Keywords: nerve regeneration, Panax ginseng extract, chronic cerebral hypoperfusion, vascular dementia, learning and memory, vascular endothelial growth factor, basic fibroblast growth factor, apoptosis, neural regeneration
Abstract
Panax ginseng is a slow-growing perennial plant. Panax ginseng extract has numerous biological activities, including antitumor, anti-inflammatory and antistress activities. Panax ginseng extract also has a cognition-enhancing effect in rats with alcohol-induced memory impairment. In this study, we partially occluded the bilateral carotid arteries in the rat to induce chronic cerebral hypoperfusion, a well-known model of vascular dementia. The rats were then intragastrically administered 50 or 100 mg/kg Panax ginseng extract. Morris water maze and balance beam tests were used to evaluate memory deficits and motor function, respectively. Protein quantity was used to evaluate cholinergic neurons. Immunofluorescence staining was used to assess the number of glial fibrillary acidic protein-positive cells. Western blot assay was used to evaluate protein levels of vascular endothelial growth factor, basic fibroblast growth factor, Bcl-2 and Bax. Treatment with Panax ginseng extract for 8 weeks significantly improved behavioral function and increased neuronal density and VEGF and bFGF protein expression in the hippocampal CA3 area. Furthermore, Panax ginseng extract reduced the number of glial fibrillary acidic protein-immunoreactive cells, and it decreased apoptosis by upregulating Bcl-2 and downregulating Bax protein expression. The effect of Panax ginseng extract was dose-dependent and similar to that of nimodipine, a commonly used drug for the treatment of vascular dementia. These findings suggest that Panax ginseng extract is neuroprotective against vascular dementia induced by chronic cerebral hypoperfusion, and therefore might have therapeutic potential for preventing and treating the disease.
Introduction
Chronic cerebral hypoperfusion is a pathological condition associated with diseases such as Alzheimer's disease and vascular dementia (VD) (Li and Zhang, 2015). Animal models of chronic cerebral hypoperfusion can be prepared by performing two-vessel occlusion, resulting in a pathology similar to VD (Sun et al., 2015; Huang et al., 2016; Lv et al., 2016) In the rat with two-vessel occlusion, cerebral blood flow is disrupted, resulting in chronic hypoperfusion in the cortex, hippocampus and white matter. The ensuing neuronal injury produces suboptimal metabolism and cognitive dysfunction. Accumulating evidence shows that cerebral ischemia is a major cause of the development of cognitive decline and dementia in the elderly, which involves multiple pathophysiological processes (Ma et al., 2016). The brain regions supplied by the middle cerebral artery, such as the parietal cortex, hippocampus and striatum, are strongly affected by cerebral ischemia. In particular, hippocampal neurons, known to play an important role in learning and memory processes, are vulnerable to ischemic neuronal injury, thereby resulting in severe learning and memory deficits. Functional and morphological alterations in the hippocampus, such as changes in neurons, astrocytes and synapses, are the most important factors contributing to cognitive dysfunction. Neuronal death in the hippocampus is a major contributor to memory decline in the elderly (Burke et al., 2014). In addition, the vulnerability of hippocampal CA3 pyramidal neurons plays a key role in the onset of cognitive impairment (Counts et al., 2014). Astrocytes also perform critical functions in the brain, such as promoting neovascularization, regulating neuronal activity, and supporting synaptogenesis and neurogenesis, which play a role in recovery following ischemic injury. Changes in astrocytes following ischemia may result from direct cellular injury or might occur in response to injury in other central nervous system structures (Lana et al., 2017). However, the pathogenic mechanisms that underlie VD remain to be identified.
There is a great demand for the development of disease-modifying drugs for VD that could attenuate or even reverse the neurodegenerative process by targeting a major hallmark of the disease. Chinese medicine has a long history of preventing and treating cardiovascular and cerebrovascular diseases as well as other related brain diseases (Gao et al., 2017; Guo et al., 2017). These medicines are therapeutically effective and are deemed safe because of their low toxicity and few side effects (Lin et al., 2017; Mei et al., 2017).
Ginseng is a slow-growing perennial plant that belongs to the Araliaceae family and Panax genus. Panax ginseng extract (PGE), is taken orally as a traditional medicine in Asian countries (Lin et al., 2017; Ma et al., 2017). It is one of the most renowned herbs, with more than 5,500 years of use in East Asia (Lin et al., 2017). It has been reported to possess a variety of biological activities, including antitumor (Zhang et al., 2008), anti-inflammatory (Wang et al., 2015) and antistress activities (Kaneko and Nakanishi, 2004). PGE also has a cognition-enhancing effect in rats with alcohol-induced memory impairment (Shin et al., 2016). In addition, its safety and tolerability for long-term human consumption is well-established (Ohkita et al., 2011; Furumura et al., 2012).
In this study, to help evaluate the effect of PGE on VD, we used nimodipine, an L-type voltage-dependent Ca2+ channel antagonist, as a positive control. Nimodipine is commonly used for treating VD (Baskys and Cheng, 2012). A previous study reported that nimodipine improves the symptoms of cognitive impairment, increases regional cerebral blood flow, reduces hippocampal inflammatory factor levels, and alleviates neuronal injury (Zhang et al., 2012).
In this study, we examine the effects and mechanism of action of PGE in a rat model of VD produced by bilateral common carotid artery occlusion. Our aim is to advance our understanding of the mechanisms by which PGE protects against the development and progression of VD.
Materials and Methods
Animals
A total of 100 healthy clean male Wistar rats, 4–5 months old and weighing 320–350 g, were purchased from the Experimental Animal Center of Guizhou Medical University, China [License No. SCXK (qian) 2018-0001]. Rats were housed at 50–70% humidity and a temperature of 22–24°C under a 12/12-hour light/dark cycle. All experiments were conducted in accordance with the guidelines of the Ministry of Health of China and the Animal Care Committee of Guizhou Medical University in China. The study protocol was approved by the Experimental Animal Research Committee of Guizhou Medical University in China. Food and water were freely available during all stages of the experiment. The rats were randomly divided into the following five groups (n = 20 each): sham, VD model, 50 mg/kg PGE, 100 mg/kg PGE and nimodipine.
