Abstract
This review summarizes and describes the use of curcumin in diagnosis, prevention, and treatment of Alzheimer's disease. For diagnosis of Alzheimer's disease, amyloid-β and highly phosphorylated tau protein are the major biomarkers. Curcumin was developed as an early diagnostic probe based on its natural fluorescence and high binding affinity to amyloid-β. Because of its multi-target effects, curcumin has protective and preventive effects on many chronic diseases such as cerebrovascular disease, hypertension, and hyperlipidemia. For prevention and treatment of Alzheimer's disease, curcumin has been shown to effectively maintain the normal structure and function of cerebral vessels, mitochondria, and synapses, reduce risk factors for a variety of chronic diseases, and decrease the risk of Alzheimer's disease. The effect of curcumin on Alzheimer's disease involves multiple signaling pathways: anti-amyloid and metal iron chelating properties, antioxidation and anti-inflammatory activities. Indeed, there is a scientific basis for the rational application of curcumin in prevention and treatment of Alzheimer's disease.
Keywords: nerve regeneration, curcumin, Alzheimer's disease, senile dementia, early diagnosis, positron emission tomography, magnetic resonance imaging, biological availability, chemical components, neurodegeneration, neural regeneration
Introduction
Alzheimer's disease (AD) is a common progressive neurodegenerative disorder prevalent worldwide, yet with no effective cure. It affects about 35 million individuals and cost more than $226 billion in 2016 alone. A conservative estimate of its prevalence is one in nine people aged 65 years and older, being almost three times higher for people aged 85 and older (Alzheimer's Association, 2015). By 2050, a new case of AD is expected to develop every 33 seconds (Lopez, 2011). Indeed, AD is now imposing a tremendous impact on society and is a costly burden that will be a modern epidemic in the near future (Hampel et al., 2011). Consequently, there is an urgent need for global diagnostic, preventive, and therapeutic measures to control the impact of this devastating disease.
Like other chronic diseases, AD develops as a result of multiple factors rather than a single cause. However, its etiology and pathology remain unclear (LaFerla and Green, 2012). Clinically, AD is characterized by memory and cognitive impairments, and personality and behavior changes (Huang and Mucke, 2012). The pathological hallmarks observed in AD brain include extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs). Amyloid-β (Aβ) is generated a cleavage of the amyloid precursor protein by β- and γ-secretases. This results in native Aβ monomers that have prosurvival effects on neurons and protect mature neurons against excitotoxic death (Giuffrida et al., 2009). In contrast, under pathological conditions, excessive accumulation of monomers results in their assembly into soluble, diffusible toxic oligomeric Aβ species: low-molecular-weight aggregates consisting of 2–30 Aβ peptides. When the oligomers reach a critical concentration, they form insoluble fibrils/aggregates and plaques. It is important to note that soluble Aβ oligomers are more toxic than insoluble deposits (Verma et al., 2015). In particular, Aβ dimers (the major form of soluble oligomers isolated from AD cortex) directly induce tau hyperphosphorylation and neurite degeneration (Jin et al., 2011). NFTs are another hallmark of AD, and are composed of hyperphosphorylated tau, which disrupts microtubules and impairs axonal transport (Beharry et al., 2014; Metaxas and Kempf, 2016; Ye et al., 2017). In addition to these pathologies, extensive neuroinflammation and oxidative damage are also observed at sites of neurodegeneration. Indeed, these pathological factors act together, resulting in progressive neuronal damage and cognitive deficits. Importantly, a vicious cycle develops among Aβ, NFTs, oxidative stress, and inflammation. Aβ and NFTs activate microglia and induce production of reactive oxygen species and inflammatory factors. Conversely, reactive oxygen species and inflammatory cytokines directly act on neurons, further promoting Aβ and NFT formation (Glass et al., 2010; Broussard et al., 2012; Luque-Contreras et al., 2014). Therefore, seeking effective therapeutics with multiple targets is highly desirable (Frautschy and Cole, 2010). Fortunately, accumulating evidence suggests that curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) may play a significant role in AD therapy, exerting pleiotropic properties. Curcumin can directly bind to Aβ in the central nervous system and prevent its assembly into neurotoxic species (Kozmon and Tvaroška, 2015; Rao et al., 2015). In addition, curcumin can reduce oxidative stress and inflammatory responses, and has beneficial effects on neuronal and vascular functions (DiSilvestro et al., 2012). Extensive lines of evidence indicate that Aβ oligomer production, oxidative markers, and neuroinflammation are attenuated by administration of curcumin (Hu et al., 2015; Nasir Abbas Bukhari and Jantan, 2015).
Curcumin is a component of the Indian spice turmeric, and is extracted from the rhizome of Curcuma longa, which is widely cultivated in south and southeast Asia, especially China and India (Wanninger et al., 2015). Commercial curcumin refers to curcumin complex, which is composed of curcumin (77%), demethoxycurcumin (17%), and bisdemethoxycurcumin (3%). Curcumin is the major component of three curcuminoids that give turmeric its distinctive yellow color, and is used as a food colorant, flavoring, and additive (Goel et al., 2008). In herbal medicine, turmeric and natural curcuminoids have been used to treat respiratory conditions, abdominal pain, sprains and swelling (Araujo and Leon, 2001). Recent studies indicate that curcumin may have a critical role in management of AD, and is particularly useful as a sensitive diagnostic agent, health-promoting life-long nutraceutical, as well as a multi-target-directed drug (Belkacemi et al., 2011; Goozee et al., 2016).
This review discusses the multifaceted functions of curcumin, including its use in diagnosis, prevention, and therapy at different stages of AD.
Curcumin: a Sensitive Fluorochrome for AD Diagnosis
Diagnosis of AD in patients is based on clinical examination, which is mainly suitable for late-stage disease (Dubois et al., 2007). Indeed, no definite early diagnostic test at the asymptomatic stage is currently available. The first diagnostic criteria for AD were established in 1984, and included progressive deterioration of language, memory, and cognition, as well as progressive cerebral atrophy detectable by brain imaging (Alzheimer's Association, 2010). However, these criteria were revised as they are too general. The new AD diagnostic criteria now require a gradual onset and fast-progressing cognitive function impairment, which cannot be explained by other diseases (Dubois et al., 2007). Pathological features such as cerebrovascular changes, Aβ, and NFTs, are believed to precede or coexist with AD (Parnetti et al., 2006). Thus, use of biomarkers may increase diagnostic specificity and reliability, which are included in the updated criteria (Reitz et al., 2011). At the time of diagnosis, patients are usually at a mild to moderate stage, which cannot be prevented by current treatments. To overcome this disadvantage, more sensitive diagnostic probes is highly desirable (Bateman et al., 2012; Chase, 2014).
With its recent success as a “multi-anti” agent, curcumin has attracted considerable interest from researchers in the fields of physics, chemistry, biology, and medicine. Curcumin comprises two phenols connected by a linear β-diketone linker, which also induces keto–enol tautomerism. Because of its special structure, curcumin exhibits many interesting photophysical and photochemical properties (Priyadarsini, 2009). Curcumin effectively binds to Aβ plaques and emits a strong fluorescence signal, making it a powerful diagnostic reagent for AD (Garcia-Alloza et al., 2007). During the last two decades, extensive research has been performed to develop curcumin probes for targeting Aβ with available imaging modalities, including positron emission tomography (PET), two-photon microscopy, magnetic resonance imaging (MRI), and near-infrared fluorescence (NIRF) (Tu et al., 2015).
