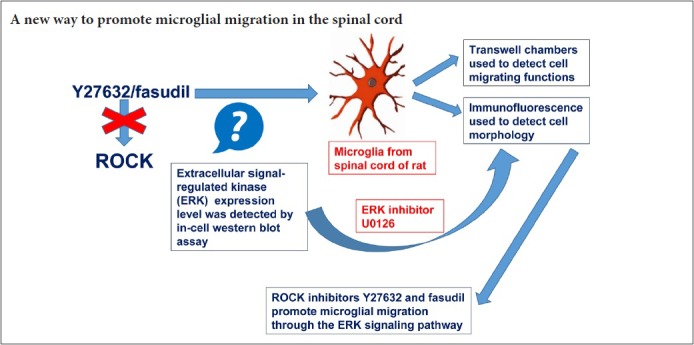
Keywords: nerve regeneration, spinal cord injury, microglia, ROCK, Y27632, fasudil, migration, morphology, ERK, U0126, in-cell western blot assay, Transwell chambers, neural regeneration
Abstract
Rho-associated kinase (ROCK) is a key regulatory protein involved in inflammatory secretion in microglia in the central nervous system. Our previous studies showed that ROCK inhibition enhances phagocytic activity in microglia through the extracellular signal-regulated kinase (ERK) signaling pathway, but its effect on microglial migration was unknown. Therefore, in this study, we investigated the effects of the ROCK inhibitors Y27632 and fasudil on the migratory activity of primary cultured microglia isolated from the spinal cord, and we examined the underlying mechanisms. The microglia were treated with Y27632, fasudil and/or the ERK inhibitor U0126. Cellular morphology was observed by immunofluorescence. Transwell chambers were used to assess cell migration. ERK levels were measured by in-cell western blot assay. Y27632 and fasudil increased microglial migration, and the microglia were irregularly shaped and had many small processes. These inhibitors also upregulated the levels of phosphorylated ERK protein. The ERK inhibitor U0126 suppressed these effects of Y27632 and fasudil. These findings suggest that the ROCK inhibitors Y27632 and fasudil promote microglial migration in the spinal cord through the ERK signaling pathway.
Introduction
Microglia are the main immune cells in the central nervous system and have functional properties similar to peripheral macrophages (Lawson et al., 1990; Streit et al., 2005). Microglia can respond within a few minutes and converge at the site of injury. The pro-inflammatory cytokines released by these cells promote the influx of polymorphonuclear cells and macrophages from the blood (Davalos et al., 2005). Microglia have a dual function in spinal cord injury (SCI); while they secrete inflammatory factors that worsen tissue damage, they also mediate phagocytosis of tissue debris and pathogens (Gitik et al., 2010). Rapid and efficient phagocytosis can reduce neuronal damage, help tissue remodeling and regulate the immune response (Tosello-Trampont et al., 2003). The mechanical forces generated by stress fibers initiate the movement of microglia (Clark et al., 2007; Pellegrin and Mellor, 2007), allowing them to migrate to the site of injury. The small GTPase Rho and its downstream effector Rho-associated kinase (ROCK) regulate the assembly/disassembly and function of stress fibers. There are two isoforms of ROCK: ROCK1 and ROCK2 (Matsui et al., 1996). Activated ROCK phosphorylates the regulatory myosin light chain and the myosin-binding subunit of the myosin light chain phosphatase to inhibit its activity. Numerous actin-myosin-related processes are regulated by ROCK, including adhesion, cell motility, phagocytosis, differentiation, neurite retraction and proliferation (Riento and Ridley, 2003; Yu et al., 2012; Kanno et al., 2015; Kim et al., 2015). Migration is regulated by the cytoskeleton through myosin light chain kinase. Accumulating evidence suggests that ROCK plays a major role in the migration of myoblasts, glioblastoma cells and melanoma cells (Mertsch et al., 2013; Goetsch et al., 2014; Wilhelm et al., 2014; Wallert et al., 2015). However, the role of ROCK in microglial migration and the underlying mechanisms are unclear.
ROCK inhibition is reported to promote the proliferation and cell cycle progression of primary astrocytes (Yu et al., 2012). Here, we cultured primary microglia from the spinal cord and investigated the effect of ROCK inhibition on the migration of these cells. ROCK inhibition-mediated microglial migration might be particularly important in SCI. Y27632, a ROCK-specific inhibitor, and fasudil, a nonspecific ROCK inhibitor, have been widely used to study the function of ROCK signaling (Miyata et al., 2000; Olson, 2008). Using Y27632 and fasudil, we investigate the effect of inhibiting the ROCK signaling pathway on the migration and morphology of cultured microglia, and we examine the underlying mechanisms.
Materials and Methods
Culture of primary microglia
Newborn Sprague-Dawley pups at postnatal day 1–2, from the Experimental Animal Center of Hubei Province of China (SCXK (E) 2015-0018), were used in this study. Ethical approval was obtained from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China (ethical approval: (2014) IACUC Number: 363).