Preparation of the VD model
Rats were acclimated to the facility for a week prior to surgery. The rat model of VD was established as previously described by Gupta et al. (2016). In brief, the rats were intraperitoneally anesthetized with 3 mL/kg of 10% (w/v) chloral hydrate. Through a midline cervical incision, the bilateral common carotid arteries were exposed and gently separated from the carotid sheath and vagus nerve. Each artery of the rats assigned to the ischemic group was ligated with a 5–0 silk suture. As a control, sham-operated rats underwent the identical surgery, but without suture insertion. After recovery from anesthesia, the rats were returned to the animal facility and given free access to food and water. An observer blinded to the identity of the groups assessed neurological deficits at 24 and 48 hours with the forelimb akinesia test, while the spontaneous rotational test was used to evaluate ischemic insult (Nishino et al., 1994). Rats not showing behavioral deficits at these time points were excluded from the study.
Drug administration
PGE in concentrated form was purchased commercially from Xi’an Season Biotechnology, Co., Ltd. (Xi’an, China). PGE was produced from the roots of fully mature six-year-old Chinese ginseng plants. PGE contains the following saponin fractions: 30.1% ginsenoside (G)-Rb1, 13.9% G-Rb2, 14.4% G-Rc, 6.1% G-Rd, 13.9% G-Re, 4.7% G-Rf, 11.5% G-Rg1, 2.6% G-Rg2 and 2.8% G-Rg3 (Ban et al., 2012). PGE was dissolved in saline solution (0.9% NaCl). After the operation, the rats in the VD model group were treated with normal saline (2.0 mL) for 8 weeks. In the 50 and 100 mg/kg PGE groups, the rats underwent middle cerebral artery occlusion (MCAO) and were treated with PGE at the indicated dose by gavage for 8 consecutive weeks. In the nimodipine group, rats underwent MCAO and were treated with nimodipine (20 mg/kg/day, gavage; Sigma-Aldrich, St. Louis, MO, USA). The extracts were filtered and concentrated under vacuum at 60°C. The residue was dissolved in 100% ethanol and filtered and concentrated again.
Behavioral testing
Morris water maze (MWM) test
Rats were tested for spatial learning and memory using the MWM as previously described (Weitzner et al., 2015). The apparatus consisted of a circular tank (opaque), and four points around the edge were arbitrarily designated north, south, east and west to divide the pool into four corresponding quadrants (northeast, southeast, northwest and southwest). An escape platform was submerged approximately 2 cm below the water (22 ± 1°C) surface and placed in the northeast quadrant of the maze. Extramaze cues consisted of laboratory furniture and lights (held constant throughout the experiment). A video camera was mounted above the center of the pool, and all behaviors were recorded for subsequent analyses. The rats were trained for 4 consecutive days with 4 trials a day from any of the two starting points separated by 90°. The maximum trial duration was 120 seconds, and the inter-trial interval was 60 seconds, during which the rat remained on the escape platform. If the rat did not find the platform within the allowed time, it was guided to the finish by the observer. In each trial, the latency to escape onto the hidden platform was recorded by the observer. A 90-second probe trial was conducted 24 hours following the last test day, and swimming distance in the target quadrant was recorded.
Balance beam test
The balance beam is a test of motor coordination (Zhou et al., 2013). After completion of the MWM test, the balance beam test was administered. Rats were trained to traverse a cylindrical beam with a length of 200 cm and a diameter of 2.5 cm. A black platform (7.0 cm × 4.0 cm) was positioned at one end of the beam at the start point, and a black plastic box (15 cm × 15 cm × 8.0 cm) was placed at the other end as an incentive to traverse the beam. The apparatus was suspended 90 cm above a cushion, which protected rats from injury due to falls, and was positioned 50 cm from a wall. Rats were moved into the testing room one hour before the test to adapt to the environment. The time required to traverse the beam during two trials was recorded. The maximal transversal time allowed was 60 seconds.
Determination of choline acetyltransferase (ChAT) and acetylcholinesterase (AchE) activities
To evaluate the neuroprotective effect of PGE on the cholinergic system, following behavioral testing, ten rats from each group were decapitated, and the brains were quickly removed. The hippocampi were dissected according to a stereotaxic brain atlas (Vállez Garcia et al., 2015). Some brain tissues were flash-frozen with liquid nitrogen and stored at −80°C for subsequent use. The others were rinsed in cold physiological saline to remove blood, and a 10% (wt/vol) homogenate was prepared using a tissue homogenizer (3 × 10 seconds, with 30-second intervals). The homogenate was centrifuged at 4000 × g for 30 minutes at 4°C. The supernatant was assayed for ChAT and AchE activities according to the manufacturer's protocol. Protein concentrations were determined using the Quantity Protein assay kit (Jiancheng Institute of Biotechnology, Nanjing, China).
Western blot assay
The hippocampal tissues were frozen and cut into 1.0-mm sections using a stainless-steel rat brain slicer, and homogenized in 0.5 mL of radioimmunoprecipitation assay buffer (150 mM NaCl, 1% N-40, 0.5% deoxycholate, 0.1% sodium dodecylsulfate, 50 mM Tris hydrochloride, 2 mM phenylmethylsulfonyl fluoride, pH 7.4). The homogenate was then transferred to small tubes and mixed by rotation at 4°C overnight. Solubilized protein was collected after centrifugation at 10,000 × g for 30 minutes. The supernatant from each tube was collected, and protein concentrations were quantified with the enhanced bicinchoninic acid protein assay kit (Boster Biotechnology, Wuhan, China). To detect vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), Bcl2 and Bax protein levels, tissue protein lysates from each group were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis using a 10% gel and electrotransferred onto a polyvinylidene fluoride membrane. The membranes were blocked with 5% skim milk powder in 0.1% phosphate buffered saline (PBS) at room temperature for 2 hours. Immunoblotting was performed using 2.0 μg/mL mouse anti-rat VEGF, bFGF, Bcl2 or Bax monoclonal antibody (1:200 dilution; Sigma-Aldrich) overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:200 dilution; Sigma) overnight at 4°C. Blots probed for β-actin or GAPDH served as a loading control. Immunoreactive proteins were visualized using enhanced chemiluminescence and a western blotting detection system (Beyotime Biotechnology, Haimen, China). Optical density values were quantified using BIO-1D software (Vilber Lourmat, Eberhardzell, Germany).
