Abstract
Background
The excitatory amino acid domoic acid, a glutamate and kainic acid analog, is the causative agent of amnesic shellfish poisoning in humans. No studies to our knowledge have investigated the potential contribution to short-term neurotoxicity of the brain microglia, a cell type that constitutes circa 10% of the total glial population in the brain. We tested the hypothesis that a short-term in vitro exposure to domoic acid, might lead to the activation of rat neonatal microglia and the concomitant release of the putative neurotoxic mediators tumor necrosis factor-α (TNF-α), matrix metalloproteinases-2 and-9 (MMP-2 and -9) and superoxide anion (O2-).
Results
In vitro, domoic acid [10 μM-1 mM] was significantly neurotoxic to primary cerebellar granule neurons. Although neonatal rat microglia expressed ionotropic glutamate GluR4 receptors, exposure during 6 hours to domoic acid [10 μM-1 mM] had no significant effect on viability. By four hours, LPS (10 ng/mL) stimulated an increase in TNF-α mRNA and a 2,233 % increase in TNF-α protein In contrast, domoic acid (1 mM) induced a slight rise in TNF-α expression and a 53 % increase (p < 0.01) of immunoreactive TNF-α protein. Furthermore, though less potent than LPS, a 4-hour treatment with domoic acid (1 mM) yielded a 757% (p < 0.01) increase in MMP-9 release, but had no effect on MMP-2. Finally, while PMA (phorbol 12-myristate 13-acetate) stimulated O2- generation was elevated in 6 hour LPS-primed microglia, a similar pretreatment with domoic acid (1 mM) did not prime O2- release.
Conclusions
To our knowledge this is the first experimental evidence that domoic acid, at in vitro concentrations that are toxic to neuronal cells, can trigger a release of statistically significant amounts of TNF-α and MMP-9 by brain microglia. These observations are of considerable pathophysiological significance because domoic acid activates rat microglia several days after in vivo administration.
Background
Microglia are leukocytes derived outside the central nervous system in the bone marrow, from where they enter the circulation as monocytes and migrate into the brain during late embryonic life establishing permanent residency [1]. Two different phenotypic forms of microglia are known: the activated but nonphagocytic microglia, which play a role in inflammatory pathologies, and the reactive or phagocytic microglia which participate actively in trauma, infection and neuronal degeneration [2].
In vivo as well as in vitro administration of LPS rapidly and potently activates rat adult and neonatal microglia (for review see Ref. [3]). Accompanying both adult and neonatal microglia activation by LPS [4] is a concomitant release of numerous secretory products (for review see Ref. [3]) that include tissue plasminogen activator [5], reactive oxygen species such as O2- [6,7], cytokines like TNF-α [7,8] and proteases, in particular MMP-9 [7,9], in a time- and concentration-dependent manner. These mediators have been implicated in sublethal and lethal neuronal and glial injury [10], neuronal degeneration [3,11,12] that could potentially affect neurodevelopment in the immature brain.
Exposure to the marine toxin domoic acid, an excitotoxic amino acid structurally similar to kainic acid and to the neurotransmitter glutamic acid, has been shown to lead to amnesic shellfish poisoning in humans, a condition that can lead to death in extreme cases [13]. Currently, the Center for Food Safety and Applied Nutrition, US. Food and Drug Administration has included amnesic shellfish poisoning as one of the "...five recognized fish poisoning syndromes in the United States...", together with paralytic, neurotoxic, diarrhetic and ciguatera fish poisoning (FDA website: http://vm.cfsan.fda.gov/~dms/haccp-2f.html) [14]. Recent behavioral toxicity studies in rodents, have confirmed that domoic acid is a very rapid and potent neurotoxin in newborn rats, at doses far lower than in adult animals [15-17]. Even though hippocampal pyramidal neurons and mossy fiber terminals appear to be selectively targeted in vivo by domoic acid in rat [18] and cynomolgus monkey brain [19], recently domoic acid was shown to affect glutamate uptake by astrocytes in vitro and "...thus produced neurotoxicity..." [20]. Of interest is the fact that no studies to our knowledge have investigated the potential contribution of brain microglia to neonatal neurotoxicity after a short-term exposure to domoic acid [21].
The possibility that domoic acid may activate neonatal microglia both in vitro and in vivo is suggested by recent observations with kainic acid, a domoic acid and glutamate analog that can induce upregulation of neonatal microglia scavenger receptor mRNA [22] and tissue plasminogen activator release [23]. The putative molecular explanation for these observations has been that kainic acid activates the ionotropic AMPA glutamate GluR4 subunit which has recently been shown to be expressed by activated microglia following transient forebrain ischemia in vivo[24]. Because this subunit of the AMPA glutamate receptor has affinity for domoic acid [25-27], we hypothesized that domoic acid might activate neonatal microglia in vitro and lead to concomitant mediator release [21].
The purpose of this investigation was to determine if a short-term in vitro exposure to domoic acid might activate rat neonatal microglia and cause mediator release. Using LPS, which we have previously shown is a rapid and potent in vitro activator of neonatal microglia [7] as our positive control, we focused our investigation on three microglia mediators which are released upon LPS activation, namely TNF-α, MMP-9 and O2- [7]. Our present study demonstrates that in contrast to LPS, during a 6 hour in vitro exposure to domoic acid, neonatal rat microglia release small, but statistically significant amounts of TNF-α and MMP-9. Furthermore our observations suggest that in vivo, brain microglia as well as the mediators released by this cell type in brain tissue, would appear to play a limited role in the rapid and potent domoic acid neurotoxicity observed in neonatal rats after a short-term administration of domoic acid.