Primary microglial cultures were prepared according to a modification of a previously published method (Gingras et al., 2007). Briefly, spinal cords were removed from pups on postnatal day 1–2 and rinsed with ice-cold Hank's balanced salt solution. After removing the meninges, the spinal cord was cut into pieces and incubated in 0.125% trypsin for 15 minutes. The trypsin solution was aspirated carefully and Dulbecco's modified Eagle's medium (DMEM)/F12 containing 10% fetal bovine serum (FBS) was added to terminate digestion. Spinal cord cells were dissociated by gentle trituration with a 1-mL pipette until the cell suspension was homogenous and no big chunks of tissue remained. After centrifugation at 200 ×g for 8 minutes at 4°C, the supernatant was removed. The cell pellet was resuspended in DMEM/F12 containing 10% FBS and placed in culture flasks precoated with poly-D-lysine at 1 × 106/mL density. Cultures were incubated at 37°C in a 5% CO2 atmosphere. After 2 days, the medium was refreshed with high-glucose DMEM containing 20% FBS. Ten days later, without medium change, astrocytes formed a congested monolayer on the surface of the flask, whereas microglia grew on the surface of the astrocytes. To isolate microglia, the culture flasks were sealed tightly and shaken on a horizontal orbital shaker at 200 r/min and 37°C for 1.5 hours. The cell suspension was collected and transferred to new flasks. The flasks were incubated at 37°C for 20 minutes to facilitate the adherence of the microglia. Twenty minutes after the microglia attached to the flasks, the medium was removed carefully and replaced with high-glucose DMEM containing 10% FBS for further purification.
In the central nervous system, each type of microglia expresses CD11b and IBA1 (ionized calcium-binding adaptor molecule 1). These two proteins are specific markers for microglia in the central nervous system. After labeling with anti-CD11b and 4′,6-diamidino-2-phenylindole (DAPI; Jackson ImmunoResearch Laboratories Inc.), cultures more than 95% pure were used for experiments. ROCK1 and ROCK2 are expressed in heart and vascular smooth muscle. ROCK1 is more abundant in testes, liver and kidney, while ROCK2 is more highly expressed in skeletal muscle and brain (Lawson et al., 1990). In our preliminary study, we found that ROCK1 was not expressed in spinal cord microglia. Therefore, in the present study, only ROCK2 expression was assessed, by immunostaining with rabbit anti-ROCK2 antibody (1:300; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
A specific inhibitor of extracellular signal-regulated kinase (ERK), U0126, was used, and microglia were divided into six groups: control, Y27632-treated, fasudil-treated, U0126-treated, U0126 + Y27632-treated and U0126 + fasudil-treated groups (n = 4 per group).
Lactate dehydrogenase release and ROCK assays
Lactate dehydrogenase release assay was used to assess toxicity caused by treatment with 10 μM Y27632 (Sigma Chemical, St. Louis, MO, USA) (John et al., 2004; Racchetti et al., 2012) and 41 μM fasudil (Sigma Chemical) (Ding et al., 2010). The CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI, USA) was used to measure lactate dehydrogenase release according to the manufacturer's instructions. The ROCK Activity Assay kit (Millipore/Chemicon, Temecula, CA, USA) was used to assess ROCK activity. Briefly, after shaking, the primary microglia were resuspended in high-glucose DMEM containing 10% FBS, adjusted to a concentration of 1 × 106 cells/mL, plated in 6-well cell culture plates in a volume of 2 mL/well, and incubated for 12 hours. The cells were randomly divided into three groups: control (phosphate-buffered saline [PBS]), Y27632 (10 μM) and fasudil (41 μM) groups. The culture medium was collected at 1, 3 and 6 hours for lactate dehydrogenase assay. The cells were collected for ROCK activity assay. The cells were extracted in radioimmunoprecipitation assay buffer containing 1 mM phenylmethyl sulfonylfluoride. The lysates were centrifuged at 12,000 × g at 4°C for 15 minutes, and the protein concentration in the supernatants was determined using the Bicinchoninic Acid Protein Assay Kit (Pierce, Cheshire, UK) with bovine serum albumin as the standard. The same amount of protein for each group was used for the ROCK activity assay, which was carried out according to the manufacturer's instructions. The ROCK activity in the Y27632 and fasudil groups was normalized to the corresponding control (taken as 100%). Cells were plated in triplicate wells for each experiment, and four independent experiments were performed.
Immunocytochemical staining
Cells were incubated in high-glucose DMEM containing 10% FBS with or without 10 μM Y27632 and 41 μM fasudil, or preincubated in 10 μM U0126 (Promega) for 30 minutes before Y27632 and fasudil treatment for 1 hour. Cells were then fixed with 100% methanol for 15 minutes. After three rinses in PBS, the cells were permeabilized in 1% Triton X-100 in PBS for 15 minutes. Subsequently, the cells were incubated with 5% bovine serum albumin for blocking nonspecific sites for 2 hours. Cells were incubated overnight with rabbit anti-IBA1 polyclonal antibody (1:300; Wako, Richmond, VA, USA), mouse anti-CD11b monoclonal antibody (1:100; BD Biosciences, San Jose, CA, USA), rabbit anti-ROCK2 polyclonal antibody (1:300; Santa Cruz Biotechnology) or negative control (PBS) at 4°C. On the second day, cells were washed with PBS and then incubated with CY3-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 1 hour at 37°C. The cells were thereafter washed in PBS and incubated with DAPI (10 μg/mL) for 10 minutes. The morphology of microglia was examined by fluorescence microscopy (DP50; Olympus, Tokyo, Japan).