PET
PET imaging with Aβ-specific tracers has been widely applied in clinical trials, with three Aβ PET tracers approved by the US Food and Drug Administration for clinical use: 18F-flutemetamol (Vizamyl), 18F-florbetapir (Amyvid), and 18F-florbetaben (Neuraceq). Moreover, this approach is an emerging tool for AD research and numerous new PET probes are under development (Mathis et al., 2012). Curcumin derivatives can be labeled with radioactive nuclides (including several radioiodinated ligands and 18F fluoropegylated ligands), making it applicable for PET (Cui et al., 2011). Ryu et al. (2006) synthesized fluoropropyl-substituted curcumin (Figure 1A), which shows high binding affinity (Ki = 0.07 nM) to Aβ. Furthermore, its radiolabeled form shows suitable lipophilicity and reasonable brain uptake. These results suggest that 18F fluoropropyl-substituted curcumin is a promising radioligand for imaging Aβ. In addition, Rokka and coworkers synthesized the [18F]curcumin derivative (Figure 1A), with high binding affinity to Aβ plaques in transgenic APP23 mouse brain cryosections. Studies have demonstrated that 18F curcumin derivative can be efficiently removed from blood (1–5 minutes), but has low blood-brain barrier (BBB) penetration, with 18F-radioactivity concentrations of only 0.04% ID/g in mouse brain and 0.03% ID/g in rat brain (Rokka et al., 2014). To overcome this low BBB permeability, Mourtas et al. (2014) designed a lipid-polyethylene glycol (PEG)-curcumin derivative to increase BBB penetration and fluorescence intensity. These nanoliposomes were loaded with curcumin derivative and immobilized to a BBB transport mediator (monoclonal anti-transferrin antibody [MAb]). As anticipated, these multifunctional nanoliposomes were more efficient at labeling Aβ deposits in postmortem tissue of AD patients, with fluorescence was enhanced by almost six times. Uptake of MAb-decorated nanoliposomes loaded with curcumin-derivative was increased (almost two-fold) compared with curcumin-conjugated nanoliposomes (Mourtas et al., 2014). Altogether, these findings indicate that curcumin derivatives entrapped in multifunctional nanoliposomes represent a useful approach in AD diagnosis.
Figure 1.
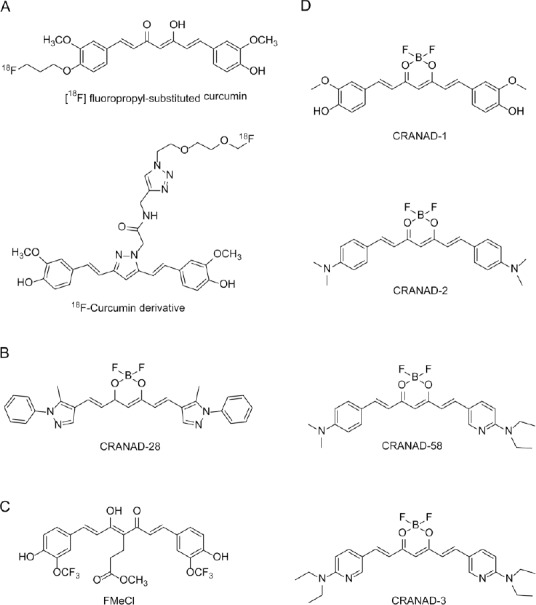
Curcumin is a sensitive fluorochrome for AD diagnosis.
(A) Curcumin derivatives for PET imaging. [18F] fluoropropyl-substituted curcumin, Ki = 0.07 nM to Aβ (Ryu et al., 2006); 18F-curcumin derivative (Rokka et al., 2014). (B) Curcumin analogue CRANAD-28 for two-photon microscopy imaging (Zhang et al., 2014). Aβ40 monomers: Kd = 68.80 nM, Aβ42 monomers: Kd = 159.70 nM, Aβ42 dimers: Kd = 162.90 nM, Aβ42 oligomers: Kd = 85.70 nM, Aβ40 aggregates: Kd = 52.40 nM. (C) Curcumin analogue FMeC1 for magnetic resonance imaging (Yanagisawa et al., 2011). (D) Curcumin analogues for near-infrared fluorescence imaging. CRANAD-1, Kd =38.00 nM; CRANAD-58, Aβ40: Kd = 105.80 nM, Aβ42: Kd = 45.80 nM; CRANAD-3, Aβ40 monomers: Kd = 24.00 nM, Aβ42 monomers: Kd = 23.00 nM (Ran et al., 2009; Zhang et al., 2013, 2015). AD: Alzheimer's disease; Aβ: amyloid-β; PET: positron emission tomography.
Two-photon microscopy
Two-photon microscopy is an important technique for investigating Aβ species, and provides insight into the dynamics of individual plaque expansion and disruption of the microenvironment (Condello et al., 2011). Zhang et al. (2014) designed and synthesized CRANAD-28 (Figure 1B) by introducing a pyrazole ring into curcumin. With this replacement, CRANAD-28 improved tissue penetration because of its longer excitation (498 nm) and emission (578 nm), and displayed high quantum yield in both phosphate-buffered saline (PBS) (0.32) and ethanol (> 1.00). When tested in vitro towards different Aβ species, CRANAD-28 showed high affinity, with Kd values ranging from 52.40 to 162.90 nM. In vivo two-photon microscopy clearly demonstrated that CRANAD-28 not only labeled Aβ plaques and cerebral amyloid angiopathies in 9-month old APP/PS1 mice, but also attenuated Aβ crosslinking in brain. These results suggest the potential use of CRANAD-28 in both diagnosis and therapy for AD (Zhang et al., 2014).
MRI
MRI is cheaper, easier, and nonradioactive in comparison with PET, but its sensitivity needs to be improved before it can be used clinically. Fortunately, recent studies have tested curcumin derivatives as MRI probes for Aβ imaging. Yanagisawa et al. (2011) developed a perfluoro curcumin analog, FMeCl (Figure 1C), for 19F MRI to facilitate visualization of Aβ in vivo. They found that compared with wild-type mice, 19F MRI showed marked 19F signal levels in the brain of Tg2576 mice after injection of FMeCl (200 mg/kg). Moreover, 19F signal in Tg2576 mice aligned with the distribution of Aβ deposits (Yanagisawa et al., 2011). Interestingly, FMeCl not only labeled Aβ plaques, but also inhibited Aβ aggregates, glial cell activity, and cognitive deficits in APP/PS1 mice (Yanagisawa et al., 2015). Subsequently, a new formulation of FMeCl was developed to increase its bioavailability. Thus, FMeCl may be a promising theranostic agent owing to its dual role in imaging and therapy, similar to CRANAD-28. In addition to curcumin analogues, several curcumin-conjugated nanoparticles have been approved for early diagnosis of AD (Patil et al., 2015). Cheng et al. (2015) used magnetic nanoparticles (MNPs) comprised of super paramagnetic iron oxide conjugated to curcumin to develop a nanoimaging agent (Cur-MNPs). Cur-MNPs show low cytotoxicity (up to 167 mg/mL) and considerable BBB penetration potential. Many dark spots were found by MRI in Tg2576 brain in vivo, while almost no such spots were found in control brain. Therefore, Cur-MNPs are another successful example of nanoparticles for Aβ imaging (Cheng et al., 2015).