Results
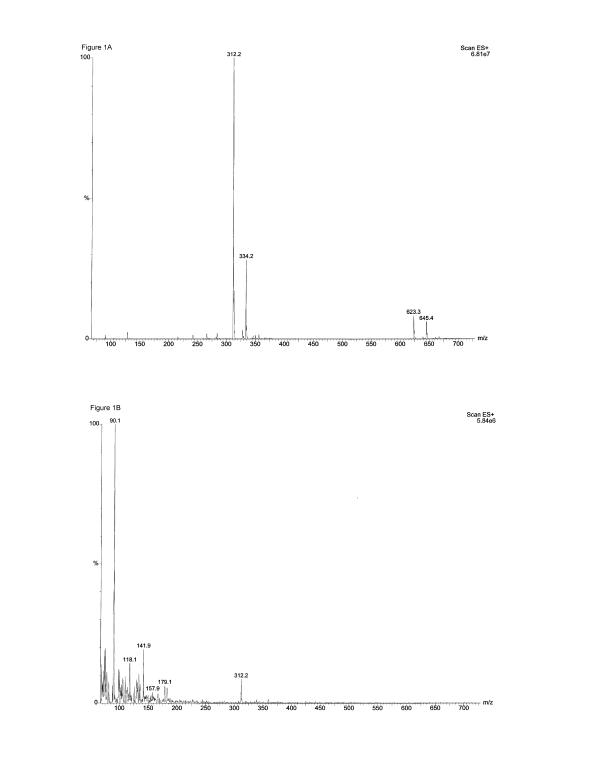
Determination of domoic acid by electrospray mass spectrometry
The presence of undegraded domoic acid in both stock solution (12.5 mM) and dilutions prepared with LPS-free water was confirmed by electrospray mass spectrometry prior to the experiments. As shown for the 12.5 mM domoic acid stock solution in Fig. 1A, the dominate ion in the spectrum was m/z 312.2 which is the pseudomolecular ion [M+H]+ of domoic acid. Also present in the spectrum were m/z 334.2, the sodium adduct [M+Na]+ of domoic acid, m/z 623.3, the dimer [2M+H]+ of domoic acid, and m/z 645.4 [2M+Na]+, the sodium adduct of the dimer. The dimer was formed in the gas phase due to the high concentration of the sample. Mass spectra for dilution of 2.5, 0.25 and 0.025 mM also showed as the dominate ion m/z 312, domoic acid [M+H]+ (data not shown). As shown in Fig. 1B, for the lowest domoic acid concentration used in our studies, namely 0.0025 mM domoic acid, although background noise dominated the sample, m/z 312.2, the domoic acid [M+H]+ was clearly seen in the spectrum.
Figure 1.
Determination of Domoic acid by electrospray mass spectrometry.(A) The electrospray mass spectrometry spectrum of 12.5 mM Domoic acid shows that the dominate ion in the spectrum is m/z 312.2, the pseudomolecular ion [M+H+] of Domoic acid, demonstrating that undegraded Domoic acid was present in the 12.5 mM stock solution; (B) The electrospray mass spectrometry spectrum of 0.0025 mM Domoic acid, the highest dilution used in the experiments, also shows the presence of undegraded Domoic acid.
Immunofluorescent visualization of CD11b/c and glutamate receptor subunit GluR4 in rat neonatal microglia
It has recently been reported that transient forebrain ischemia leads to the expression of the inotropic AMPA glutamate receptor GluR4 subunit in vivo[24] in rat neonatal microglia. In order to ascertain that under our experimental conditions rat neonatal microglia expressed the GluR4 subunit, we studied the expression of the AMPA receptor GluR4 by immunohistochemistry. The top left photo in Fig. 2 shows a bright field, phase contrast view of microglia that were incubated in the absence of the primary antibodies against GluR4 and CD11b/c. Note that the microglia are irregular in shape and show numerous elongated processes extending from the bodies of the cells. In addition, numerous vesicular structures are present in the cytoplasm of the microglia. The top right photo shows the same microglia when viewed using the FITC filter configuration. Note that the vesicular structures exhibit an intense autofluorescence, but that little or no fluorescence is evident over most of the microglia cell bodies or in the elongated processes. Interestingly, this same pattern of autofluorescence was evident when the microglia were viewed with the TRITC filter configuration (not shown). The middle left photo shows a bright field, phase contrast view of microglia that had been labeled with the antibodies against the glutamate receptor GluR4 subunit and the leukocyte marker CD11b/c. Again, the microglia are irregular in shape, with elongated processes extending from the cell bodies and numerous vesicles present in the cytoplasm. The middle right photo (green) shows this same field when viewed using the FITC filter configuration. As evident, the microglia not only contain fluorescent vesicles, but also exhibit a prominent speckled pattern of fluorescence over the cell bodies and the elongated processes. The fact that this speckled pattern of labeling is not present in the samples that were incubated without the primary GluR4 antibody (compare with top right photo) strongly indicates that the speckled pattern represents specific labeling of GluR4 subunit. The lower left photo shows that these same microglia exhibited intense labeling of CD11b/c (red), which confirms that the microglia are of leukocytic origin. In the bottom right photo the images showing the GluR4 and the CD11b/c labeling have been superimposed (yellow). Together, these results indicate that GluR4 glutamate receptor subunits are present on the cell surface and that these cells are, in fact, microglia.
Figure 2.
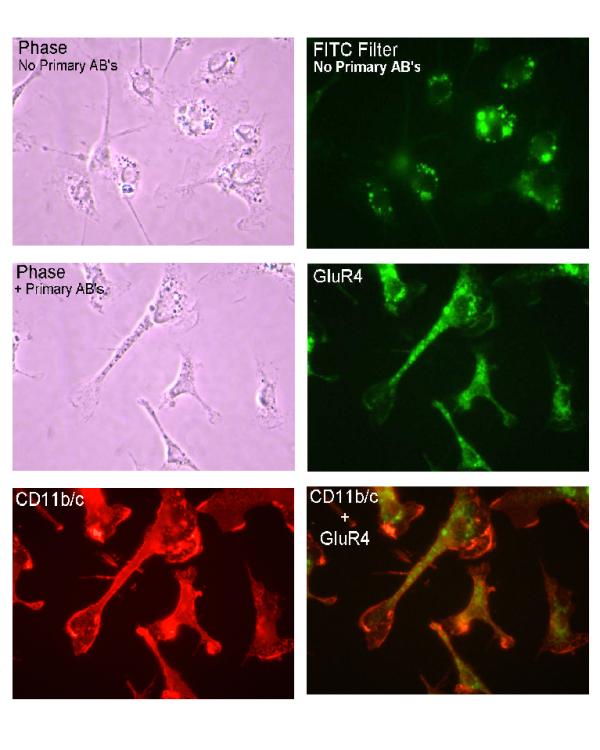
Immunofluorescent visualization of CD11b/c and ionotropic glutamate receptor subunit GluR4 in neonatal rat brain microglia. The integrin CD11b/c and the ionotropic glutamate receptor subunit GluR4 were visualized using the dual-labeling indirect immunofluorescent technique (See Materials and Methods). (Top left) bright field, phase contrast view of microglia incubated in the absence of the primary antibodies against GluR4 and CD11b/c. (Top right) same microglia viewed using FITC filter configuration. Note vesicular structures exhibiting intense autofluorescence, but little or no fluorescence over most of the microglia cell bodies or the elongated processes. (Middle left) bright field, phase contrast view of microglia labeled with anti-glutamate receptor GluR4 subunit and the leukocyte marker CD11b/c. (Middle right)(green) same field viewed using the FITC filter configuration. Microglia not only contain fluorescent vesicles, but exhibit a prominent speckled pattern of fluorescence over the cell bodies and the elongated processes. Absence of speckled pattern of labeling in cells incubated without primary GluR4 antibody (compare with top right photo) suggests the speckled pattern represents specific labeling of GluR4 subunit. (Lower left) same microglia exhibited intense labeling of CD11b/c (red), which confirms microglia are of leukocytic origin. (Bottom right) GluR4 and CD11b/c superimposed labeling (yellow). Approximate magnification of the original was × 525.