Migration ability determined by the Transwell migration assay
Cell migration ability was evaluated using Transwell chambers (No. 3422, 8 μm pore size, Corning Costar, Cambridge, MA, USA) (Koro et al., 2011; Liu et al., 2012). Briefly, cells were suspended in culture medium, adjusted to a concentration of 1 × 106 cells/mL, plated (100 μL/well) in the upper compartment of the Transwell chamber, and incubated for 6 hours. Microglia were randomly divided into three groups: control, Y27632 and fasudil groups. The lower and upper compartments contained the same medium. After 6 hours, the transwell chambers were fixed with 100% methanol for 15 minutes, washed in PBS, and incubated with Coomassie brilliant blue at room temperature for 20 minutes. The cells in the upper compartment of the transwell chamber were carefully removed using cotton swabs, and the number of microglia in the lower compartment was assessed with an inverted microscope (Olympus). Four fields were selected for statistical analysis by another experimenter. Triplicate plates were used for each experiment, and four independent experiments were conducted.
In-cell western blot assay
Given the limited number of microglia in primary culture available for protein extraction, the in-cell western blot assay was performed as previously described (Egorina et al., 2006). Briefly, cells were seeded in 96-well plates (Black wall, Corning 3631) at 5 × 105 cells/well and cultured for 12 hours. Cell were washed twice in PBS, fixed with 4% paraformaldehyde for 20 minutes, incubated with 0.1% Triton X-100 for 20 minutes, and then washed twice in PBS. Blocking was carried out with 5% bovine serum albumin for 1 hour at room temperature. The cells in the wells were incubated overnight at 4°C with the negative control or a mixture of anti-phospho-ERK1/2 polyclonal antibody (1:500; Cell Signaling Technology, Beverly, MA, USA) and anti-β-actin monoclonal antibody (1:1,500; Santa Cruz Biotechnology), and then with secondary antibodies (Alexa Fluor 800-conjugated goat anti-mouse IgG and/or IRDye 800-conjugated goat anti-rabbit IgG, 1:5,000; Li-COR Biosciences Inc., Lincoln, NE, USA) at room temperature for 1 hour. The immunoreactivity of cells was quantitatively analyzed using an Odyssey IR imaging system (Li-COR Biosciences Inc.) and expressed as the mean optical density. The amount of each protein was normalized to that of β-actin as follows: optical density of target protein/optical density of the corresponding β-actin.
Statistical analysis
All data are expressed as the mean ± SEM. SPSS 19.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. The paired t-test was used to compare differences between two groups, and one-way analysis of variance with Tukey's post hoc test was used to compare differences between more than two groups. P < 0.05 was considered statistically significant.
Results
Y27632 and fasudil reduced ROCK activity in microglia
The purity of microglial cultures and ROCK2 expression were determined by immunocytochemical staining. Double labeling with ROCK2 and anti-CD11b antibodies showed that over 95% of the cells were CD11b-positive microglia, and almost all cells were positive for ROCK2 (Figure 1A).
Figure 1.
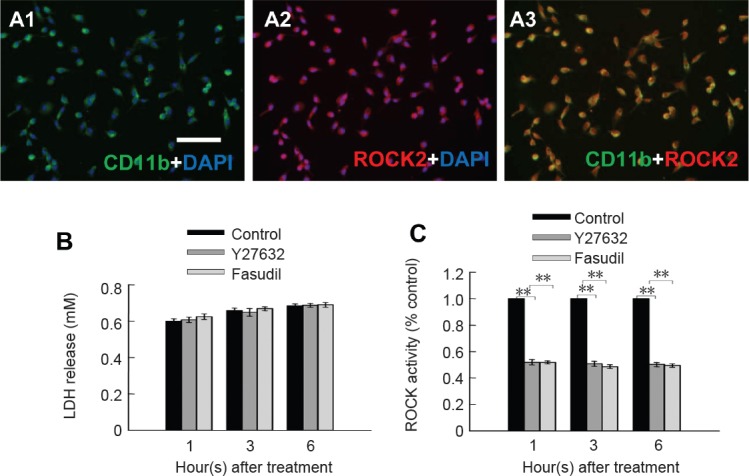
Microglia expressed ROCK2, and the ROCK inhibitors were not cytotoxic to cultured spinal cord microglia at the concentrations used.