Histopathological examination
The remaining rats were intraperitoneally anesthetized with 3.5% chloral hydrate, 35 mg/100 g, and perfused through the aorta with pre-cooled physiological saline, followed by 4% paraformaldehyde in PBS (0.1 M, pH 7.4). The brains were immediately removed and postfixed in 4% paraformaldehyde for 2–4 hours, then dehydrated overnight in graded sucrose solutions (15%, 20% and 30%) until completely submerged. The dehydrated brains were embedded in Tissue-Tek Optimal Cutting Temperature Compound (Sakura Finetek, Tokyo, Japan) under frozen conditions. Coronal slices were cut at 6 μm using a cryostat, and unbiased cell estimation was performed for the hippocampal CA3 region on every sixth section according to a systematic random sampling procedure. Approximately 105–137 consecutive sections were collected from the hippocampus in each rat and subjected to Nissl staining and glial fibrillary acidic protein (GFAP) immunofluorescence labeling.
Nissl staining
For Nissl staining, sections were immersed in 0.01% toluidine blue (Leagene Biotechnology, Beijing, China) for 15–20 minutes at room temperature, dehydrated twice using a graded series of ethanols (70%, 80%, 90% and 100%), permeabilized with xylene, wet-mounted onto glass slides, and immediately mounted using neutral resin. Sections were photographed using a light microscope (Olympus, Tokyo, Japan).
Immunofluorescence staining
To detect GFAP-immunoreactive cells in the hippocampal CA3 of the ischemic hemisphere, brain sections were incubated for 30 minutes in 2.0 N HCl to denature the DNA, and the reaction was neutralized in 0.1 M boric acid for 10 minutes. Thereafter, brain sections were rinsed in PBS containing 0.3% Triton for 30 minutes, preincubated in 10% normal goat serum for 2 hours at room temperature, incubated with a mouse anti-GFAP antibody (1:200; Beyotime Biotechnology) at 4°C overnight, and then incubated with a Cy5-conjugated affinity-purified goat anti-mouse IgG (1:100; Sigma-Aldrich) in a humidified chamber for 1 hour at 37°C. GFAP-immunoreactive cells were counted with a laser scanning confocal microscope (Olympus).
Statistical analyses
All statistical analyses were performed by an observer blinded to the experimental groupings. All data were analyzed using SPSS 19.0 software (IBM, Armonk, NY, USA). The data were expressed as the mean ± SD. One-way analysis of variance followed by Student's t-test was used to assess statistical significance. A value of P < 0.05 was considered statistically significant.
Results
PGE treatment prevented chronic cerebral hypoperfusion- induced learning and memory deficits in the rat model of VD
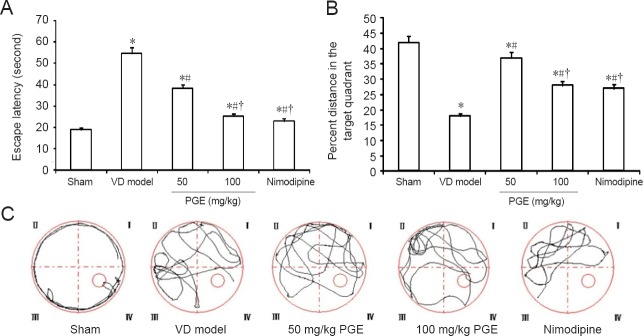
To investigate the effects of PGE treatment on spatial learning in VD rats, the MWM test was performed, and learning ability was assessed by measuring escape latency (Figure 1). Rats in the sham group rapidly learned the location of the escape platform and quickly reached it. In contrast, rats in the VD model group spent a relatively greater amount of time exploring the margin of the pool during the testing period. In comparison, in the 50 and 100 mg/kg PGE groups as well as in the nimodipine group, the escape latency was significantly shortened compared with the VD model group (P < 0.05; Figure 1A). These results show that PGE alleviates the learning and memory impairment in rats with VD in a dose-dependent manner. In probe trials, the percent time in the target quadrant was shorter in the VD model group than in the sham group, but was longer after treatment with PGE. The PGE and nimodipine-treated rats swam a greater distance over the platform compared with rats in the VD model group (P < 0.05). There were no significant differences between the 100 mg/kg PGE and nimodipine groups (P > 0.05) Figure 1B). The swimming paths recorded on the last day are shown in Figure 1C. Additionally, there was no significant difference in swimming speed among the groups (data not shown) (P > 0.05).
Figure 1.
Effect of PGE on performance in the Morris water maze.
(A) Escape latency in the training trials. (B) Percent distance in the target quadrant in the probe trials. (C) Representative pathways on the last day of training trials. Data are shown as the mean ± SD (n = 20; one-way analysis of variance followed by Student's t-test). *P < 0.05, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, vs. 50 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia.
PGE treatment did not affect balance in the rat model of VD
In the balance beam test, rats were placed on the beam to assess their balance and coordination by measuring the time required to traverse the beam. No statistically significant differences in traversal time were found among the five groups (P > 0.05; Figure 2).
Figure 2.
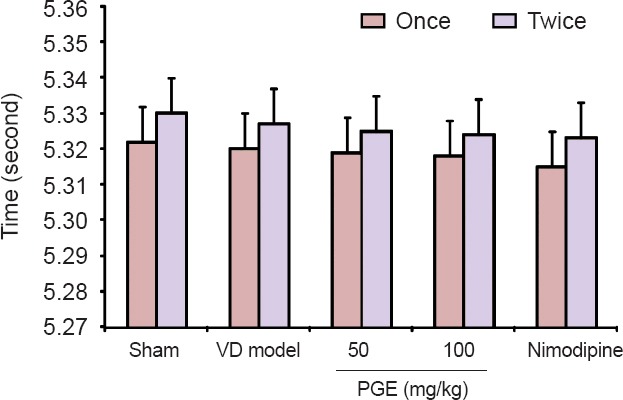
Effect of PGE on balance beam test scores.