Effect of domoic acid on cerebellar granule cell and rat neonatal microglia viability
Domoic acid has been reported to be neurotoxic to neuronal tissue in vitro in concentrations ranging from 10 μM to 20 mM [28-33]. In order to determine a concentration of domoic acid that was neurotoxic in vitro during a 6 hour exposure, we investigated the concentration-dependent effect of domoic acid on cerebellar granule neurons using the WST-1 viability assay. This assay measures cell viability as a function of cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells to formazan. As shown in Fig. 3C and 3D the viability of primary cerebellar granule cells exposed for up to 6 hours to domoic acid [10 μM-1 mM] was significantly affected (P < 0.05). In contrast to our observations with cerebellar granule neurons, domoic acid even at the highest neurotoxic concentration tested in our in vitro study, namely [1 mM] did not affect microglia viability during a 6 hour exposure (Fig 3A and 3B). Because 1 mM domoic acid was clearly neurotoxic in our studies, this concentration was selected for all in vitro experiments with microglia. LPS (10 ng/ml) did not affect microglia viability in vitro during a 6 hour exposure (data not shown).
Figure 3.
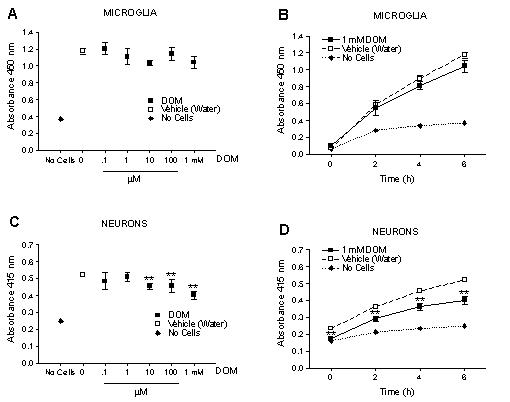
Effect of a 6 hour exposure of Domoic acid on neonatal rat brain microglia and primary cerebellar granule cell viability as determined by the WST-1 assay (See Materials and Methods). Domoic acid did not affect viability of neonatal rat brain microglia in either a (A) concentration or (B) time-dependent manner. Domoic acid [10 μM – 1 mM] significantly affected viability of primary cerebellar granule cells in both a (C) concentration and (D) time-dependent manner. Data (absorbance of formazan formation at 450 nM) are expressed as mean ± SD of values obtained from one representative experiment (n = 4–8). *p < 0.05 vs vehicle control.
Effect of LPS and domoic acid on TNF-α mRNA expression from rat neonatal microglia
Activation of microglia has been shown to cause the release of cytokines such as TNF-α [7,8]. As shown in Fig. 4A, stimulation of microglia with LPS [10 ng/ml] resulted in a time-dependent increase in TNF-α mRNA expression relative to controls as determined by semi-quantitative RT-PCR at the linear range of amplification (20 cycles). Without LPS treatment (control), TNF-α mRNA expression was undetectable. As shown in Fig. 5, the relative levels of TNF-α expression increased 4.5 fold by 1 hour and remained elevated throughout the 6 hour observation period. In contrast to the marked increase in TNF-α expression induced by LPS, as shown in Fig. 4B, domoic acid [1 mM] induced a less potent yet time-dependent increase in TNF-α mRNA expression as determined by semi-quantitative RT-PCR over the linear range of amplification (25 cycles). As shown in Fig. 5, although not statistically significant, the relative levels of TNF-α expression increased two-fold by 4 hours and remained elevated during the next two hours.
Figure 4.
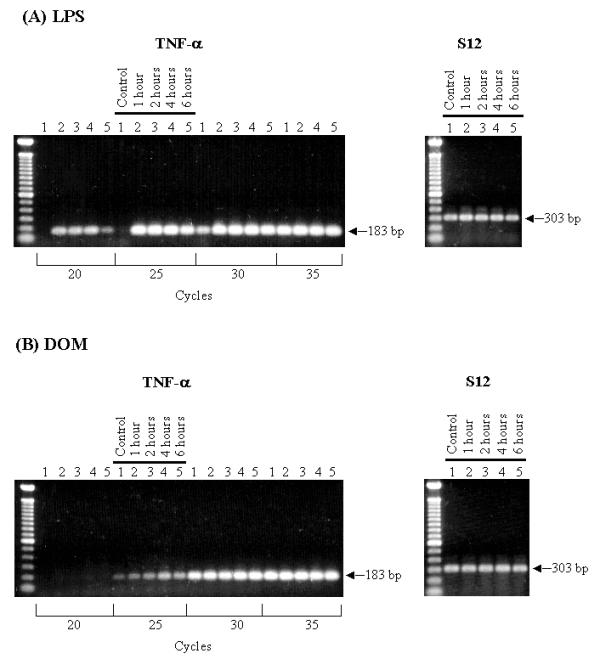
RT-PCR transcript analysis of TNF-α gene expression. Neonatal rat brain microglia (2.8–5 × 106 cells/culture dish) were treated with (A) LPS [10 ng/mL] or (B) Domoic acid [1 mM] for 1 to 6 hours. (See Materials and Methods). Amplification of TNF-α and S12 genes shows the predicted fragment size after separation on a 1.5 % agarose gel and visualization by SYBRR Gold nucleic acid staining. The S12 rRNA gene product was amplified for 25 cycles and demonstrates equal loading of the gels. Quantification of the TNF-α product was done after 20 cycles (LPS) or 25 cycles (Domoic acid). The cycle numbers used for quantification were shown to be in the linear range.