(A1) CD11b + DAPI double staining; (A2) ROCK2 + DAPI double staining; (A3) CD11b + ROCK2 double staining. Scale bar: 25 μm. (B) Mean LDH release after 1, 3 and 6 hours of treatment with Y27632 (10 μM), fasudil (41 μM) or control (phosphate-buffered saline). (C) ROCK activity in microglia after Y27632 (10 μM) and fasudil (41 μM) treatment. Data are shown as the mean ± SEM (n = 4; one-way analysis of variance followed by Tukey's post hoc test). **P < 0.01. ROCK: Rho-associated kinase; LDH: lactate dehydrogenase; DAPI: 4′,6-diamidino-2-phenylindole.
The lactate dehydrogenase release assay was used to assess cytotoxicity after treatment with Y27632 and fasudil. There was no significant difference between cells treated with Y27632, cells treated with fasudil and control cells (P > 0.05; Figure 1B). Hence, Y27632 and fasudil were not cytotoxic to primary microglia. To evaluate the inhibitory effect of Y27632 and fasudil on ROCK activity, we used the ROCK Activity Assay Kit to measure ROCK activity in microglia. After treatment with Y27632 or fasudil for 1, 3 and 6 hours, we observed significant reductions in ROCK activity in microglia (Figure 1C; P < 0.01).
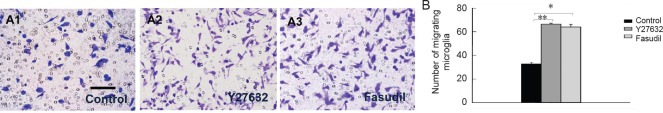
Y27632 and fasudil promoted microglial migration
We used the Transwell chamber assay to investigate the effects of Y27632 and fasudil on microglial migration. Treatment with Y27632 (Figure 2A2) or fasudil (Figure 2A3) resulted in a higher number of migrating cells compared with the control (Figure 2A1). These findings show that ROCK inhibition by Y27632 and fasudil results in increased microglial migration (Figure 2B; P < 0.01 or P < 0.05).
Figure 2.
Y27632 and fasudil promoted migration of cultured spinal cord microglia.
(A) Transwell chamber assay using control (A1, phosphate-buffered saline), Y27632 (A2, 10 μM) and fasudil (A3, 41 μM)-treated microglia at 6 hours (Coomassie brilliant blue staining). Scale bar: 25 μm. (B) Y27632 and fasudil treatments increase the number of migrating microglia at 6 hours. Data are shown as the mean ± SEM (n = 4; one-way analysis of variance followed by Tukey's post hoc test). *P < 0.05, **P < 0.01.
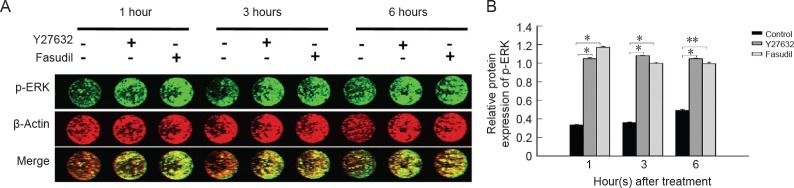
The ERK signaling pathway played a role in mediating the effects of Y27632 and fasudil on microglia
Our previous study showed that Y27632 and fasudil significantly increased levels of phosphorylated (p)-ERK in BV2 microglial cells, without significantly impacting the levels of p-JNK or p-p38 (Fu et al., 2016a). In this study, in-cell western blot assay was used to determine whether Y27632 and fasudil modulate the levels of p-ERK. After treatment with Y27632 or fasudil for 1, 3 and 6 hours, p-ERK levels were significantly upregulated compared with the control (Figure 3A, B; P < 0.01 or P < 0.05).
Figure 3.
Effects of Y27632 and fasudil on p-ERK protein expression levels in cultured spinal cord microglia.
(A) In-cell western blot assay of the changes in p-ERK protein expression levels in response to Y27632 (10 μM) and fasudil (41 μM) treatments at 1, 3 and 6 hours. (B) Statistical analysis of p-ERK protein levels. Data are shown as the mean ± SEM (n = 4; one-way analysis of variance followed by Tukey's post hoc test). *P < 0.05, **P < 0.01. p-ERK: Phosphorylated extracellular signal-regulated kinase.
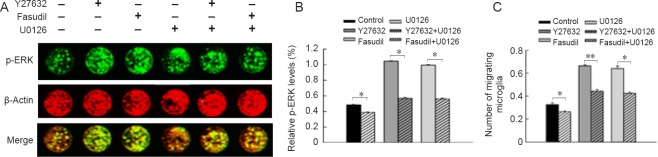
The ERK signaling pathway was involved in Y27632- and fasudil-induced microglial migration
ERK is downstream of MEK. To determine whether the MEK1/2 inhibitor U0126 affects the levels of p-ERK, in-cell western blot assay was performed. Microglia were preincubated with U0126 (Caron and Hall, 1998) at 10 μM for 30 minutes before Y27632 or fasudil treatment. p-ERK was significantly downregulated at 6 hours for each pairwise comparison (U0126 group compared with control group; Y27632 + U0126 group versus Y27632 group; fasudil + U0126 group versus fasudil group) (Figure 4A, B; P < 0.05). The number of migrating cells (Figure 4C; P < 0.05) varied in a manner consistent with the p-ERK level in each group.
