiating factors is though to be NM, released from the affected neurons and capable both to migrate through the brain and deliver iron ions and inflammatory substrates to adjacent/remote brain areas. Additional involvement of the Aβ peptide in these processes is thought to evoke pathological “chain-reaction” in proliferation and neurogenesis of the affected neurons (Chinta et al., 2015), especially in the hippocampal dentate gyrus. Recently, a specific expression of genes and the functioning of epigenetic mechanisms, involved in SC-mediated changes both in microenvironment and, through SASP, in remote brain areas, have been described. The profiles of genes expression in aging are thought to be closely linked with consecutive changes in heterochromatin of SC in various tissues (Tan et al., 2014). However, the epigenetic mechanisms of SC formation in both aging and neurodegeneration are still unknown and need to be discovered. This is especially important for DAS where modifications in expression of peptides, in particular, α-synuclein, have been shown to induce SC formation and, hence, the development of neurodegenerative diseases.
It should be mentioned, however, that this paper does not introduce all aspects of the association between the aging, neurodegeneration, and the senescent cells. In particular, the question how the damaged cells avoid the apoptosis in aging remains unclear. There are evidences that FOXO4 protein is involved in this process through the binding with p53 protein, which is accumulated in the nucleus because of DNA damage (Baar et al., 2017). Furthermore, the aging has been mentioned is associated with numerous pathologies, and genetic clearance of SC is able to retard the aging symptoms.
In conclusion, we are hypothesizing that the interactions between SC and DAS have to be involved in mechanisms of both aging and initiation of neurodegenerative pathologies, in particular, PD and AD (see Figure 1). The role of SC in these mechanisms is associated with specificity of the affected brain area(s) and/or the metabolic pathways chain(s). This suggestion allows the substitution of the conventional symptomatic therapy of the neurodegenerative disorders by approaches that could target their intimate mechanisms. Thus, the results of studies of SC generation and SC spatio-temporal distribution in the cerebral DAS could be a breakthrough in the development of novel pharmacological approaches in the control of aging and neurodegeneration.
Figure 1.
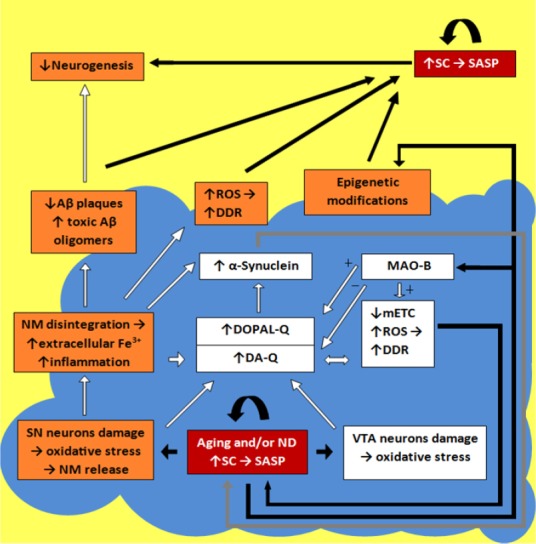
Schematic imaging of pathways and factors transforming dopaminergic cells into their senescent forms in aging and/or neurodegeneration (ND).
Blue fragment is the DA-producing areas (SN and VTA) in the brain (open boxes mark factors functioning inside these areas); red boxes mark areas where cells are transformed into their senescent forms; orange boxes mark the main critical factors involved in the cell senescence (the boxes, overlapping the blue-yellow border, mark the factors usable on each side). The details of the senescence pathways functioning are described in the text. SC: Senescent cells; DA-Q: dopamine-quinone; DOPAL-Q: 3,4-dihydroxyphenylacetaldehyde-quinone; DDR: damage DNA response; Aβ: amyloid-beta; MAO-B: monoamine oxidase B; NM: neuromelanin; mETC: mitochondrial electron transport chain; ROS: reactive oxygen species; SASP: senescence-associated secretory phenotype; SN: substantia nigra; VTA: ventral tegmental area.
This study was supported by RFBR 16-04-00942 (Russia).
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Robert L. Haining, Georgia Gwinnett College, USA.
Comments to authors: I believe this paper highlights a potentially very important aspect of neurodegeneration. It has certainly got me thinking and I believe it should get much more attention. My understanding is that MAO-B is largely responsible for eliminating excess dopamine which leaches from active DA neurons. As this enzyme is predominant in glial cells, the loss of such cells, or conversion to a senescent form, would likely have profound implications on neuroprotection/neurodegeneration. There is strong evidence that DA neurons down-regulate tyrosine hydroxylase upon accumulation of neuromelanin pigment, something I have often thought implied a lower demand for dopamine synthesis in later stages of cellular life, yet such cells are presumably still storing and releasing dopamine. The toxin MPTP seems to be activated by MAO-B into the pyridinium ion, which is the active Parkinsonian-inducing toxin, highlighting the interplay between glial cells and DA neurons. Indeed, there are several pieces of this puzzle still missing.
Reviewer 2: Myung Koo Lee, Chungbuk National University, Korea.
Comments to authors: The invited paper reviewed the roles of senescent cells in dopaminergic neuronal system of the brain aging and neurodegenerative diseases. This paper will give the good information for readers and also suggest the future directions.
References
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64:1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson's disease: a premature demise. Trends Neurosci. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Kumar MJ, Andersen JK. Perspectives on MAO-B in aging and neurological disease: where do we go from here. Mol Neurobiol. 2004;30:77–89. doi: 10.1385/MN:30:1:077. [DOI] [PubMed] [Google Scholar]
- Liu B, Moloney A, Meehan S, Morris K, Thomas SE, Serpell LC, Hider R, Marciniak SJ, Lomas DA, Crowther DC. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J Biol Chem. 2011;286:4248–4256. doi: 10.1074/jbc.M110.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana A, Koch G. “Is dopamine involved in Alzheimer's disease”? Front Aging Neurosci. 2014;6:252. doi: 10.3389/fnagi.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan FC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders. Biogerontology. 2014;15:643–660. doi: 10.1007/s10522-014-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