No statistically significant differences were found among the five groups. PGE treatment did not alter balance, as indicated by the time taken to traverse the elevated 2.5-cm (diameter) cylindrical beam (P > 0.05). Data are expressed as the mean ± SD (n = 20; one-way analysis of variance followed by Student's t-test). Once: The first test; twice: the second test. PGE: Panax ginseng extract; VD: vascular dementia.
PGE treatment restored cholinergic neuron levels in the hippocampus of rats with VD
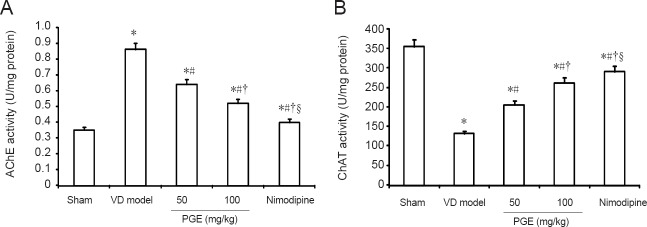
We examined AChE activity levels in the hippocampus to assess cholinergic function (Figure 3A). AChE activity was significantly higher in the VD model group compared with the sham group (P < 0.05). AChE activity was significantly lower in animals treated with PGE or nimodipine, compared with the VD model group. Furthermore, AChE activity was significantly lower in the 100 mg/kg PGE group than in the 50 mg/kg PGE group (P < 0.05). The hippocampal CA3 ChAT activity assay results are shown in Figure 3B. ChAT activity was significantly lower in the VD model group than in the sham group (P < 0.05). In rats administered PGE or nimodipine, ChAT activity was significantly higher compared with the VD model group (P < 0.05). PGE treatment dose-dependently reduced AChE activity and increased ChAT activity. In addition, AChE activity was lower and ChAT activity was significantly higher in the nimodipine group compared with the 100 mg/kg PGE group (P < 0.05).
Figure 3.
Effect of PGE on AChE and ChAT activities in the hippocampus.
Data are presented as the mean ± SD (n = 10; one-way analysis of variance followed by Student's t-test). *P < 0.05, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, vs. 50 mg/kg PGE group; §P < 0.05, vs. 100 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia; AChE: acetylcholinesterase; ChAT: choline acetyltransferase.
PGE treatment increased the expression of VEGF and bFGF in the brain of rats with VD
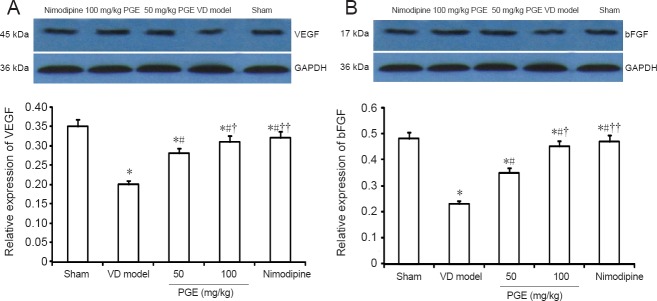
To clarify the mechanisms by which PGE alleviates neural tissue damage and promotes repair, we measured the expression of VEGF and bFGF proteins in the five groups. As shown in Figure 4, VEGF and bFGF protein levels were decreased in the VD model group compared with the sham group, and treatment with PGE (50 or 100 mg/kg) significantly blocked this reduction in expression (P < 0.05). Furthermore, protein levels of VEGF and bFGF were significantly higher in the 100 mg/kg PGE group compared with the 50 mg/kg PGE group (P < 0.01). However, there were no significant differences between the 100 mg/kg PGE and nimodipine groups (P > 0.05).
Figure 4.
Western blot assay for VEGF and bFGF protein expression in the brain.
Expression was calculated as the ratio of the optical density of the target protein to that of GAPDH. Values were presented as the mean ± SD (n = 10; one-way analysis of variance followed by Student's t-test). *P < 0.05, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, ††P < 0.01, vs. 50 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor.
PGE treatment impacted the expression of neuronal apoptosis-related proteins in the hippocampus
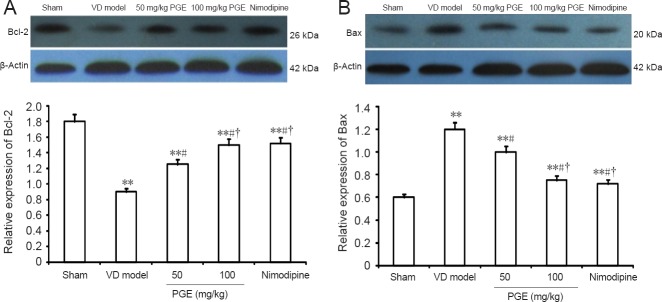
To further clarify the molecular mechanisms underlying the neuroprotective effect of PGE, we focused on two proteins involved in apoptotic death by western blot assay. Bcl-2 expression was significantly decreased in the VD model group compared with the sham group (P < 0.01), and treatment with PGE (50 or 100 mg/kg) significantly suppressed this reduction in expression level (P < 0.05) in a dose-dependent manner. In addition, Bax expression was significantly increased in the VD model group compared with the sham group (P < 0.01), and this was significantly prevented by treatment with PGE (50 or 100 mg/kg; P < 0.05) in a dose-dependent manner. No difference was found in the expression of Bcl-2 or Bax between the nimodipine and 100 mg/kg PGE groups (Figure 5).
Figure 5.
Western blot assay for Bax and Bcl-2 protein expression in the hippocampus.
Expression was calculated as the ratio of the optical density of the target protein to that of β-actin. Values are presented as the mean ± SD (n = 10; one-way analysis of variance followed by Student's t-test). **P < 0.01, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, vs. 50 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia.