Figure 4.
Involvement of the ERK signaling pathway in Y27632- and fasudil-induced microglial migration.
(A) In-cell western blot assay shows the changes in p-ERK levels in cultures treated with Y27632 (10 μM), fasudil (41 μM), U0126 (10 μM), Y27632 (10 μM) + U0126 (10 μM) and fasudil (41 μM) + U0126 (10 μM) at 6 hours. Odyssey infrared imaging system was used to assess the fluorescence staining. (B) p-ERK levels at 6 hours after treatment (mean ± SEM; n = 4; one-way analysis of variance followed by Tukey's post hoc test). (C) The number of migrating microglia varied in accordance with the levels of p-ERK protein at 6 hours after treatment (mean ± SEM; n = 4; one-way analysis of variance followed by Tukey's post hoc test). *P < 0.05, **P < 0.01. ERK: Extracellular signal-regulated kinase; p-ERK: phosphorylated extracellular signal-regulated kinase.
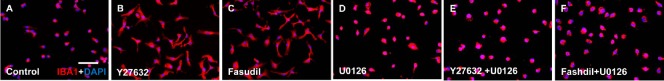
The ERK signaling pathway played a role in the Y27632- and fasudil-induced changes in microglial morphology
Using IBA1 staining, we examined morphological changes. Compared with control cells, Y27632- and fasudil-treated microglia had a larger size, an irregular shape and more small processes (Figure 5A–C). These results indicate that ROCK inhibition affects the actin cytoskeleton, impacting retraction and cell spreading, thereby inducing morphological changes.
Figure 5.
The ERK signaling pathway is involved in the morphological changes induced by Y27632 and fasudil in microglia.
Fluorescence double staining (Olympus DP50) with anti-IBA1 (red)/DAPI (blue) shows morphological changes in microglia after a 1-hour treatment with control (A, PBS), Y27632 (B, 10 μM), fasudil (C, 41 μM), U0126 (D, 10 μM), Y27632 (10 μM) + U0126 (10 μM) (E), and fasudil (41 μM) + U0126 (10 μM) (F). Scale bar: 25 μm. ERK: Extracellular signal-regulated kinase; DAPI: 4′,6-diamidino-2-phenylindole.
Next, U0126 was used to assess whether the morphological changes were dependent on ERK signaling. We observed no morphological change in cells treated only with U0126 (Figure 5D), compared with control cells (Figure 5A). Pretreatment with U0126 blocked the morphological changes induced by Y27632 and fasudil, presumably by inhibiting the ERK pathway (Figure 5E, F). Thus, the ERK signaling pathway plays a role in Y27632- and fasudil-induced morphological changes in microglia, and these changes are associated with increased microglial migration.
Discussion
ROCK regulates cellular morphology and inflammatory cytokine secretion in microglia (Schwab et al., 2004, Hoffmann et al., 2008; Takenouchi et al., 2008; Bernhart et al., 2010; Xin et al., 2015). ROCK-mediated microglial motility promotes phagocytosis of dying dopaminergic neurons (Barcia et al., 2012). Lysophosphatidylcholine-induced neuroinflammation is mediated by ROCK-dependent pathways in glia, and inhibition of these pathways confers neuroprotection (Sheikh et al., 2009). Thrombin-treated microglia exhibit enhanced phagocytosis and increased tumor necrosis factor-α, nitric oxide and ROCK levels. Thrombin-treated microglia that are pretreated with argatroban or Y-27632 display decreased phagocytotic activity, reduced ROCK and tumor necrosis factor-α expression, and lowered nitric oxide levels (Cui et al., 2013). ROCK inhibition by fasudil protects against lipopolysaccharide-mediated degeneration of dopaminergic neurons and might enhance the treatment of Parkinson's disease (He et al., 2016). Nitric oxide and proinflammatory cytokine production is induced in brain tissue after hypoxia/reoxygenation, and fasudil lowers secretion of nitric oxide and pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin-6 and interleukin-1β, and promotes production of anti-inflammatory cytokines, such as IL-10, in primary microglia (Ding et al., 2010). In our previous study, we found that ROCK inhibition enhances microglial phagocytic activity via the ERK signaling pathway (Fu et al., 2016a). Nevertheless, its role in microglial migration and the underlying mechanisms remained largely unknown. In the present study, we demonstrate that microglial migration is enhanced by ROCK inhibition.
Microglia are the main immune cells in the central nervous system. After central nervous system injury, microglia form a barrier between healthy and damaged tissue by rapidly converging at the site of injury (Davalos et al., 2005; Lu et al., 2011; Doncel-Pérez and Nieto-Sampedro, 2016; Wang et al., 2017). Microglial branch dynamics mediate this rapid response. After injury, astrocytes release adenosine triphosphate, which regulates microglial branch dynamics (Davalos et al., 2005). Our present findings show that ROCK inhibition increases migration activity in microglia, and in our previous studies, we found that ROCK inhibition enhances microglial phagocytosis and promotes early functional recovery after SCI (Fu et al., 2016a, b). Therefore, ROCK inhibition promotes functional recovery from SCI by various mechanisms, including by enhancing microglial migration.