NIRF
NIRF is an attractive tool for early AD detection, and presents several advantages including acceptable photon penetration, noninvasive exposure, and inexpensive instrumentation. In past years, Ran's group has designed and synthesized a series of curcumin analogues (CRANAD-X) as NIRF imaging probes (Ran et al., 2009).First, they synthesized CRANAD-1 (Figure 1D) by introducing a difluoroboronate ring into curcumin. With this replacement, CRANAD-1 emission was red-shifted to λmax (em) = 560 nm in methanol, which is not in the NIRF wavelength range (> 650 nm). To further increase emission wavelength, CRANAD-1 was modified by substituting the N,N′-dimethyl group for a phenolic hydroxyl group to yield the compound, CRANAD-2, (Figure 1D). As anticipated, CRANAD-2 showed longer emission at λmax (em) = 760 nm. In vitro, CRANAD-2 effectively interacted with Aβ (Kd = 38.00 nM) and increased fluorescence brightness by 70-fold. In vivo, CRANAD-2 showed a significant fluorescence difference between 19-month-old wild-type and transgenic mice. However, as a limitation, CRANAD-2 was not able to detect soluble dimeric and oligomeric Aβ species, which are more neurotoxic than insoluble deposits. To overcome this limitation, Ran et all. (2009) designed and synthesized another curcumin analogue, CRANAD-58 (Figure 1D), which detects both insoluble and soluble Aβ species. As expected, CRANAD-58 not only displayed sufficient long emission (750 nm), but also exhibited strong binding to soluble Aβ monomers. Notably, CRANAD-58 detected soluble Aβ species in 4-month-old APP/PS1 mice, a younger age than with CRANAD-2. Consequently, CRANAD-58 can be considered the first NIRF imaging probe that is sensitive to both soluble and insoluble Aβ species in vitro and in vivo. Importantly, Aβ imaging is not only a means for early diagnosis, but also an approach for monitoring the efficacy of therapy. However, none of these NIRF probes have been used for this purpose. To fill this gap, Ran et al. (2009) designed CRANAD-3 (Figure 1D) by replacing the two aromatic rings with pyridyls to increase Aβ affinity. In vitro spectral testing and in vivo NIRF imaging indicated that CRANAD-3, like CRANAD-58, was sensitive to both soluble and insoluble Aβ, but with higher sensitivity than CRANAD-58. Crucially, owing to its excellent ability to detect both soluble and insoluble Aβ, CRANAD-3 can be used to monitor the effectiveness of Aβ-lowering therapeutics, suggesting a dual role of CRANAD-3 in AD (Ran et al., 2009; Zhang et al., 2013, 2015).
Compared with traditional diagnostic agents, synthesized curcumin analogues (CRANAD-58 and CRANAD-3) can detect not only insoluble Aβ plaques but also soluble Aβ oligomers in vitro and in vivo (Zhang et al., 2013, 2015). Moreover, CRANAD-3 can monitor and evaluate the effectiveness of anti-amyloid interventions, enabling selection of patients for treatment (Zhang et al., 2015). Importantly, curcumin analogues (CRANAD-28 and FMeC1) combine diagnostic and therapeutic properties in a single molecule (Yanagisawa et al., 2011; Zhang et al., 2014), leading to time-saving and cost-effective optimization. Apart from the theranostic role of curcumin for Aβ, curcumin has been reported to detect tau pathology. For example, Mohorko et al. (2010) have shown that curcumin can label tau aggregates in brain sections that coincides with routine thioflavine S and Gallyas silver staining. This suggests curcumin has diagnostic potential in tauopathies. Similarly, Park et al. (2015) designed and synthesized a novel curcumin-based molecular probe by introducing a (4-dimethylamino-2,6-dimethoxy) phenyl moiety to the aromatic rings of CRANAD-2. This probe showed a significant fluorescence response to tau fibrils (quantum yield = 0.32; Kd = 0.77 µM; λmax(em) = 620 nm), encouraging further development of curcumin in AD theranostics for both Aβ and NFTs.
Curcumin: a Health-Promoting Nutraceutical for AD Prevention
At present, there is no cure for AD, yet the impact of this disease can be lessened by delaying its onset. Delayed onset of 6 months would result in a reduction of 100,000 cases after 10 years, highlighting the importance of prevention (Brookmeyer et al., 1998). Epidemiological and experimental data suggest that optimal diet, physical exercise, and intellectual activity may promote brain health (Vivar, 2015). In particular, an optimal diet with rich phenolic compounds may provide preventive effects on development of AD (Yamada et al., 2015). Safouris et al. (2015) reported that consumption of a Mediterranean-type diet reduced the incidence of AD. This diet is characterized by a high proportion of plant foods and fish, a moderate proportion of wine, and a low proportion of red meat. They found that higher adherence to the Mediterranean-type diet was associated with lower risk for AD (hazard ratio of 0.60, compared with 0.91 in non-Mediterranean countries) (Safouris et al., 2015). Similarly, Ng et al. (2006) reported that consumption of an Asian-type diet that is rich in soy and turmeric (containing considerable amounts of isoflavones and curcumin, respectively) and high levels of seaweed, also reduced the incidence of AD. These diets are rich in fruits and vegetables, which are primary sources of dietary polyphenols, glucosinolates, and vitamins. Curcumin is a natural phenolic substance with beneficial effects on various chronic conditions including obesity, diabetes, and depression (Arun and Nalini, 2002; Kim and Kim, 2010; Rinwa et al., 2013). Importantly, such chronic diseases may be risk factors for AD, and are linked to the etiology or outcome of AD (Jorm, 2001; Gustafson et al., 2003). For example, diabetes promotes the formation of advanced glycosylation end products, leading to activation of receptors for advanced glycosylation end products on the surface of glial cells, vascular endothelial cells, and neurons. In turn, this induces inflammatory responses and increases Aβ influx, giving rise to further brain damage and ensuing cognitive impairment (Yan et al., 1996). This suggests that curcumin intake may prevent AD progression by reducing AD risk (Reitz et al., 2011). Additionally, curcumin improves memory function in healthy-aged rodents by enhancing synaptic plasticity and neurogenesis (Kim et al., 2008; Dong et al., 2012; Belviranlı et al., 2013). It may also increase docosahexaenoic acid synthesis, resulting in better plasma membrane integrity, which further maintains normal mitochondrial and synaptic function (Pinkaew et al., 2015; Wu et al., 2015). Several studies have examined curcumin supplementation in healthy older people. DiSilvestro et al. (2012) demonstrated that a low dose of lipidated curcumin produced diverse potential health benefits in healthy middle-aged people by increasing nitric oxide levels and lowering soluble intercellular adhesion molecule. Both molecules have relevance for cardiovascular disease risk. Also, curcumin suppressed alanine aminotransferase activity, a general marker of liver injury, and raised plasma myeloperoxidase, an effect associated with inflammation (DiSilvestro et al., 2012). More recently, Cox et al. (2015) showed that supplementation with solid lipid curcumin formulation (80 mg as Longvida®) improved cognitive function, reduced fatigue, and lessened the detrimental impact of psychological stress on mood, which may improve quality of life for the growing elderly population. Therefore, dietary uptake of curcumin may reduce AD risk, enhance cognitive function, and delay and counteract the effect of aging and neurodegenerative disease.
Curcumin: a Pleiotropic Agent for AD Treatment
Considering the multifactorial etiology and complex pathological mechanisms involved in AD, it is quite reasonable that treatments targeting a single causal or modifying factor will have limited benefits (Figure 2). Therefore, growing interest is focused on therapeutic agents with pleiotropic activity, targeting several affected processes (Bajda et al., 2011). Several compounds described here fulfill these properties, with curcumin showing strong anti-Aβ properties and considerable anti-inflammatory and antioxidant activities (Belkacemi et al., 2011).