PGE treatment attenuated histopathological changes in the hippocampal CA3 region of rats with VD
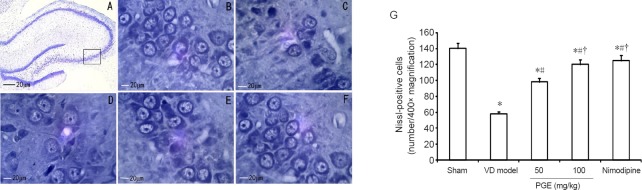
Histological changes in the hippocampal CA3 region were examined by Nissl staining (Figure 6). In the sham group, the neurons exhibited a normal morphology, with distinct round or oval nuclei and nucleoli, and clear Nissl bodies in the cytoplasm, with no signs of interstitial edema (Figure 6A, B). In contrast, in the VD model group, degeneration and necrosis of a great number of neurons, with cell body shrinkage, pyknosis, breaking and dissolution of the nucleus, and interstitial edema were observed (Figure 6C). In comparison, in rats treated with PGE or nimodipine for 8 weeks, the numbers of degenerating and necrotic neurons were substantially reduced (Figure 6D–G).
Figure 6.
Effect of PGE on hippocampal histopathology.
(A) Neurons in the hippocampal CA3 area. The box indicates the analyzed area (Nissl staining, ×100). (A, B) Sham group: The neurons were arranged normally, and Nissl bodies in the cytoplasm were abundant (× 400). (C) VD model group: the number of neurons was substantially reduced, and they were sparsely arranged. Nissl bodies in the cytoplasm were decreased (× 400). (D) 50 mg/kg PGE group (× 400). (E) 100 mg/kg PGE group (× 400). (F) Nimodipine group (× 400). In rats given PGE or nimodipine treatment, the number of neurons was increased, with a greater number of Nissl bodies in the cytoplasm. (G) Quantitation of Nissl-positive cells. Data are shown as mean ± SD (n = 10; one-way analysis of variance followed by Student's t-test). *P < 0.05, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, vs. 50 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia.
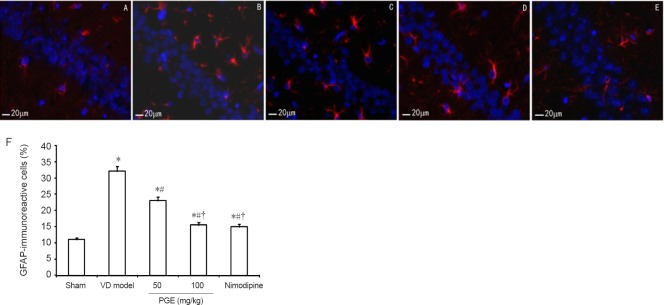
PGE treatment reduced GFAP-immunoreactive cells in the hippocampal CA3 region in the rat model of VD
To provide further insight into the molecular mechanisms underlying the neuroprotective effect of PGE, we examined GFAP-immunoreactive cells by immunolabeling. The expression of GFAP in the rat hippocampal CA3 area is shown and quantified in Figure 7A–E. Compared with the weak constitutive expression in the sham group, GFAP-immunoreactive cells were significantly increased in the VD model group (P < 0.05). Treatment with 50 or 100 mg/kg PGE significantly reduced the number of GFAP-immunoreactive cells compared with the VD model group (P < 0.05). Moreover, compared with the 50 mg/kg PGE group, the number of GFAP-immunoreactive cells was significantly lower in the 100 mg/kg PGE group (P < 0.05). However, there were no significant differences between the 100 mg/kg PGE and nimodipine groups (P > 0.05; Figure 7F).
Figure 7.
Effect of PGE on hippocampal GFAP-immunoreactive cells.
(A–E) GFAP-immunoreactive cells were examined in the hippocampal CA3 region by immunofluorescence labeling. GFAP-positive cells are labeled red, and the labeling was mainly localized to the cytoplasm. Scale bars: 20 μm. (A) Sham group. (B) VD model group. (C) 50 mg/kg PGE group. (D) 100 mg/kg PGE group. (E) Nimodipine group. (F) Quantitation of GFAP-immunoreactive cells. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by Student's t-test). *P < 0.05, vs. sham group; #P < 0.05, vs. VD model group; †P < 0.05, vs. 50 mg/kg PGE group. PGE: Panax ginseng extract; VD: vascular dementia; GFAP: glial fibrillary acidic protein.
Discussion
In the present study, we used a rat model of VD which is widely used to simulate human ischemic injury-induced learning and memory impairment. The ischemic injury produced by MCAO had a strong impact on the brain. Administration of PGE significantly attenuated neurological injury by ameliorating central cholinergic function, improving blood circulation in the brain, delaying neuronal cell death, and by decreasing apoptosis in the hippocampal region.
Cognitive dysfunction as a result of Alzheimer's disease or VD is a major contributor to morbidity in Western society (Brookmeyer et al., 2007). Although the underlying etiology of dementia is likely multifactorial, dysfunction of the cerebrovasculature is undoubtedly a contributing factor, especially in VD (Du et al., 2017). To date, pharmacological and immunologic interventions have met with limited success in reducing mild cognitive impairments and dementia in patient populations (Venkat et al., 2015). Patients with VD generally experience a decline in cognitive function due to ischemic, ischemic-hypoxic (Blair et al., 2017) or hemorrhagic brain lesions caused by cerebrovascular disease and cardiovascular pathologic changes (Gorelick et al., 2011). The hippocampus is one of the most important brain regions associated with learning and memory. In recent years, investigators have found that the hippocampus, especially the hippocampal CA3 area, is particularly susceptible to ischemic insult (Jung et al., 2012). Functional and morphological perturbations in the hippocampus, including changes in neurons, astrocytes and synapses, are among the most important factors contributing to cognitive dysfunction. Neuronal death in the hippocampus is a major contributor to memory decline in the elderly (Burke et al., 2014). Astrocytes also perform critical functions in the brain, such as promoting neovascularization, regulating neuronal activity, and supporting synaptogenesis and neurogenesis (Kim et al., 2017), which may influence recovery from ischemic injury. Changes in astrocytes following ischemia can result from direct cellular injury or in response to injury in other central nervous system structures (Becerra-Calixto and Cardona-Gómez, 2017).