The Rho/ROCK pathway is a major signaling pathway in the central nervous system, transducing inhibitory signals to block regeneration. After central nervous system injury, factors near the injury site inhibit neural regeneration through the Rho/ROCK pathway. Thus, an understanding of the Rho/ROCK pathway is important for advancing studies on the regeneration and repair of the central nervous system (Liu et al., 2015).
It is well known that various activated membrane receptors can activate the Rho/ROCK signaling pathway, such as G protein-coupled receptors, tyrosine kinase receptors and intracellular receptors (Tan et al., 2011; Walchli et al., 2013). The ROCK signaling pathway plays a particularly important role in cell migration (Mertsch et al., 2013; Goetsch et al., 2014; Wilhelm et al., 2014; Wallert et al., 2015). Various actin-myosin-related processes, such as cell motility, adhesion, phagocytosis and morphological changes, are promoted by ROCK activation (Honjo et al., 2001; Leemhuis et al., 2002; Khyrul et al., 2004; Mammoto et al., 2004; Kitzing et al., 2007; Rousseau et al., 2011; Zhou et al., 2011). In our study, Y27632 and fasudil produced striking changes in the morphology of microglia that were associated with increased migration activity. After ROCK inhibition, microglia had enlarged cell bodies and an irregular shape with many small processes. The expansion and shrinkage of the cell body along with the disappearance and outgrowth of branched processes depend on cytoskeletal remodeling induced by ROCK (Matsui et al., 1996).
ROCK is a well-known downstream effector of Rho. Rho proteins constitute a subgroup of the Ras superfamily of GTP hydrolases. Ras family members are small GTP-binding proteins. Ras activates the cascade composed of a MAPKKK (Raf), a MAPKK (MEK1/2) and a MAPK (Erk). The MAPK signaling pathway plays an important role in microglial phagocytosis, proliferation, inflammatory factor secretion and migration (Calvo et al., 2011; Martin et al., 2012; Miao et al., 2012; Ellert-Miklaszewska et al., 2013). ROCK signaling plays a key role in many neurodegenerative diseases and models, and inhibition of ROCK has beneficial effects. ROCK as well as ERK regulate dynamic changes in cellular morphology through actin cytoskeletal reorganization, and both the ROCK and ERK pathways are potential therapeutic targets for spinal muscular atrophy (Hensel et al., 2014). In the present study, ERK signaling was found to be involved in the migratory and morphological changes induced by Y27632 and fasudil. Y27632 and fasudil, which inhibit ROCK, increased the phosphorylation of ERK1/2 in microglia. However, U0126, an inhibitor of ERK (Villanueva et al., 2007), suppressed the Y27632- and fasudil-induced changes in microglial migration. These results strongly suggest that the ERK signaling pathway mediates the ROCK inhibitor-induced enhancement of microglial migration and associated morphological changes.
SCI is a catastrophic injury, including multiple events. We previously reported that Rho/ROCK signaling is involved in microglial phagocytosis and migration, making it a potential therapeutic target for treatment of SCI. Fasudil is used clinically for the treatment of ischemic cerebrovascular disease and is therefore an attractive candidate for clinical trials of treatments for SCI.
In summary, ROCK inhibition by Y27632 and fasudil effectively promotes microglial migration and initiates cell morphological changes through the ERK signaling pathway. Further studies on the underlying molecular and cellular mechanisms should provide additional insight into the role of ROCK in spinal cord injury and disease.
Acknowledgments
We are very grateful to Miss Li Xu from the Key Laboratory of Neurological Diseases (Huazhong University of Science and Technology) in China for her excellent technical supporting.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81471200, 81771341.