Figure 2.
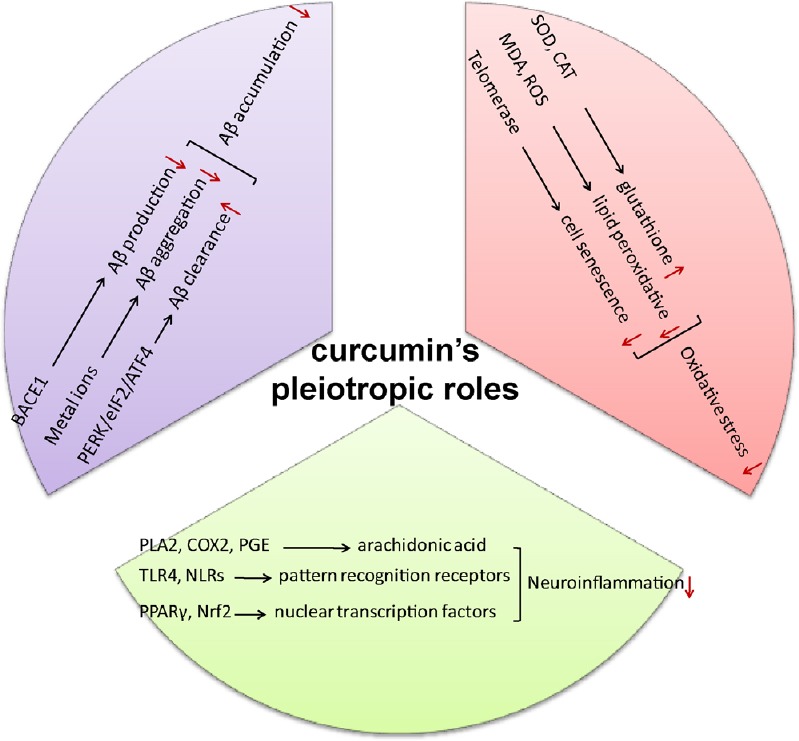
Curcumin: a pleiotropic agent for treatment of Alzheimer's disease.
Curcumin decreases Aβ production, inhibits Aβ aggregation, and promotes Aβ clearance. Besides, curcumin inhibits inflammatory signal pathways and decreases the production of inflammatory cytokines. Additionally, curcumin reduces oxidative stress and scavenge radicals. Aβ: Amyloid β-protein.
Effect of curcumin on Aβ protein
Over the past decades, the amyloid hypothesis has been widely accepted and been the focus of AD research (Soto, 1999). Consequently, one current strategy for treating AD is anti-amyloid treatments including decreasing Aβ production, inhibiting Aβ aggregation, and promoting Aβ clearance. In vitro studies have shown that curcumin lowers Aβ levels by attenuating amyloid precursor protein maturation and suppressing beta-secretase 1 (BACE1) expression, which is the sole β-secretase enzyme (Liu et al., 2010). Moreover, in vivo studies using a drosophila AD model have shown that demethoxycurcumin has strong inhibitory BACE-1 activity (IC50 = 17 μM), contributing to rescue of morphological and behavioral defects caused by overexpression of amyloid precursor protein maturation and BACE1 (Wang et al., 2014). Recent studies have investigated the molecular mechanism of BACE-1 inhibition by curcumin. Curcumin was found to repress BACE-1 transcription by activating the Wnt/β-catenin pathway, which binds to T-cell factor-4, a repressor of the BACE1 gene (Zhang et al., 2011; Parr et al., 2015). Apart from its role in amyloid precursor protein maturation, studies have indicated that curcumin can attach to Aβ peptides and prevent Aβ aggregation in vitro and in vivo. In vitro curcumin displays high-affinity binding to Aβ aggregates (Kd = 0.20 nM), with EC50 of curcumin for Aβ destabilization being approximately 1 µM (Ono et al., 2004). In APPswe/PS1dE9 mice, Garcia-Alloza et al. (2007) suggested that curcumin (7.5 mg/kg intravenously, 7 days) clears or reduces the size of senile plaques (Garcia-Alloza et al., 2007). In Tg2576 mice, a daily single dose (500 ppm) of curcumin administered orally for 5 months significantly reduced levels of insoluble Aβ (85%) and Aβ plaques (32.50%) (Yang et al., 2005). Based on comprehensive structure–activity analysis, coplanarity of two phenol rings, length and rigidity of the linker, and substitution conformation of the phenol rings were shown to contribute to the inhibitory potency of curcumin (Reinke and Gestwicki, 2007). Further studies have investigated the atomistic mechanism of curcumin inhibition on Aβ aggregation. By molecular docking and molecular dynamic simulations, Rao et al. (2015) demonstrated that curcumin binding to Aβ-aggregates leads to significant amino acid fluctuations, with a shift in equilibrium towards non-toxic Aβ aggregates. Moreover, curcumin binds to Aβ via strong hydrophobic interactions and H-bonding, which disrupts preformed fibrils and prevents oligomerization (Kundaikar and Degani, 2015). Interestingly, alternative theories suggest that curcumin blocks Aβ aggregation by chelating metal ions, such as Cu2+, Zn2+, and Fe3+, likely agonists of Aβ aggregation and oxidative stress (Perrone et al., 2010; Banerjee, 2014). Kozmon investigated interactions between Aβ peptide and Cu2+ ions and/or curcumin by molecular dynamic simulations. They found that curcumin not only chelated Cu2+ ions, but also directly attached to Aβ, forming curcumin–Cu2+–Aβ and curcumin–Aβ complexes that decrease toxic β-sheet structures (Kozmon and Tvaroška, 2015). Crucially, the effects of curcumin are not limited to modulation of Aβ production and aggregation, and further studies have shown that curcumin accelerates Aβ clearance. Curcumin increases expression of autophagy- and lysosome-related protein markers, such as heat shock proteins, LC3A/B-II, and beclin-1, which are essential for Aβ phagocytosis in neurons (Maiti et al., 2017). Moreover, a curcumin derivative, CNB-001, serves as a 5-lipoxygenase inhibitor, inducing activation of the PERK/eIF2/ATF4 arm of the unfolded protein response and accelerating degradation of Aβ aggregates (Valera et al., 2013). These studies not only indicate that curcumin plays a critical role in the Aβ cascade, but also identify several new targets for AD treatment, such as Wnt/β-catenin and PERK/eIF2/ATF4 of the unfolded protein response.