Figure 5.
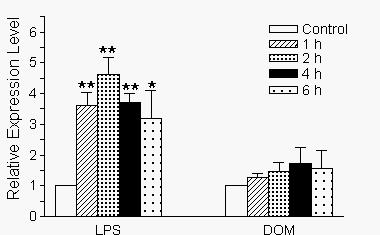
Alterations in the time-dependent expression levels of TNF-α and ribosomal S12 genes in neonatal rat brain microglia after treatment with LPS or Domoic acid. Levels of gene expression were quantified as described in Materials and Methods and were normalized to sl2 rRNA. Relative expression levels were calculated by dividing the experimental level at each time point by the level observed in the control. Expression is relative to steady-state levels in control (1.00). Data (relative expression level) is expressed as mean ± SE of 3 independent experiments. *p < 0.05 or **p < 0.01 vs. vehicle control.
Effect of domoic acid and LPS on rat neonatal microglia release of TNF-α
In order to determine if the increase in TNF-α mRNA after a short term stimulation with either domoic acid [1 mM] or LPS [10 ng/mL] led to a concomitant production of TNF-α protein, the cell-free media corresponding to the same microglia used for TNF-α mRNA analysis were assayed with an ultrasensitive rat-specific ELISA. As expected and shown in Fig. 6, LPS [10 ng/mL] stimulated microglia time-dependent TNF-α release: by 4 hours TNF-α levels increased 2,233 % and were 298.7 ± 3.9 pg/mL (LPS-treated, n = 4) vs. 12.8 ± 2.6 pg/mL (untreated controls, n = 8), P < 0.01. In contrast to LPS, levels of immunoreactive TNF-α in the tissue culture media increased 53.3 % 4 hours after microglia were treated with domoic acid [1 mM] and were 28.2 ± 3.6 pg/mL (domoic acid-treated, n = 6) vs. 18.4 ± 1.99 pg/mL (untreated controls, n = 10), P < 0.01. Interestingly, the 4 hour time point corresponded to the highest level of TNF-α mRNA expression shown in Fig. 5.
Figure 6.
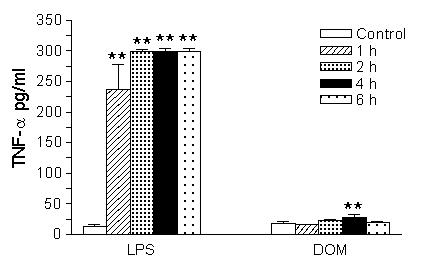
The time-dependent effect of LPS and Domoic acid on neonatal rat brain microglia TNF-α release. Neonatal rat brain microglia (2.8–5 × 106 cells/culture dish) were treated with Domoic acid [1 mM] or LPS [10 ng/mL] for 1–6 hours. TNF-α was determined as described under Materials and Methods. Data (pg/mL) are expressed as mean ± SE of 2 independent experiments. **p < 0.01 vs. vehicle control.
Effect of domoic acid and LPS on rat neonatal microglia release of MMP-2 and MMP-9
We have recently shown that a stimulation of microglia with LPS will lead to both TNF-α generation and MMP-9 production, a mediator that has been reported to be toxic to neuronal tissue [7]. As shown in Fig. 7, when microglia were stimulated with LPS [10 ng/mL], an increase in MMP-9 and MMP-2 release was observed: by 4 hours MMP-9 inverse O.D. levels increased 2,100 % and were respectively 0.198 ± 0.009 (LPS-treated, n = 3) vs. 0.009 ± 0.001 (untreated controls, n = 3), P < 0.01. In contrast to LPS, a 757 % increase in MMP-9 levels was observed in the tissue culture media 4 hours after microglia were treated with domoic acid [1 mM]: inverse O.D. levels were respectively 0.06 ± 0.01 (domoic acid-treated, n = 3) vs. 0.007 ± 0.001 (untreated controls, n = 3), P < 0.01. Domoic acid [1 mM] had no effect on MMP-2 release.
Figure 7.
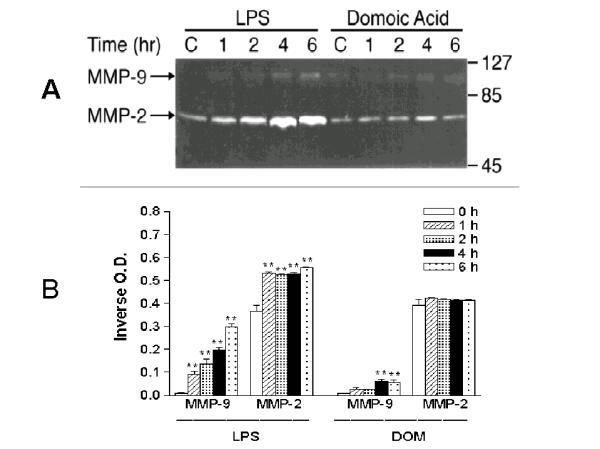
The time-course of MMP-2 and MMP-9 expression in neonatal rat brain microglia cultured in the presence of LPS or Domoic acid. MMP-2 and MMP-9 were determined as described in Materials and Methods. (A) SDS-PAGE zymography (B) bar-graph depicting the quantitated results. Neonatal rat brain microglia (2.8–5 × 106 cells/culture dish) were treated with LPS [10 ng/ml] or Domoic acid [1 mM] for 1–6 hours. Data (inverse o.d. units) are expressed as mean ± SE of values obtained from 3 independent experiments. **p < 0.01 vs. vehicle control.