Conflicts of interest: None declared.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81471200, 81771341. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Research ethics: Ethical approval was obtained from the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (ethical approval: [2014] IACUC Number: 363).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Patel B, Maxwell R, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Barcia C, Ros CM, Annese V, Carrillo-de Sauvage MA, Ros-Bernal F, Gomez A, Yuste JE, Campuzano CM, de Pablos V, Fernandez-Villalba E, Herrero MT. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci Rep. 2012;2:809. doi: 10.1038/srep00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhart E, Kollroser M, Rechberger G, Reicher H, Heinemann A, Schratl P, Hallstrom S, Wintersperger A, Nusshold C, DeVaney T, Zorn-Pauly K, Malli R, Graier W, Malle E, Sattler W. Lysophosphatidic acid receptor activation affects the C13NJ microglia cell line proteome leading to alterations in glycolysis, motility, and cytoskeletal architecture. Proteomics. 2010;10:141–158. doi: 10.1002/pmic.200900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–568. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cui G, Zuo T, Zhao Q, Hu J, Jin P, Zhao H, Jing J, Zhu J, Chen H, Liu B, Hua F, Ye X. ROCK mediates the inflammatory response in thrombin induced microglia. Neurosci Lett. 2013;554:82–87. doi: 10.1016/j.neulet.2013.08.065. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Ding J, Li QY, Wang X, Sun CH, Lu CZ, Xiao BG. Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice. J Neurochem. 2010;114:1619–1629. doi: 10.1111/j.1471-4159.2010.06876.x. [DOI] [PubMed] [Google Scholar]
- Doncel-Pérez E, Nieto-Sampedro M. Aldynoglia cells and modulation of RhoGTPase activity as useful tools for spinal cord injury repair. Neural Regen Res. 2016;11:1043–1045. doi: 10.4103/1673-5374.187020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Dabrowski M, Lipko M, Sliwa M, Maleszewska M, Kaminska B. Molecular definition of the pro-tumorigenic phenotype of glioma-activated microglia. Glia. 2013;61:1178–1190. doi: 10.1002/glia.22510. [DOI] [PubMed] [Google Scholar]
- Fu P, Tang R, Yu Z, Li C, Chen X, Xie M, Wang W, Luo X. Rho-associated kinase inhibitors promote microglial uptake via the ERK signaling pathway. Neurosci Bull. 2016a;32:83–91. doi: 10.1007/s12264-016-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu PC, Tang RH, Wan Y, Xie MJ, Wang W, Luo X, Yu ZY. ROCK inhibition with fasudil promotes early functional recovery of spinal cord injury in rats by enhancing microglia phagocytosis. J Huazhong Univ Sci Technolog Med Sci. 2016b;36:31–36. doi: 10.1007/s11596-016-1537-3. [DOI] [PubMed] [Google Scholar]
- Gingras M, Gagnon V, Minotti S, Durham HD, Berthod F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. J Neurosci Meth. 2007;163:111–118. doi: 10.1016/j.jneumeth.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Gitik M, Reichert F, Rotshenker S. Cytoskeleton plays a dual role of activation and inhibition in myelin and zymosan phagocytosis by microglia. FASEB J. 2010;24:2211–2221. doi: 10.1096/fj.09-146118. [DOI] [PubMed] [Google Scholar]
- Goetsch KP, Snyman C, Myburgh KH, Niesler CU. ROCK-2 is associated with focal adhesion maturation during myoblast migration. J Cell Biochem. 2014;115:1299–1307. doi: 10.1002/jcb.24784. [DOI] [PubMed] [Google Scholar]
- He Q, Li YH, Guo SS, Wang Y, Lin W, Zhang Q, Wang J, Ma CG, Xiao BG. Inhibition of Rho-kinase by Fasudil protects dopamine neurons and attenuates inflammatory response in an intranasal lipopolysaccharide-mediated Parkinson's model. Eur J Neurosci. 2016;43:41–52. doi: 10.1111/ejn.13132. [DOI] [PubMed] [Google Scholar]
- Hensel N, Stockbrugger I, Rademacher S, Broughton N, Brinkmann H, Grothe C, Claus P. Bilateral crosstalk of rho- and extracellular-signal-regulated-kinase (ERK) pathways is confined to an unidirectional mode in spinal muscular atrophy (SMA) Cell Signal. 2014;26:540–548. doi: 10.1016/j.cellsig.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Hofmann F, Just I, Lehnardt S, Hanisch UK, Bruck W, Kettenmann H, Ahnert-Hilger G, Holtje M. Inhibition of Rho-dependent pathways by clostridium botulinum C3 protein induces a proinflammatory profile in microglia. Glia. 2008;56:1162–1175. doi: 10.1002/glia.20687. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophth Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Hirano S, Chiba S, Takeshita H, Nagai T, Takada M, Sakamoto K, Mukai T. The role of Rho-kinases in IL-1beta release through phagocytosis of fibrous particles in human monocytes. Arch Toxicol. 2015;89:73–85. doi: 10.1007/s00204-014-1238-2. [DOI] [PubMed] [Google Scholar]
- Khyrul WA, LaLonde DP, Brown MC, Levinson H, Turner CE. The integrin-linked kinase regulates cell morphology and motility in a rho-associated kinase-dependent manner. J Biol Chem. 2004;279:54131–54139. doi: 10.1074/jbc.M410051200. [DOI] [PubMed] [Google Scholar]