It is becoming increasingly clear that traditional Chinese herbs can play an important role in alleviating symptoms and dementia (Lin et al., 2017). In traditional oriental medicine, many herbal drugs and prescriptions have been used clinically for the treatment of stroke (Mei et al., 2017), Alzheimer's disease and VD (Lin et al., 2017). Chinese medicine incorporates centuries of experience in dealing with dementia. Ginseng is a slow-growing perennial plant that belongs to the Araliaceae family and Panax genus. PGE is used frequently as a crude substance that is taken orally as a traditional medicine in Asian countries (Lin et al., 2017). PGE contains various ginseng saponin fractions, including ginsenoside (G)-Rb1, G-Rb2, G-Rc, G-Rd, G-Re, G-Rf, G-Rg1, G-Rg2 and G-Rg3 (Ban et al., 2012). These components might act synergistically with each other to enhance the activity of or counteract the toxic effect of other factors (Yun et al., 2001; Goudarzvand et al., 2016), resulting in cognitive and behavioral improvement.
The MWM test is a hippocampus-dependent memory task that is commonly used in the evaluation of cognitive status in rodents. The training trials are used to assess spatial or place learning, and the probe trials evaluate whether the animal remembers the position of the platform (Vorhees and Williams, 2006). Rodents with VD exhibit significant learning and memory deficits during the MWM test, with a longer escape latency (Huang et al., 2017). In this experiment, we found that the sham group rapidly learned the location of the platform and quickly reached the escape platform. In contrast, the VD model group exhibited a swimming behavior in which rats wasted time exploring the margin of the pool during the testing period. In comparison, the escape latency was significantly shorter in the PGE treatment groups and in the nimodipine group. There were no significant differences in body weight or swimming ability among the different experimental groups. Our findings indicate that PGE improves learning and memory abilities in rats with VD in a dose-dependent manner.
A study on the pathogenetic mechanisms of VD showed that, similar to AD, cholinergic abnormalities are associated with a disturbance in cognitive function in VD patients (Naddafi et al., 2013). Cholinergic neurons that project into the hippocampus play a critical role in learning and memory functions, and the cholinergic terminals in the presynaptic membrane are sensitive to ischemic insults (Jia et al., 2004). In this study, we examined the brain tissue levels of two critical central cholinergic markers, ChAT and AChE. PGE treatment increased ChAT activity while decreasing AChE levels. This suggests that drugs that target the cholinergic system might have therapeutic efficacy in VD patients. Inhibition of brain AChE increases synaptic concentrations of acetylcholine, which might alleviate cognitive dysfunction and neuropathology in patients with cerebral ischemic dementia.
Whether in the normal or ischemic brain, neurogenesis and angiogenesis usually accompany each other, and both are important for functional recovery (Beck and Plate, 2009). In recent years, it has become clear that strategies that enhance angiogenesis and improve cerebral blood circulation are critical for the treatment of VD (Tarkowski et al., 2002). VEGF is a key angiogenic factor in the ischemic brain (Tarkowski et al., 2002), and it also has neurotrophic and neuroprotective effects that promote recovery (Dzietko et al., 2013). bFGF induces cell proliferation and neovascularization in an autocrine and/or paracrine fashion (Sun et al., 2009). Previous studies have demonstrated that bFGF promotes axonal branch formation (Shi et al., 2002) and stimulates the proliferation and differentiation of neural precursors (Zhang et al., 2014). Therefore, bFGF has great potential for the treatment of central nervous system disorders. Our results show that VEGF and bFGF levels decreased dramatically after ischemic brain injury, and that PGE treatment significantly diminished this reduction, helping to maintain their levels.
The Nissl body is a structure unique to neurons, and the density of Nissl staining in the neuronal cytoplasm is used to evaluate neuronal damage (Kadar et al., 2009). Cognitive deficits are associated with damage to the hippocampal CA1 (Sugawara et al., 2002). Permanent occlusion of the bilateral common carotid arteries in rats results in significant pyramidal neuron loss in the hippocampal CA1 (Li et al., 2014). In addition, chronic cerebral hypoperfusion triggers reactive astrocytosis with detectable morphological changes and accumulation of GFAP in these glia (Lana et al., 2017). GFAP, one of the most highly synthesized proteins in the brain, is widely used to study the activation state of astrocytes and might play a role in the ischemic process (Panickar and Norenberg, 2005). In the current study, we found that PGE treatment significantly increased pyramidal neuron numbers and reduced astrocyte activation and proliferation in the hippocampal CA3. These changes were associated with improved cognitive performance in our rat model of VD. However, the mechanisms underlying the various neuroprotective effects of PGE require further investigation.
It is now well known that apoptosis during VD plays a major role in brain injury associated with dementia (Liu et al., 2017). Bcl-2 and Bax are the two primary proteins regulating apoptotic cell death, with opposing functions (Min et al., 2014). Bcl-2 is functionally an apoptosis suppressing factor, whereas Bax is an apoptosis promoting factor (Hwang et al., 2013). Bcl-2 inhibits cytochrome c release from mitochondria elicited by the pro-apoptotic molecule Bax, resulting in the inhibition of caspase activation and apoptotic death (Zhao et al., 2017). VD is a multifactorial disease, and the mechanisms of neuronal apoptosis in the hippocampus are important for an understanding of its pathogenesis. Therefore, we also examined the effects of PGE on the expression of Bcl-2 and Bax in the hippocampal CA3 region. VD significantly decreased Bcl-2 expression and increased Bax protein expression. PGE up-regulated Bcl-2 and down-regulated Bax, suggesting that the herbal medicine inhibits apoptosis, and therefore might have therapeutic potential for ischemic brain injury.