Effect of domoic acid and LPS on rat neonatal microglia release of O2-
We have shown that while unprimed naive microglia release low levels of O2-, pretreatment with LPS results in an increase of PMA-stimulated O2- [7]. As is depicted in Fig. 8A, when microglia were pretreated for 6 hours with LPS [10 ng/mL], there was, as expected, an increase in PMA [1 μM]-stimulated O2- production: 6.5 ± 0.9 nmoles/2 hours (LPS-pretreated, n = 3) vs. 1.7 ± 0.07 nmoles/2 hours (untreated controls, n = 3), P < 0.01. In contrast, a 6 hour pretreatment of microglia with domoic acid [1 mM], yielded no statistically significant increase in PMA [1 μM]-stimulated O2- generation: 2.97 ± 0.44 nmoles/2 hours (domoic acid pretreated, n = 3) vs. 1.7 ± 0.07 nmoles/2 hours (untreated controls, n = 3). Furthermore, domoic acid did not affect PMA [1 μM]-stimulated O2- generation from 6 hour LPS-pretreated microglia: 6.5 ± 0.9 nmoles/2 hours (LPS-pretreated, n = 3) vs. 6.26 ± 0.4 nmoles/2 hours (LPS + domoic acid pretreated, n = 3). Finally, as shown in Fig. 8B, though not statistically significant, a 2 hour stimulation with increasing concentrations of domoic acid and PMA [1 μM], reduced O2- generation from 6 hours LPS-pretreated microglia: 4.16 ± 0.58 nmoles/2 hours (domoic acid [1 mM] + PMA [1 μM], n = 3) vs 6.5 ± 1.0 nmoles/2 hours (untreated controls, n = 3).
Figure 8.
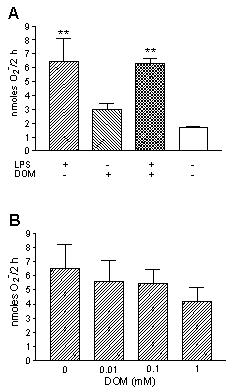
The effect of LPS and Domoic acid on neonatal rat brain microglia O2- release. O2- generation was determined as described in Materials and Methods. (A) Neonatal rat brain microglia (250,000 cells/well) were pretreated with LPS [10 ng/mL] and/ or Domoic acid [1 mM] for 6 hours and then stimulated with PMA [1 μM] for 2 hours. (B) Neonatal rat brain microglia (250,000 cells/well) were first pretreated with LPS [10 ng/mL] for 6 hours and then stimulated with PMA [1 μM] ± Domoic acid [0–1 mM] for 2 hours. Data (O2- nanomoles/2 hours) are expressed as mean ± SD of values obtained from three culture wells of one representative experiment. **p < 0.01 vs. untreated control.
Discussion
Although domoic acid's excitotoxicity to adult neuronal tissue in humans, rats, mice and monkeys is well documented (for review see Ref. [21]), a growing body of research also suggests that there might be increased vulnerability during the neonatal period [15-17]. The fact that the blood-brain barrier is incomplete [34], provides a putative explanation as to why neonates and fetal mice in utero appear to be more sensitive to neurotoxins like domoic acid than adult animals [35-37]. Although microglia contribute to circa 10% of the total glial cell population in the central nervous system (For review see Ref. [38]), to our knowledge, no previous studies have investigated the potential role of microglia in mediating the neurotoxic effects of domoic acid in the developing rat brain. We have recently hypothesized that the marine toxin domoic acid might affect the developing brain by directly activating neonatal rat microglia and causing a subsequent release of potentially neurotoxic mediators [21].
Prior to testing our hypothesis experimentally, and determining whether domoic acid had the potential to directly activate rat neonatal microglia in vitro, we thought that it was of critical importance to establish that: (1) the domoic acid preparation used in these experiments was stable, (2) rat neonatal microglia isolated by our methodology expressed the AMPA glutamate receptor GluR4 subunit which has been observed in activated rat microglia in vivo[24] and, (3) to confirm that the domoic acid concentration selected for our in vitro studies with rat neonatal microglia was one that was clearly toxic to neuronal cells in vitro after a short-term exposure.
Although it has been reported that the solubility of domoic acid in water is approximately 7.6 g/L (24.4 mM) [39], rapid and complete dissolution of domoic acid was only consistently observed in our laboratory when preparing a stock domoic acid solution of 12.5 mM in certified LPS-free water. To rule out the possibility that domoic acid might have degraded as a consequence of either our preparative method or the subsequent freezing (-80°C) and thawing of the domoic acid stock solution, electrospray mass spectrometry was run on both the domoic acid stock solution (12.5 mM) and the dilutions prepared prior to the experiments. Our electrospray mass spectrometry results clearly showed that undegraded domoic acid was present, demonstrating the stability of domoic acid in the aqueous solutions that had been frozen at -80°C and thawed prior to use in the experiments. Interestingly, our observations complement those recently reported on domoic acid's stability in saline solutions [40].
The ionotropic AMPA glutamate receptor GluR4 subunit has been shown to be expressed by activated rat adult microglia in vivo following transient forebrain ischemia [24] and also in vitro by rat neonatal microglia [41]. Because it is well established that AMPA glutamate receptors bind domoic acid with high affinity in the nanomolar range [25], we investigated the presence of GluR4 in rat neonatal microglia used in our in vitro experiments by a dual-labeling indirect immunofluorescence technique. Our results clearly confirmed that GluR4 was present in the rat neonatal microglia used in our in vitro studies with domoic acid, demonstrating that the protocol used in our laboratory to isolate microglia did not appear to affect the presence of the AMPA glutamate receptor GluR4 subunits and their colocalization with the CD 11 b/c integrin.