Effect of curcumin on neuroinflammation
Neuroinflammation is one of the pathological factors in the vicious circle of AD pathogenesis, and is characterized by extensive glial activation and robust cytokine production at the site of damage. Curcumin targets numerous inflammatory signaling pathways, including biosynthesis and metabolism of arachidonic acid, pattern recognition receptor pathways on the surface of glial cells, and nuclear transcription factors (He et al., 2015). For example, IC50 values of curcumin for secretory phospholipase A2, cyclooxygenases-2, lipo-oxygenase, and microsomal prostaglandin E synthase-1 (which are involved in arachidonic acid metabolism) are 11.10, 93.36, 57.77, and 4.88 µM, respectively (Ahmad et al., 2014). Similarly, curcumin serves as a repressor of both toll-like receptors and NOD-like receptors (NLRs), sensors of Aβ and NFTs during neuroinflammation. Curcumin inhibits dimerization of toll-like receptor 4, resulting in marked reduction of proinflammatory cytokines (Youn et al., 2006). Recent studies have suggested that curcumin attenuates neurotoxicity and the related inflammatory response by suppressing nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome activation (Gong et al., 2015; Li et al., 2015). Curcumin may also act as an agonist of both peroxisome proliferator-activated receptor γ and nuclear factor erythroid-2 related factor 2, which regulate expression of various inflammatory cytokines (Innamorato et al., 2008; Wang et al., 2010). In vitro studies suggest that curcumin attenuates Aβ-induced inflammatory responses in microglia by suppressing the ERK1/2 and p38 signaling pathways (Shi et al., 2015). Moreover, in vivo studies using an AD rat model have shown that curcumin exerts a significant reduction in glial fibrillary acidic protein expression and astrocyte activity, contributing to the rescue of behavioral defects caused by Aβ intracerebral injection (Wang et al., 2013). These results imply that the powerful anti-inflammatory properties of curcumin may be responsible for inhibiting glial cell activation and alleviating Aβ pathology in AD.
Effect of curcumin on oxidative stress
As described, Aβ and phosphorylated-tau aggregation, inflammation, and oxidative stress form a vicious cycle in the brain, contributing to neuronal apoptosis and cognitive decline in AD. Thus, interventions to attenuate oxidative stress have been postulated as another approach in prevention and treatment of AD. Curcumin has excellent antioxidant properties, which elevate superoxide dismutase and catalase activity to conserve glutathione levels and decrease malonyldialdehyde accumulation in mouse models and humans (Soni and Kuttan, 1992; Soudamini et al., 1992; Ak and Gülçin, 2008; Dkhar and Sharma, 2010). A study using a homocysteine-induced rat aging model showed that curcumin (5, 15, or 45 mg/kg) treatment improved learning and memory function by significantly decreasing malonyldialdehyde and super oxide anion levels in the hippocampus (Ataie et al., 2010). However, elevated homocysteine plasma levels also led to abnormal DNA methylation, resulting in decline of cognitive performance (Fux et al., 2005). Curcumin inhibits DNA methyltransferase and may be responsible for its ability to improve cognitive impairment (Fang et al., 2007). Moreover, curcumin inhibits Aβ-induced oxidative stress and cell toxicity, which are dependent on telomerase. Telomerase is a ribonuclear protein complex that synthesizes and elongates telomeric DNA, protecting cells against senescence (Fang et al., 2007). These data suggest that telomerase may be a novel target of curcumin, providing a potential new therapeutic strategy for treating AD.
Curcumin in the Clinic
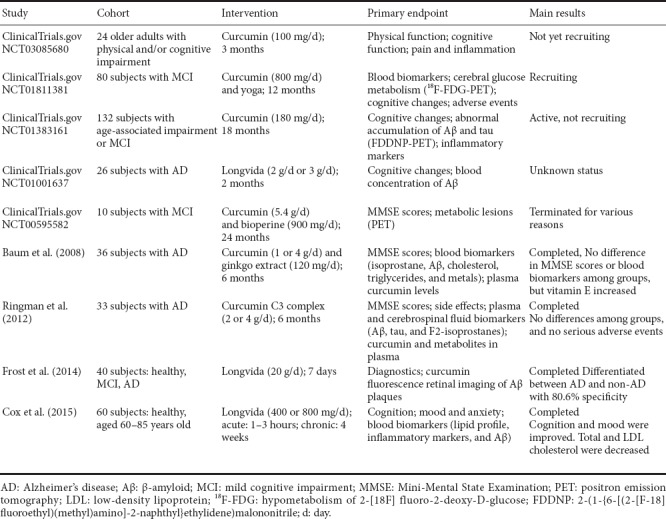
Extensive preclinical studies over the past decades have indicated the therapeutic potential of curcumin against a wide range of human chronic diseases. In addition, curcumin directly interacts with numerous cell signaling molecules such as pro-inflammatory cytokines, apoptotic proteins, and phosphorylase kinases. These studies provide a solid foundation for evaluating the efficacy of curcumin in clinical trials (Gupta et al., 2013). Until now, nine human trials of curcumin in AD interventions (including diagnosis, prevention and therapy) have been performed (Table 1). In diagnosis, a pilot study using curcumin as a fluorochrome for retinal imaging found that curcumin enabled Aβ visualization with excellent fluorescence properties. The retinal Aβ test was able to differentiate between AD and non-AD with 80.6% specificity (Frost et al., 2014). Additionally, Cox et al. (2015) showed that supplementation with solid lipid curcumin formulation (80 mg as Longvida®) improved cognitive function and reduced fatigue and psychological stress in a healthy older population, suggesting a protective potential of curcumin. Nevertheless, clinical studies on mild cognitive impairment and AD found no significant differences in cognitive function and biomarker measurements between placebo and intervention groups, although curcumin increased vitamin E levels and did not cause any adverse effects at a high dose (Baum et al., 2008). These studies suggest that curcumin may delay disease progression rather than improve biomarkers and cognitive function. It is possible that poor bioavailability of curcumin, selection of cohorts at an advanced stage of AD, and differences in the biology of rodent models and AD patients may be responsible for these failures in clinical trials. It is interesting to note that one clinical trial combining curcumin and Bioperine was terminated, while another trial of high-bioavailability curcumin formulation (Longvida) was not updated, yet both had enhanced bioavailability of curcumin (Table 1). Additionally, to the best of our knowledge, none of the existing models fully reproduce the complete pathology and process of AD. Many interventions, although successful in animal models, have failed in clinic trials (LaFerla and Green, 2012). This highlights the urgent need for a next-generation of animal models, which better recapitulate critical aspects of the disease spectrum and facilitate success in preclinical studies and human clinical trials. Thus, it is premature to conclude that there is no effect of curcumin in AD patients. More studies with better bioavailability and delivery strategies, larger numbers of patients at the asymptomatic stage, and longer treatment durations are highly desirable.
Table 1.