In summary, PGE possesses a potent neuroprotective activity against brain damage in VD. The ability of PGE to alleviate learning and memory deficits suggests a multifactorial mechanism that likely involves modulating neurotransmitter levels, protecting cerebral vessels from damage, promoting angiogenesis, and inhibiting apoptosis. These processes ultimately stimulate repair mechanisms that alleviate brain damage. Therefore, PGE may be a novel promising alternative medicine for the treatment of VD. However, further studies are needed to clarify the mechanisms underlying its neuroprotective effects.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81660243; the Joint Foundation of Department of Science and Technology of Guizhou Province of China, No. LG [2012] 028; the Science and Technology Department of Guizhou Province of China, No. qian SY[2015]3041.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81660243; the Joint Foundation of Department of Science and Technology of Guizhou Province of China, No. LG (2012) 028; the Science and Technology Department of Guizhou Province of China, No. qian SY(2015)3041. Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics: The study protocol was approved by the Experimental Animal Research Committee of Guizhou Medical University of China. The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Nahid Aboutaleb, Iran University of Medical Sciences, Iran.
(Copyedited by Patel B, Frenchman B, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Ban JY, Kang SW, Lee JS, Chung JH, Ko YG, Choi HS. Korean red ginseng protects against neuronal damage induced by transient focal ischemia in rats. Exp Ther Med. 2012;3:693–698. doi: 10.3892/etm.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol. 2012;47:887–891. doi: 10.1016/j.exger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Becerra-Calixto A, Cardona-Gómez GP. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci. 2017;10:88. doi: 10.3389/fnmol.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- Blair GW, Appleton JP, Law ZK, Doubal F, Flaherty K, Dooley R, Shuler K, Richardson C, Hamilton I, Shi Y, Stringer M, Boyd J, Thrippleton MJ, Sprigg N, Bath PM, Wardlaw JM. Preventing cognitive decline and dementia from cerebral small vessel disease: The LACI-1 Trial. Protocol and statistical analysis plan of a phase IIa dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int J Stroke. 2017 doi: 10.1177/1747493017731947. doi: 10.1177/1747493017731947. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Burke MJ, Nelson L, Slade JY, Oakley AE, Khundakar AA, Kalaria RN. Morphometry of the hippocampal microvasculature in post-stroke and age-related dementias. Neuropathol Appl Neurobiol. 2014;40:284–295. doi: 10.1111/nan.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ. Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology. 2014;79:172–179. doi: 10.1016/j.neuropharm.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SQ, Wang XR, Xiao LY, Tu JF, Zhu W, He T, Liu CZ. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017;54:3670–3682. doi: 10.1007/s12035-016-9915-1. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumura M, Sato N, Kusaba N, Takagaki K, Nakayama J. Oral administration of French maritime pine bark extract (Flavangenol((R))) improves clinical symptoms in photoaged facial skin. Clin Interv Aging. 2012;7:275–286. doi: 10.2147/CIA.S33165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhou Y, Jiang Z, Zhao Y, Zhang D, Cong X, Cao R, Li H, Tian W. Cytotoxic and chemosensitization effects of Scutellarin from traditional Chinese herb Scutellaria altissima L. in human prostate cancer cells. Oncol Rep. 2017;38:1491–1499. doi: 10.3892/or.2017.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzvand M, Afraei S, Yaslianifard S, Ghiasy S, Sadri G, Kalvandi M, Alinia T, Mohebbi A, Yazdani R, Azarian SK, Mirshafiey A, Azizi G. Hydroxycitric acid ameliorates inflammation and oxidative stress in mouse models of multiple sclerosis. 2016;11:1610–1616. doi: 10.4103/1673-5374.193240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Su Z, Wang Z, Luo X, Lai R. The effect of chinese herbal medicine Banxia Baizhu Tianma Decoction for the treatment of vertebrobasilar insufficiency vertigo: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2017;31:27–38. doi: 10.1016/j.ctim.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Gupta S, Singh P, Sharma B. Neuroprotective effects of nicorandil in chronic cerebral hypoperfusion-induced vascular dementia. J Stroke Cerebrovasc Dis. 2016;25:2717–2728. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu FF, He X, Li JP, Yan XX, Pan AH, Li ZY. Effects of enriched environment on immature neurons in piriform cortex of a rat model of vascular dementia. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4006–4012. [Google Scholar]
- Huang W, Li Z, Zhao L, Zhao W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer's disease via modulating the expression of miR-106b. Biomed Pharmacother. 2017;92:46–57. doi: 10.1016/j.biopha.2017.05.060. [DOI] [PubMed] [Google Scholar]
- Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, Kim SH, Jin JJ, Chung JY, Yi JW. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–1056. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- Jia JP, Jia JM, Zhou WD, Xu M, Chu CB, Yan X, Sun YX. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer's disease and vascular dementia. Chin Med J (Engl) 2004;117:1161–1164. [PubMed] [Google Scholar]
- Jung YJ, Suh EC, Lee KE. Oxygen/glucose deprivation and reperfusion cause modifications of postsynaptic morphology and activity in the ca3 area of organotypic hippocampal slice cultures. Korean J Physiol Pharmacol. 2012;16:423–429. doi: 10.4196/kjpp.2012.16.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissl staining. J Neurosci Methods. 2009;184:115–118. doi: 10.1016/j.jneumeth.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ko PW, Lee HW, Jeong JY, Lee MG, Kim JH, Lee WH, Yu R, Oh WJ, Suk K. Astrocyte-derived lipocalin-2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia. 2017;65:1471–1490. doi: 10.1002/glia.23174. [DOI] [PubMed] [Google Scholar]