The cytotoxicity of domoic acid to neuronal tissue in vitro has been investigated over a wide range of concentrations, namely at 10 μM with primary cultures of cerebellar neurons [30,31] as well as hippocampal slices [33], at 100 μM with cultured prenatal rat hippocampal neurons [29] and at 20 mM with rat lumbar spinal interneurons [28]. In order to determine a concentration of domoic acid for our in vitro studies that was toxic to cerebellar granule cells after a short-term exposure in our experimental conditions, we completed a viability study using the WST-1 method. The results of this study demonstrated that even though the highest concentration of domoic acid tested, namely 1 mM, did not affect rat neonatal microglia viability in vitro, domoic acid concentrations as low as 10 μM were clearly cytotoxic to cerebellar granule neurons after a 6 hour exposure. Because cerebellar granule cells appear to be inherently resistant to the cytotoxic effects of excitatory amino acids and neurotoxicity appears to be "...facilitated when cellular energy is limited in cultured cerebellar neurons..." [42], perhaps manipulating the experimental conditions (e.g. lowering the temperature to 22°C) might have enhanced the intensity of the observed cytotoxicity to cerebellar neurons even further, as was reported recently when cerebellar granule cells were exposed to 10 μM domoic acid for up to 24 hours [43]. Future work is necessary to determine if similar changes in the experimental conditions might also increase the toxicity of domoic acid to rat neonatal microglia in vitro. It is interesting to note, however, that in newborn mice a brief local application of 2 mM domoic acid to the exposed cortical surface led to a massive degeneration of AMPA/KA GluR receptor positive Cajal-Retzius neurons [44], severe impairment of cortical neuronal migration in vivo and malformation of the domoic acid-treated neocortex [44]. Thus, because 1 mM domoic acid was clearly toxic to cerebellar granule neurons both in vitro (our current study) and in vivo[44], we selected 1 mM domoic acid for the experiments with rat neonatal microglia reported herein.
Having determined that our domoic acid preparation was stable and undegraded, that rat neonatal microglia expressed GluR4 in vitro and that 1 mM domoic acid was toxic to cerebellar granule neurons in vitro but did not affect the viability of rat neonatal microglia, we then investigated whether a short term 1–6 hour exposure to 1 mM domoic acid would activate rat neonatal microglia in vitro. In all our experiments we compared domoic acid's effect with that of 10 ng/ml LPS, a potent and rapid microglia activator [7], to clearly show that rat neonatal microglia were able to become rapidly activated and release mediators, i.e. TNF-α, MMP-9 and O2- upon short-term in vitro treatment. We have recently reported on the kinetics of LPS activation of rat neonatal microglia and the concomitant release of TNF-α protein, MMP-9 and O2- [7]. These three microglia mediators are currently thought to participate in neuronal and glial injury in vivo[10,45] .
TNF-α is a cytokine that can mediate myelin and oligodendrocyte damage in vitro [10] and thought to play a key role in central nervous system pathologies [3]. In vitro E. coli LPS can trigger rat neonatal microglia to rapidly activate TNF-α gene expression and subsequent protein release [46] in a concentration- and time-dependent manner. Although in vitro exposure of rat neonatal microglia to 10 ng/ml LPS resulted, as expected, in a rapid elevation of both TNF-α gene expression and protein release, we observed only a small effect of 1 mM domoic acid on TNF-α gene expression. Interestingly, the slight rise in TNF-α expression yielded a 53 % (p < 0.01) increase of TNF-α protein release 4 hours after stimulation with 1 mM domoic acid. Although we feel it may be premature to speculate on the putative pathophysiological significance of this increase in immunoreactive TNF-α, the fact that TNF-α can induce MMP-9 expression in macrophages [47], suggests that TNF-α may play an as yet undetermined role in the mechanism of MMP-9 release observed in our studies. Futhermore immuno-PCR techniques, which have allowed investigators to study the kinetics of TNF-α release at the femtomolar range [48], might help to study the kinetics of picomolar microglia TNF-α release triggered by a short-term exposure to domoic acid. These proposed studies might be of considerable significance in view of the fact that similar amounts of TNF-α release have also been observed after a 2 hour stimulation of rat neonatal microglia with kainic acid, a domoic acid and glutamate analog [41]. Taken together, our results with domoic acid as well as those of Noda et al. [41]with kainic acid support the recently proposed notion that TNF-α might not be neurotoxic in some situations when the release of this mediator is low, and therefore might not be a necessary requirement for neuronal death involving excitatory amino acids [49].
Proteases such as MMP-9 [9], which have been implicated in sublethal and lethal neuronal and glial injury in vivo in "...central nervous system diseases..." [50] are released by LPS-activated rat neonatal microglia in vitro[7], by a mechanism that may involve TNF-α [47,51]. Clearly because in our studies 1 mM domoic acid stimulated both an increase in TNF-α release, as well as MMP-9, further investigation of a possible relationship between these two events may be important. Furthermore, though the magnitude of MMP-9 secretion stimulated by domoic acid was lower than the one observed with LPS, this is the first observation to our knowledge that an excitatory amino acid like domoic acid is able to trigger MMP-9 release in vitro. Although at the present time it is difficult to comment on the pathophysiological significance of increased MMP-9 release, it is perhaps noteworthy that increased MMP-9 expression has been correlated with increased apoptosis [52] and increased microvascular permeability [53], events possibly involved in the opening of the blood-brain barrier [54] and potential injury to the brain [50].
Microglia constitute the main leukocyte-dependent source of reactive oxygen species in the central nervous system [55]. Since microglia were first shown to release O2- [6] numerous investigations have been undertaken over the past 14 years to characterize agents than can either prime and/or trigger the microglia O2- generating system (for review see Ref. [3]). In our current experiments, we observed that 1 mM domoic acid did not prime rat neonatal microglia for PMA-stimulated O2- release, again in marked contrast with LPS. Interestingly though, domoic acid appeared to modulate LPS-primed rat neonatal microglia O2- release, suggesting that domoic acid might potentially play a role in the in vivo modulation of activated rat neonatal microglia, and perhaps other leukocytes capable of releasing O2-.
In summary, in our experiments the in vitro effect of a 1–6 hour exposure to domoic acid on rat neonatal microglia activation and concomitant TNF-α, MMP-9 and O2- release was clearly distinct in its magnitude from that observed after exposure to LPS, an agent involved in endotoxic shock and other central nervous pathologies [3]. Even though this present study was limited to the study of the in vitro effects of domoic acid on three microglia mediators, namely TNF-α, MMP-9 and O2-, it provides the first experimental evidence to our knowledge that domoic acid, at in vitro concentrations that are toxic to neuronal cells, can trigger a limited activation of rat neonatal microglia and the concomitant release of modest though statistically significant amounts of TNF-α and MMP-9.