Clinical trials with curcumin in diagnosis, prevention, and therapy
Pharmacokinetic Studies and Commercial Formulations
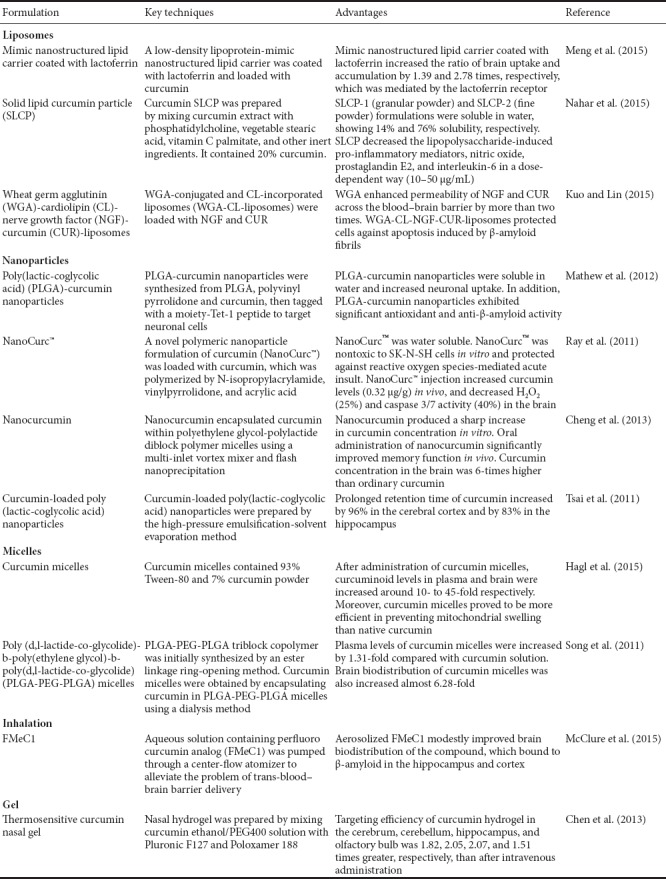
Curcumin is soluble in organic solvents, but insoluble in water (Wang et al., 1997). Although curcumin is safe and well-tolerated, absorption of curcumin is quite poor. Clinical studies in humans have shown that curcumin is generally safe even at high doses up to 8 g/d (Cheng et al., 2001), but with no detectable levels of the parent compound in the plasma unless patients ingest > 8 g (Lao et al., 2006). Moreover, curcumin availability is lower in the brain than other organs (Vareed et al., 2008). Curcumin undergoes extensive first-pass glucuronidation, resulting in rapid elimination in bile and urine (Ireson et al., 2001). Approximately 75% of curcumin can be detected in feces after a dietary dose (1 g/kg) administered to rats (Sharma et al., 2007). Similarly, curcumin declined rapidly and was unquantifiable within 3–6 hours after intake (Vareed et al., 2008). The main factors limiting curcumin bioavailability are low solubility, poor absorption, and rapid metabolism and elimination. Therefore, numerous studies have been directed at increasing curcumin bioavailability, including use of phospholipid complex formation, loading curcumin into liposomes and nanoparticle encapsulation, and intranasal administration (Table 2).
Table 2.
Commercial formulations of curcumin
Conclusions
Curcumin is one of the most studied phytochemical agents in the spice turmeric, displaying complex and multifaceted activities. There have been many reports on curcumin and its roles in AD. This review highlights the unique photophysical, chemical, and biological activities of curcumin as well as its properties throughout the course of AD. It shows high-affinity binding to Aβ and a strong fluorescence signal, making it a powerful diagnostic tool for AD. Many curcumin tracers have been developed to assess Aβ deposits in vivo, including the 18F curcumin derivatives, FMeCl and CRANAD-X. These probes have sufficiently long excitation and emission wavelengths for deep brain imaging, reasonable BBB permeability, low toxicity, and reasonable stability. Moreover, CRANAD-58 and CRANAD-3 can detect both soluble and insoluble Aβ species. Further, CRANAD-28 and FMeC1 play a dual role in imaging and therapy. There is concern that reduction of Aβ burden by curcumin derivatives may interfere with Aβ imaging. However, Aβ imaging is performed in the early hours after administration of curcumin derivatives, and its effect on Aβ levels is likely to be minimal at 6 months (Yanagisawa et al., 2011; Zhang et al., 2014). In contrast, prevention and treatment of Aβ requires long-term curcumin administration (Yanagisawa et al., 2015). Curcumin is abundant in an Asian-type diet and may reduce AD risk, consistent with lower AD prevalence in India. Evidence also suggests that curcumin consumption has diverse potential health benefits in the aged population. Apart from its role in diagnosis and prevention, curcumin acts in AD therapies as an antioxidant, anti-inflammatory agent, inhibitor of Aβ aggregation, and chelator of metal ions. Taken together, current research suggests that curcumin is one of the most promising and exciting compounds for development of AD therapeutics.
To date, one clinical study has evaluated the sensitivity and specificity of curcumin fluorochrome in retinal Aβ imaging. This study obtained positive results and has encouraged more clinical trials of curcumin-related Aβ probes in brain imaging. Besides, curcumin shows potential beneficial effects on human health, which may reduce risk factors of AD, and make it a life-long anti-aging nutraceutical. Although curcumin has multifaceted biological activity in AD animal models, its treatment in AD patients remains a challenge, and development of early AD diagnosis and new curcumin formulations are an active area of research.
Acknowledgments
We are grateful to Wei Zhou from the Institute of Natural Medicinal Chemistry & Green Chemistry, College of Light Industry and Chemical Engineering, Guangdong University of Technology in China for his useful suggestions.
Footnotes
Funding: This study was supported by a grant from the Department of Education of Guangdong Province of China, No. 2016KCXTD005.
Conflicts of interest: None declared.
Financial support: This study was supported by a grant from the Department of Education of Guangdong Province of China, No. 2016KCXTD005. The funder had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by James R, Stow A, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Ahmad W, Kumolosasi E, Jantan I, Bukhari SN, Jasamai M. Effects of novel diarylpentanoid analogues of curcumin on secretory phospholipase A2, cyclooxygenases, lipo-oxygenase, and microsomal prostaglandin e synthase‐1. Chem Biol Drug Des. 2014;83:670–681. doi: 10.1111/cbdd.12280. [DOI] [PubMed] [Google Scholar]
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of Curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimer's Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Araujo C, Leon L. Biological activities of curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–728. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- Arun N, Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr. 2002;57:41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B. Neuroprotective effects of the polyphenolic antioxidant agent, curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav. 2010;96:378–385. doi: 10.1016/j.pbb.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Bajda M, Guzior N, Ignasik M, Malawska B. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem. 2011;18:4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- Banerjee R. Effect of curcumin on the metal ion induced fibrillization of amyloid-β peptide. Spectrochim Acta Part A. 2014;117:798–800. doi: 10.1016/j.saa.2013.09.064. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L, Lam CWK, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Beharry C, Cohen LS, Di J, Ibrahim K, Briffa-Mirabella S, Alonso AdC. Tau-induced neurodegeneration: mechanisms and targets. Neurosci Bull. 2014;30:346–358. doi: 10.1007/s12264-013-1414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi A, Doggui S, Dao L, Ramassamy C. Challenges associated with curcumin therapy in Alzheimer disease. Expert Rev Mol Med. 2011;13:e34. doi: 10.1017/S1462399411002055. [DOI] [PubMed] [Google Scholar]
- Belviranlı M, Okudan N, Atalık KEN, Öz M. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology. 2013;14:187–196. doi: 10.1007/s10522-013-9422-y. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard GJ, Mytar J, Li RC, Klapstein GJ. The role of inflammatory processes in Alzheimer's disease. Inflammopharmacology. 2012;20:109–126. doi: 10.1007/s10787-012-0130-z. [DOI] [PubMed] [Google Scholar]
- Chase A. Alzheimer disease: advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10:239–239. doi: 10.1038/nrneurol.2014.71. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhi F, Jia X, Zhang X, Ambardekar R, Meng Z, Paradkar AR, Hu Y, Yang Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65:807–816. doi: 10.1111/jphp.12043. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer's disease Tg2576 mice. AAPS J. 2013;15:324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KK, Chan PS, Fan S, Kwan SM, Yeung KL, Wáng Y-XJ, Chow AHL, Wu EX, Baum L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer's disease mice using magnetic resonance imaging (MRI) Biomaterials. 2015;44:155–172. doi: 10.1016/j.biomaterials.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Condello C, Schain A, Grutzendler J. Multicolor time-stamp reveals the dynamics and toxicity of amyloid deposition. Sci Rep. 2011;1:19. doi: 10.1038/srep00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Cui M, Ono M, Kimura H, Liu B, Saji H. Synthesis and structure−affinity relationships of novel dibenzylideneacetone derivatives as probes for β-amyloid plaques. J Med Chem. 2011;54:2225–2240. doi: 10.1021/jm101404k. [DOI] [PubMed] [Google Scholar]