- Kim K, Ossipova O, Sokol SY. Neural crest specification by inhibition of the ROCK/Myosin II pathway. Stem Cells. 2015;33:674–685. doi: 10.1002/stem.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Gene Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Leemhuis J, Boutillier S, Schmidt G, Meyer DK. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J Pharmacol Exp Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao HY, Wang XF. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res. 2015;10:1892–1896. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, van Mil A, Aguor EN, Siddiqi S, Vrijsen K, Jaksani S, Metz C, Zhao J, Strijkers GJ, Doevendans PA, Sluijter JP. MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J Cell Mol Med. 2012;16:2379–2386. doi: 10.1111/j.1582-4934.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MJ, Zhu Y, Sun J, Yang XR. Microglia mediates inflammation injury in mouse models of Parkinson's disease. Zhongguo Zuzhi Gongcheng Yanjiu. 2011;15:1945–1948. [Google Scholar]
- Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- Martin R, Cordova C, Nieto ML. Secreted phospholipase A2-IIA-induced a phenotype of activated microglia in BV-2 cells requires epidermal growth factor receptor transactivation and proHB-EGF shedding. J Neuroinflamm. 2012;9:154. doi: 10.1186/1742-2094-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mertsch S, Oellers P, Wendling M, Stracke W, Thanos S. Dissecting the inter-substrate navigation of migrating glioblastoma cells with the stripe assay reveals a causative role of ROCK. Mol Neurobiol. 2013;48:169–179. doi: 10.1007/s12035-013-8429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Ding M, Zhang A, Xiao Z, Qi W, Luo N, Di W, Tao Y, Fang Y. Pleiotrophin promotes microglia proliferation and secretion of neurotrophic factors by activating extracellular signal-regulated kinase 1/2 pathway. Neurosci Res. 2012;74:269–276. doi: 10.1016/j.neures.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscl Throm Vas. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Racchetti G, D’Alessandro R, Meldolesi J. Astrocyte stellation, a process dependent on Rac1 is sustained by the regulated exocytosis of enlargeosomes. Glia. 2012;60:465–475. doi: 10.1002/glia.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Rousseau M, Gaugler MH, Rodallec A, Bonnaud S, Paris F, Corre I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun. 2011;414:750–755. doi: 10.1016/j.bbrc.2011.09.150. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ. Lesional RhoA+ cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia. 2004;47:377–386. doi: 10.1002/glia.20031. [DOI] [PubMed] [Google Scholar]
- Sheikh AM, Nagai A, Ryu JK, McLarnon JG, Kim SU, Masuda J. Lysophosphatidylcholine induces glial cell activation: role of rho kinase. Glia. 2009;57:898–907. doi: 10.1002/glia.20815. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, Sato M, Hashimoto M, Kitani H. Lysophospholipids and ATP mutually suppress maturation and release of IL-1 beta in mouse microglial cells using a Rho-dependent pathway. J Immunol. 2008;180:7827–7839. doi: 10.4049/jimmunol.180.12.7827. [DOI] [PubMed] [Google Scholar]
- Tan HB, Zhong YS, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol. 2011;4:652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- Villanueva J, Yung Y, Walker JL, Assoian RK. ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets. Mol Biol Cell. 2007;18:1457–1463. doi: 10.1091/mbc.E06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walchli T, Pernet V, Weinmann O, Shiu JY, Guzik-Kornacka A, Decrey G, Yuksel D, Schneider H, Vogel J, Ingber DE, Vogel V, Frei K, Schwab ME. Nogo-A is a negative regulator of CNS angiogenesis. PNAS. 2013;110:E1943–E1952. doi: 10.1073/pnas.1216203110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallert MA, Hammes D, Nguyen T, Kiefer L, Berthelsen N, Kern A, Anderson-Tiege K, Shabb JB, Muhonen WW, Grove BD, Provost JJ. RhoA Kinase (Rock) and p90 Ribosomal S6 Kinase (p90Rsk) phosphorylation of the sodium hydrogen exchanger (NHE1) is required for lysophosphatidic acid-induced transport, cytoskeletal organization and migration. Cell Signal. 2015;27:498–509. doi: 10.1016/j.cellsig.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HF, Liu XK, Li R, Zhang P, Chu Z, Wang CL, Liu HR, Qi J, Lv GY, Wang GY, Liu B, Li Y, Wang YY. Effect of glial cells on remyelination after spinal cord injury. Neural Regen Res. 2017;12:1724–1732. doi: 10.4103/1673-5374.217354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Fazakas C, Molnar J, Hasko J, Vegh AG, Cervenak L, Nagyoszi P, Nyul-Toth A, Farkas AE, Bauer H, Guillemin GJ, Bauer HC, Varo G, Krizbai IA. Role of Rho/ROCK signaling in the interaction of melanoma cells with the blood-brain barrier. Pigm Cell Melanom. 2014;27:113–123. doi: 10.1111/pcmr.12169. [DOI] [PubMed] [Google Scholar]
- Xin YL, Yu JZ, Yang XW, Liu CY, Li YH, Feng L, Chai Z, Yang WF, Wang Q, Jiang WJ, Zhang GX, Xiao BG, Ma CG. FSD-C10: a more promising novel ROCK inhibitor than fasudil for treatment of CNS autoimmunity. Bioscience Rep. 2015 doi: 10.1042/BSR20150032. doi: 10.1042/BSR20150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu M, Fu P, Xie M, Wang W, Luo X. ROCK inhibition with Y27632 promotes the proliferation and cell cycle progression of cultured astrocyte from spinal cord. Neurochem Int. 2012;61:1114–1120. doi: 10.1016/j.neuint.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]