Our investigation does not exclude the possibility that in vitro domoic acid may affect expression of genes and mediators that might support survival of neuronal cells. Several lines of evidence appear to support this possibility. Thus, in a recent report ionotropic AMPA glutamate receptors were shown to "...function not only as ion channels but also as cell-surface transducers by means of their interaction with the Src-family non-receptor protein kinase Lyn...", ultimately leading to "...expression of brain-derived neurotrophic factor (BDNF) messenger RNA" in cerebellar primary culture neurons [56]. In another study, basic fibroblast growth factor (bFGF) was shown to significantly protect cerebellar granule neurons in culture from the neurotoxicity by domoic acid, perhaps "...through a direct interaction of bFGF and bFGF receptors on the neuronal surface..." [32]. Interestingly, because rat neonatal microglia have been shown to express BDNF [57] and bFGF [58], and in view of the fact that LPS can affect BDNF gene expression in rat neonatal microglia cells [59], experiments are currently underway in our laboratory to determine if domoic acid may affect neonatal rat microglia expression of both BDNF and bFGF. However, we do not rule out the possibility that growth factors other than BDNF and bFGF could be released by domoic acid-stimulated neonatal microglia cells and play a role in neuroprotection from excitotoxic injury.
Conclusions
Our present results provide the first experimental evidence to support the hypothesis that a direct interaction of domoic acid with rat neonatal microglia in vitro, leads to the release of small but statistically significant amounts of two potentially neurotoxic mediators, namely TNF-α and MMP-9 after a short-term in vitro exposure. These observations are of considerable significance if future research demonstrates that higher levels of TNF-α and MMP-9 are generated by neonatal rat microglia as a result of either (1) a synergistic interaction between domoic acid "...when in association with subtoxic concentrations of excitatory amino acids..." a combination that has been shown to increase the neurotoxicity of domoic acid to primary cultures of cerebellar neurons [31] or (2) an increase in the time of exposure to domoic acid, as we have reported is the case with LPS [7]. The fact that domoic acid has been shown to activate rat microglia several days after in vivo administration [60]clearly raises this latter possibility.
An understanding of the potential neurotoxic and/or neuroprotective effects of domoic acid in neonatal rats will ultimately lead to a better understanding of domoic acid's effect in neonatal human development. This concept is supported by the established fact that glutamate receptors are highly conserved between mammals [26]. Ultimately studies contributing to the characterization of the early biochemical as well as cellular events subsequent to an intoxication with the marine toxin domoic acid will yield new insights for the rationale development of novel therapeutic interventions for the prevention and treatment of amnesic shellfish poisoning.
Materials and Methods
Reagents
LPS B (Escherichia coli 026:B6) was obtained from Difco Laboratories (Detroit, MI); crystalline lyophilized domoic acid was purchased from Diagnostics Chemicals Ltd. (Oxford, CT) and was dissolved in LPS-free water obtained from GIBCO-BRL (Grand Island, NY) to prepare a 12.5 mM stock [39] and stored at -80°C; goat anti-mouse IgG conjugated with tetramethylrhodamine isothiocyanate (TRITC) and goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC) were obtained from Sigma Chemical Co. (St. Louis, MO). PMA was maintained at -80°C as a 10 mM stock solution in DMSO. Dulbecco's modified Eagle medium (DMEM) with high glucose (4,500 mg/l), Neurobasal-A medium, serum-free B-27 supplement. Hank's balanced salt solution (HBSS), penicillin (P), streptomycin (S), trypsin (0.25%)-EDTA (1 mM) and trypan blue were purchased from GIBCO-BRL (Grand Island, NY); certified heat-inactivated fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT); mouse anti-rat CD11b/c monoclonal antibody (OX-42) and rabbit anti-glutamate receptor (GluR4) polyclonal antibody were purchased from Pharmingen (San Diego, CA). A LPS stock of 1 mg/ml was prepared in a 0.9% sodium chloride nonpyrogenic solution from Baxter Healthcare Corp. (Toronto, ONT, Canada) and then diluted with DMEM plus 10% FBS plus P and S to the appropriate concentration used in our experiments. Both the LPS stock solution [10 ng/ml] and dilutions were stored at -80°C, thawed prior to each experiment and discarded after use.
LPS containment
To inactivate extraneous LPS, all glassware and metal spatulas were baked for 4 hours at 180°C. Sterile and LPS-free 75-and 162-cm2 vented cell culture flasks, 24-well flat-bottom culture clusters, 96-well cell culture clusters and disposable serological pipettes were purchased from Costar Corporation (Cambridge, MA), while polystyrene cell culture dishes (60 × 15 mm) were obtained from Corning Glass Works (Corning, NY). Sterile and pyrogen-free Eppendorf Biopur pipette tips were purchased from Brinkmann Instruments, Inc. (Westbury, NY).
Electrospray mass spectrometry of domoic acid
The presence of undegraded domoic acid in the stock solution (12.5 mM) and dilutions prepared with LPS-free water was confirmed by electrospray mass spectrometry prior to the experiments. Electrospray mass spectrometry was performed in the Mass Spectrometry Laboratory, School of Chemical Sciences, University of Illinois. All electrospray mass spectrometry spectra were acquired on a Micromass Quattro triple quadruple mass spectrometer (Manchester, UK) employing a Megaflow probe and ion source. The data were acquired in the continuum mode. All solvents were Fisher Optima or Burdick and Jackson Distilled-in-Glass grade. Deionized water was obtained from a Barstead NANOpure system. Domoic acid samples (10 μl) were diluted with 40 μl of 50/50 H2O/AcN with 0.1% formic acid as the mobile flow phase. An aliquot (10 μl) was injected using a model 7125 Rheodyne valve with a 10 μl loop. The sample was nebulized with dry nitrogen at a flow of 15 l/hr and the nitrogen bath gas flow as 300 l/h. The electrospray mass spectrometry source was typically operated with 3.5 kV on the electrospray mass spectrometry probe and 400 volts on the counter electrode at a temperature of 65°C. The cone voltage was 25 volts. Normal electrospray mass spectrometry spectra were acquired employing MS 1 over the desired mass range in 10-s at unit resolution (50% valley). The mass scale of the instrument was calibrated employing CsI (4 mg/ml in 50/50 H2O/AcN).