- DiSilvestro RA, Joseph E, Zhao S, Bomser J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012;11:79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhar P, Sharma R. Effect of dimethylsulphoxide and curcumin on protein carbonyls and reactive oxygen species of cerebral hemispheres of mice as a function of age. Int J Dev Neurosci. 2010;28:351–357. doi: 10.1016/j.ijdevneu.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7:e31211. doi: 10.1371/journal.pone.0031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM. Why pleiotropic interventions are needed for Alzheimer's disease. Mol Neurobiol. 2010;41:392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S, Kanagasingam Y, Macaulay L, Koronyo-Hamaoui M, Koronyo Y, Biggs D, Verdooner S, Black K, Taddei K, Shah T. Retinal amyloid fluorescence imaging predicts cerebral amyloid burden and alzheimer's disease. Alzheimers Dement. 2014;10:P234–P235. [Google Scholar]
- Fux R, Kloor D, Hermes M, Röck T, Proksch B, Grenz A, Delabar U, Bücheler R, Igel S, Mörike K. Effect of acute hyperhomocysteinemia on methylation potential of erythrocytes and on DNA methylation of lymphocytes in healthy male volunteers. Am J Physiol Renal Physiol. 2005;289:F786–792. doi: 10.1152/ajprenal.00465.2004. [DOI] [PubMed] [Google Scholar]
- Garcia‐Alloza M, Borrelli L, Rozkalne A, Hyman B, Bacskai B. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A. β-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gong Z, Zhou J, Li H, Gao Y, Xu C, Zhao S, Chen Y, Cai W, Wu J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS‐induced septic shock. Mol Nutr Food Res. 2015;59:2132–2142. doi: 10.1002/mnfr.201500316. [DOI] [PubMed] [Google Scholar]
- Goozee K, Shah T, Sohrabi HR, Rainey-Smith S, Brown B, Verdile G, Martins R. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease. Br J Nutr. 2016;115:449–465. doi: 10.1017/S0007114515004687. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hagl S, Kocher A, Schiborr C, Kolesova N, Frank J, Eckert GP. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice–Impact on bioavailability. Neurochem Int. 2015;89:234–242. doi: 10.1016/j.neuint.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, Frölich L, Riepe MW, Dodel R, Leyhe T, Bertram L. The future of Alzheimer's disease: the next 10 years. Prog Neurobiol. 2011;95:718–728. doi: 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked. Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Maiti P, Ma Q, Zuo X, Jones MR, Cole GM, Frautschy SA. Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother. 2015;15:629–637. doi: 10.1586/14737175.2015.1044981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato NG, Rojo AI, García-Yagüe ÁJ, Yamamoto M, De Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr Res Pract. 2010;4:191–195. doi: 10.4162/nrp.2010.4.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmon S, Tvaroška I. Molecular dynamic studies of amyloid-beta interactions with curcumin and Cu2+ ions. Chem Papers. 2015;69:1262–1276. [Google Scholar]
- Kundaikar HS, Degani MS. Insights into the Interaction mechanism of ligands with Aβ42 based on molecular dynamics simulations and mechanics: implications of role of common binding site in drug design for alzheimer's disease. Chem Biol Drug Des. 2015;86:805–812. doi: 10.1111/cbdd.12555. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin CC. Rescuing apoptotic neurons in Alzheimer's disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int J Nanomedicine. 2015;10:2653. doi: 10.2147/IJN.S79528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006320. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Li S, Li Y, Wang X, Liu B, Fu Q, Ma S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Liu H, Li Z, Qiu D, Gu Q, Lei Q, Mao L. The inhibitory effects of different curcuminoids on β-amyloid protein, β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci Lett. 2010;485:83–88. doi: 10.1016/j.neulet.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Lopez OL. The growing burden of Alzheimer's disease. Am J Manag Care. 2011;17:S339–345. [PubMed] [Google Scholar]
- Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D, Campos-Peña V. Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer's disease? Oxid Med Cell Longev. 2014:497802. doi: 10.1155/2014/497802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Rossignol J, Dunbar G. Curcumin modulates molecular chaperones and autophagy-lysosomal pathways in vitro after exposure to Aβ42. J Alzheimer Dis Parkinsonism. 2017;7:299. [Google Scholar]
- Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer's disease. PLoS One. 2012;7:e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Mason NS, Lopresti BJ, Klunk WE. Development of positron emission tomography β-amyloid plaque imaging agents. Semin Nucl Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R, Yanagisawa D, Stec D, Abdollahian D, Koktysh D, Xhillari D, Jaeger R, Stanwood G, Chekmenev E, Tooyama I. Inhalable curcumin: offering the potential for translation to imaging and treatment of Alzheimer's disease. J Alzheimers Dis. 2015;44:283–295. doi: 10.3233/JAD-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Asghar S, Gao S, Su Z, Song J, Huo M, Meng W, Ping Q, Xiao Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer's disease. Colloids Surf B Biointerfaces. 2015;134:88–97. doi: 10.1016/j.colsurfb.2015.06.025. [DOI] [PubMed] [Google Scholar]
- Metaxas A, Kempf SJ. Neurofibrillary tangles in Alzheimer's disease: elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen Res. 2016;11:1579–1581. doi: 10.4103/1673-5374.193234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohorko N, Repovš G, Popović M, Kovacs GG, Bresjanac M. Curcumin labeling of neuronal fibrillar tau inclusions in human brain samples. J Neuropathol Exp Neurol. 2010;69:405–414. doi: 10.1097/NEN.0b013e3181d709eb. [DOI] [PubMed] [Google Scholar]
- Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem. 2014;80:175–183. doi: 10.1016/j.ejmech.2014.04.050. [DOI] [PubMed] [Google Scholar]
- Nahar PP, Slitt AL, Seeram NP. Anti-inflammatory effects of novel standardized solid lipid curcumin formulations. J Med Food. 2015;18:786–792. doi: 10.1089/jmf.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir Abbas Bukhari S, Jantan I. Synthetic curcumin analogs as inhibitors of β-amyloid peptide aggregation: potential therapeutic and diagnostic agents for Alzheimer's disease. Mini Rev Med Chem. 2015;15:1110–1121. doi: 10.2174/138955751513150923101841. [DOI] [PubMed] [Google Scholar]
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's β‐amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Park K, Seo Y, Kim MK, Kim K, Kim YK, Choo H, Chong Y. A curcumin-based molecular probe for near-infrared fluorescence imaging of tau fibrils in Alzheimer's disease. Org Biomol Chem. 2015;13:11194–11199. doi: 10.1039/c5ob01847a. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Lanari A, Silvestrelli G, Saggese E, Reboldi P. Diagnosing prodromal Alzheimer's disease: role of CSF biochemical markers. Mech Ageing Dev. 2006;127:129–132. doi: 10.1016/j.mad.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Parr C, Mirzaei N, Christian M, Sastre M. Activation of the Wnt/β-catenin pathway represses the transcription of the β-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J. 2015;29:623–635. doi: 10.1096/fj.14-253211. [DOI] [PubMed] [Google Scholar]