- Lana D, Ugolini F, Melani A, Nosi D, Pedata F, Giovannini MG. The neuron-astrocyte-microglia triad in CA3 after chronic cerebral hypoperfusion in the rat: Protective effect of dipyridamole. Exp Gerontol. 2017;96:46–62. doi: 10.1016/j.exger.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Li N, Wang P, Ma XL, Wang J, Zhao LJ, Du L, Wang LY, Wang XR, Liu KD. Effect of bone marrow stromal cell transplantation on neurologic function and expression of VEGF in rats with focal cerebral ischemia. Mol Med Rep. 2014;10:2299–2305. doi: 10.3892/mmr.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z. Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol. 2015;8:14099–14109. [PMC free article] [PubMed] [Google Scholar]
- Lin SK, Lin PH, Hsu RJ, Chuang HC, Liu JM. Traditional Chinese medicine therapy reduces the catheter indwelling risk in dementia patients with difficult voiding symptoms. J Ethnopharmacol. 2017;203:120–126. doi: 10.1016/j.jep.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu M, Lu P, Wang H, Qi Q, Xu J, Xiao Y, Fan M, Jia Y, Zhang D. Cerebrolysin alleviates cognitive deficits induced by chronic cerebral hypoperfusion by increasing the levels of plasticity-related proteins and decreasing the levels of apoptosis-related proteins in the rat hippocampus. Neurosci Lett. 2017;651:72–78. doi: 10.1016/j.neulet.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Lv YL, Wu ZZ, Chen LX, Wu BX, Chen LL, Qin GC, Gui B, Zhou JY. Neuroprotective effects of tetrandrine against vascular dementia. Neural Regen Res. 2016;11:454–459. doi: 10.4103/1673-5374.179058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma GD, Chiu CH, Hsu YJ, Hou CW, Chen YM, Huang CC. Changbai Mountain Ginseng (Panax ginseng C. A.Mey) Extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules. 2017;22:E237. doi: 10.3390/molecules22020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Bo SH, Lu XT, Xu AJ, Zhang J. Protective effects of carnosine on white matter damage induced by chronic cerebral hypoperfusion. Neural Regen Res. 2016;11:1438–1444. doi: 10.4103/1673-5374.191217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei ZG, Tan LJ, Wang JF, Li XL, Huang WF, Zhou HJ. Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen Res. 2017;12:425–432. doi: 10.4103/1673-5374.202946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JJ, Huo XL, Xiang LY, Qin YQ, Chai KQ, Wu B, Jin L, Wang XT. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep. 2014;4:5555. doi: 10.1038/srep05555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddafi F, Reza Haidari M, Azizi G, Sedaghat R, Mirshafiey A. Novel therapeutic approach by nicotine in experimental model of multiple sclerosis. Innov Clin Neurosci. 2013;10:20–25. [PMC free article] [PubMed] [Google Scholar]
- Nishino H, Czurkó A, Onizuka K, Fukuda A, Hida H, Ungsuparkorn C, Kunimatsu M, Sasaki M, Karadi Z, Lénárd L. Neuronal damage following transient cerebral ischemia and its restoration by neural transplant. Neurobiology (Bp) 1994;2:223–234. [PubMed] [Google Scholar]
- Ohkita M, Kiso Y, Matsumura Y. Pharmacology in health foods: improvement of vascular endothelial function by French maritime pine bark extract (Flavangenol) J Pharmacol Sci. 2011;115:461–465. doi: 10.1254/jphs.10r37fm. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- Shi J, Perry G, Berridge MS, Aliev G, Siedlak SL, Smith MA, LaManna JC, Friedland RP. Labeling of cerebral amyloid beta deposits in vivo using intranasal basic fibroblast growth factor and serum amyloid P component in mice. J Nucl Med. 2002;43:1044–1051. [PubMed] [Google Scholar]
- Shin K, Guo H, Cha Y, Ban YH, Seo da W, Choi Y, Kim TS, Lee SP, Kim JC, Choi EK, Yon JM, Kim YB. Cereboost, an American ginseng extract, improves cognitive function via up-regulation of choline acetyltransferase expression and neuroprotection. Regul Toxicol Pharmacol. 2016;78:53–58. doi: 10.1016/j.yrtph.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YH, Ge LJ, Zhao JY, He WY, Li BP. Changes of cholinergic neurons in the hippocampus of vascular dementia rats after neural stem cell transplantation. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:8126–8131. [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Vállez Garcia D, Casteels C, Schwarz AJ, Dierckx RAJO, Koole M, Doorduin J. A standardized method for the construction of tracer specific PET and SPECT rat brain templates: validation and implementation of a toolbox. PLoS One. 2015;10:e0122363. doi: 10.1371/journal.pone.0122363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu X, Yang X, Li Y, Yao Y, Lui EM, Ren G. Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius) Int J Biol Macromol. 2015;74:12–17. doi: 10.1016/j.ijbiomac.2014.10.062. [DOI] [PubMed] [Google Scholar]
- Weitzner DS, Engler-Chiurazzi EB, Kotilinek LA, Ashe KH, Reed MN. Morris Water Maze test: optimization for mouse strain and testing environment. J Vis Exp. 2015:e52706. doi: 10.3791/52706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun YP, Do JH, Ko SR, Ryu SY, Kim JH, Song HC, Park YD, Ahn KS, Kim SH. Effects of Korean red ginseng and its mixed prescription on the high molecular weight dextran-induced blood stasis in rats and human platelet aggregation. J Ethnopharmacol. 2001;77:259–264. doi: 10.1016/s0378-8741(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chen J, Feng C, Shao X, Liu Q, Zhang Q, Pang Z, Jiang X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer's disease. Int J Pharm. 2014;461:192–202. doi: 10.1016/j.ijpharm.2013.11.049. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu A, Zhou Y, San X, Jin T, Jin Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol. 2008;115:441–448. doi: 10.1016/j.jep.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zheng SL, Dong FR, Wang ZM. Nimodipine improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. J Int Med Res. 2012;40:1036–1045. doi: 10.1177/147323001204000322. [DOI] [PubMed] [Google Scholar]
- Zhao L, Gu Q, Xiang L, Dong X, Li H, Ni J, Wan L, Cai G, Chen G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther Clin Risk Manag. 2017;13:1099–1105. doi: 10.2147/TCRM.S141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Yu G, Chi L, Zhu J, Zhang W, Zhang Y, Zhang L. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136–145. doi: 10.1016/j.neuro.2013.07.007. [DOI] [PubMed] [Google Scholar]