- Patil R, Gangalum PR, Wagner S, Portilla‐Arias J, Ding H, Rekechenetskiy A, Konda B, Inoue S, Black KL, Ljubimova JY. Curcumin targeted, polymalic acid‐based MRI contrast agent for the detection of Aβ plaques in Alzheimer's disease. Macromol Biosci. 2015;15:1212–1217. doi: 10.1002/mabi.201500062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone L, Mothes E, Vignes M, Mockel A, Figueroa C, Miquel MC, Maddelein ML, Faller P. Copper transfer from Cu–Aβ to human serum albumin inhibits aggregation, radical production and reduces Aβ toxicity. Chembiochem. 2010;11:110–118. doi: 10.1002/cbic.200900474. [DOI] [PubMed] [Google Scholar]
- Pinkaew D, Changtam C, Tocharus C, Thummayot S, Suksamrarn A, Tocharus J. Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Aβ25-35. Neurochem Int. 2015;80:110–119. doi: 10.1016/j.neuint.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Priyadarsini KI. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J Photochem Photobiol C. 2009;10:81–95. [Google Scholar]
- Ran C, Xu X, Raymond SB, Ferrara BJ, Neal K, Bacskai BJ, Medarova Z, Moore A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-β deposits. J Am Chem Soc. 2009;131:15257–15261. doi: 10.1021/ja9047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PP, Mohamed T, Teckwani K, Tin G. Curcumin binding to beta amyloid: a computational study. Chem Biol Drug Des. 2015;86:813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- Ray B, Bisht S, Maitra A, Maitra A, Lahiri DK. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: implications for Alzheimer's disease. J Alzheimers Dis. 2011;23:61–77. doi: 10.3233/JAD-2010-101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke AA, Gestwicki JE. Structure-activity Relationships of amyloid beta-aggregation inhibitors based on curcumin: influence of linker length and flexibility. Chem Biol Drug Des. 2007;70:206–215. doi: 10.1111/j.1747-0285.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, Gylys KH, Badmaev V, Heath DD, Apostolova LG. Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS One. 2013;8:e61052. doi: 10.1371/journal.pone.0061052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rokka J, Snellman A, Zona C, La Ferla B, Nicotra F, Salmona M, Forloni G, Haaparanta-Solin M, Rinne JO, Solin O. Synthesis and evaluation of a 18F-curcumin derivate for β-amyloid plaque imaging. Bioorg Med Chem. 2014;22:2753–2762. doi: 10.1016/j.bmc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Ryu EK, Choe YS, Lee KH, Choi Y, Kim BT. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for β-amyloid plaque imaging. J Med Chem. 2006;49:6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- Safouris A, Tsivgoulis G, N Sergentanis T, Psaltopoulou T. Mediterranean diet and risk of dementia. Curr Alzheimer Res. 2015;12:736–744. doi: 10.2174/1567205012666150710114430. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. Adv Exp Biol. 2007;10:81–95. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- Shi X, Zheng Z, Li J, Xiao Z, Qi W, Zhang A, Wu Q, Fang Y. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: involvement of ERK1/2 and p38 signaling pathways. Neurosci Lett. 2015;594:105–110. doi: 10.1016/j.neulet.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354:116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Soni K, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36:273–273. [PubMed] [Google Scholar]
- Soto C. Plaque busters: strategies to inhibit amyloid formation in Alzheimer's disease. Mol Med Today. 1999;5:343–350. doi: 10.1016/s1357-4310(99)01508-7. [DOI] [PubMed] [Google Scholar]
- Soudamini K, Unnikrishnan M, Soni K, Kuttan R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J Physiol Pharmacol. 1992;36:239–239. [PubMed] [Google Scholar]
- Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Tu P, Fu H, Cui M. Compounds for imaging amyloid-β deposits in an Alzheimer's brain: a patent review. Expert Opin Ther Pat. 2015;25:413–423. doi: 10.1517/13543776.2015.1007953. [DOI] [PubMed] [Google Scholar]
- Valera E, Dargusch R, Maher PA, Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer's disease. J Neurosci. 2013;33:10512–10525. doi: 10.1523/JNEUROSCI.5183-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M, Vats A, Taneja V. Toxic species in amyloid disorders: oligomers or mature fibrils. Ann Indian Acad Neurol. 2015;18:138. doi: 10.4103/0972-2327.144284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C. Adult hippocampal neurogenesis, aging and neurodegenerative diseases: possible strategies to prevent cognitive impairment. Curr Top Med Chem. 2015;15:2175–2192. doi: 10.2174/1568026615666150610141524. [DOI] [PubMed] [Google Scholar]
- Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, Ding JQ, Chen SD. PPARγ agonist curcumin reduces the amyloid-β-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20:1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim JR, Lee SB, Kim YJ, Jung MY, Kwon HW, Ahn YJ. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer's disease drosophila models. BMC Complement Altern Med. 2014;14:88. doi: 10.1186/1472-6882-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin H, Wang L, Shuboy A, Lou J, Han B, Zhang X, Li J. Curcumin as a potential treatment for Alzheimer's disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med. 2013;41:59–70. doi: 10.1142/S0192415X13500055. [DOI] [PubMed] [Google Scholar]
- Wanninger S, Lorenz V, Subhan A, Edelmann FT. Metal complexes of curcumin–synthetic strategies, structures and medicinal applications. Chem Soc Rev. 2015;44:4986–5002. doi: 10.1039/c5cs00088b. [DOI] [PubMed] [Google Scholar]
- Wu A, Noble EE, Tyagi E, Ying Z, Zhuang Y, Gomez-Pinilla F. Curcumin boosts DHA in the brain: Implications for the prevention of anxiety disorders. Biochim Biophys Acta. 2015;1852:951–961. doi: 10.1016/j.bbadis.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. Adv Exp Med Biol. 2015;863:79–94. doi: 10.1007/978-3-319-18365-7_4. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa D, Ibrahim NF, Taguchi H, Morikawa S, Hirao K, Shirai N, Sogabe T, Tooyama I. Curcumin derivative with the substitution at C-4 position, but not curcumin, is effective against amyloid pathology in APP/PS1 mice. Neurobiol Aging. 2015;36:201–210. doi: 10.1016/j.neurobiolaging.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Yanagisawa D, Amatsubo T, Morikawa S, Taguchi H, Urushitani M, Shirai N, Hirao K, Shiino A, Inubushi T, Tooyama I. In vivo detection of amyloid β deposition using 19F magnetic resonance imaging with a 19F-containing curcumin derivative in a mouse model of Alzheimer's disease. Neuroscience. 2011;184:120–127. doi: 10.1016/j.neuroscience.2011.03.071. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Ye S, Wang TT, Cai B, Wang Y, Li J, Zhan JX, Shen GM. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer's disease model rats. Neural Regen Res. 2017;12:1479–1484. doi: 10.4103/1673-5374.215260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur J Pharm Sci. 2011;42:540–546. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tian Y, Li Z, Tian X, Sun H, Liu H, Moore A, Ran C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer's disease. J Am Chem Soc. 2013;135:16397–16409. doi: 10.1021/ja405239v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tian Y, Yuan P, Li Y, Yaseen MA, Grutzendler J, Moore A, Ran C. A bifunctional curcumin analogue for two-photon imaging and inhibiting crosslinking of amyloid beta in Alzheimer's disease. Chem Commun (Camb) 2014;50:11550–11553. doi: 10.1039/c4cc03731f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tian Y, Zhang C, Tian X, Ross AW, Moir RD, Sun H, Tanzi RE, Moore A, Ran C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer's disease. Proc Natl Acad Sci U S A. 2015;112:9734–9739. doi: 10.1073/pnas.1505420112. [DOI] [PMC free article] [PubMed] [Google Scholar]