Immunofluorescent labeling of GluR4 and CD11b/c on rat neonatal microglia
The α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate-4 receptor subunit (GluR4) and the integrin CD11b/c were visualized by indirect immunofluorescence using a dual labeling procedure. Rat neonatal microglia on glass coverslips were fixed and permeabilized in -20°C methanol for 10 min, and then blocked with 10% goat serum in phosphate buffered saline (PBS) at 25°C for 5 min. The samples were incubated for 45 min at 37°C in PBS containing the primary antibodies, a rabbit anti-rat GluR4 at a 1:50 dilution, and a mouse anti-rat CD11b/c (OX-42) at a 1:100 dilution. Control samples were incubated in the same manner except the primary antibodies were omitted from the solution. The samples were rinsed quickly in PBS and then incubated for 30 minutes at 37°C in PBS containing the secondary antibodies, a FITC-conjugated goat anti-rabbit IgG at a dilution of 1:50 and a TRITC-conjugated goat anti-mouse IgG at a dilution of 1:400. The samples were rinsed successively in PBS and deionized water and then were mounted on glass slides in Aqua Polymount from Polysciences, Inc. (Warrington, PA). The samples were allowed to dry in the dark and were viewed using a 100 × objective lens on a Nikon Eclipse E400 epifluorescence microscope from Nikon Inc. (Melville, NY). Digital images of the cells were captured with a Spot 2 digital camera from Diagnostic Instruments, Inc. (Sterling Heights, MI) and processed by using the Image-Pro Plus image analysis computer program from Media Cybernetics, Inc. (Silver Spring, MD).
Isolation and culture of rat neonatal microglia
All experiments were performed with adherence to the National Institutes of Health guidelines on the use of experimental animals and with protocols approved by Midwestern University's Research and Animal Care Committee. To isolate rat neonatal microglia, cerebral cortices of 1–2 day-old Sprague-Dawley rats purchased from Harlan (Indianapolis, IN) were surgically removed and placed in cold DMEM + 10% FBS +120 U/ml P and 12 μg/ml S, the meninges carefully removed, and brain tissue minced and dissociated with trypsin-EDTA at 36°C for 3–5 min. The mixed glial cell suspension was plated in either 75- or 162-cm2 vented cell culture flasks with DMEM medium supplemented with 10% FBS +120 U/ml P + 12 μg/ml S and grown in a humidified 5% CO2 incubator at 36°C for 12–14 days. On day 14 and every 3–4 days thereafter, microglia were detached using an orbital shaker (150 rpm, 0.5 hours, 36°C, 5% CO2), centrifuged (400 × g, 25 min, 4°C), and microglia number and viability assessed by trypan blue exclusion. Microglia were characterized as described earlier [7]. Depending on the particular experimental protocol (see below), microglia averaging > than 95% viability were plated in either 60 mm × 15 mm polystyrene cell culture dishes, 96-well cell culture clusters, or 24-well cell culture clusters, with DMEM supplemented with 10% FBS + 120 U/ml P + 12 μg/ml S, and placed in a humidified 5% CO2 incubator at 36°C 18–24 hours prior to the experiments.
Primary culture of rat cerebellar granule neurons
Primary cultures of rat cerebellar granule neurons were prepared from the cerebella of 7-day-old Sprague-Dawley rats. Cerebella were dissected and processed as described elsewhere [61]. The dissociated cells were grown in a serum-free Neurobasal-A medium with B27 supplement (10 ml/500 ml) [62], and in the presence of 25 mM KCl (Sigma). The cells were maintained in poly-L-lysine-treated (10 ug/ml; Sigma) 96-well plates (60,000 cells/0.1 ml Neurobasal A medium/well), at 37°C in an incubator with 95% air/5% CO2. This method results in a culture consisting of neuronal precursors that differentiate into neurons; the differentiated cultures were used for experiments after 10 days.
Experimental protocol to determine the viability of domoic acid-treated rat neonatal microglia or cerebellar granule neurons
A colorimetric assay for the quantitation of cell viability, based on the cleavage of the tetrazolium salt WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]1,3-benzene disulfonate) (slightly red) to formazan (red) by mitochondrial dehydrogenases in viable cells was utilized for these studies. Briefly, rat neonatal microglia or cerebellar granule cells (60,000 cells / well) were plated in LPS-free 96-well cell culture clusters 24 hours prior to the experiments in either 0.1 ml DMEM supplemented with 10% FBS + 120 U/ml P + 12 μg/ml S (microglia), or 0.1 ml serum-free Neurobasal A medium with B27 supplement (cerebellar granule neurons). Thereafter each well received 4 μl of domoic acid (0.01 μM – 1 mM final concentration) or vehicle (LPS-free water) and 10 μl of the tetrazolium salt WST-obtained from Roche Diagnostics Gmbh (Mannheim, Germany). Plates were incubated in a humidified 5% CO2 incubator at 36°C and the reduction of the WST-1 reagent to formazan was measured repeatedly after the addition of the WST-1 reagent, at either 415 nM (neurons) or 450 nM (microglia), with a microtiter plate (ELISA) reader.
Experimental protocol to determine the effect of domoic acid and LPS on rat neonatal microglia release of TNF-α, MMP-2, MMP-9 and O2-
To study the time-dependent effects of domoic acid and LPS on the generation of TNF-α, MMP-2 and MMP-9, rat neonatal microglia (2.8–5 × 106 cells/60 mm × 15 mm polystyrene cell culture dish) were treated with (1) 1 mM domoic acid, (2) LPS-free water used as a vehicle for domoic acid or (3) 10 ng/ml LPS in a final volume of 5 ml of DMEM supplemented with 10% FBS + 120 U/ml P + 12 μg/ml S and incubated in a humidified 5% CO2 incubator at 36°C for 1 to 6 h. Upon termination of the experiments, cell-free supernatants from each culture dish were assayed for TNF-α, MMP-2 and MMP-9, while rat neonatal microglia were processed for RNA isolation and RT-PCR. For the O2- assay, rat neonatal microglia (250,000 cells/ 24-well cell culture clusters) were treated with (1) 1 mM domoic acid, (2) LPS-free water used as a vehicle for domoic acid or (3) 10 ng/ml LPS in a final volume of 1 ml of DMEM supplemented with 10% FBS + 120 U/ml P + 12 μg/ml S and incubated in a humidified 5% CO2 incubator at 36°C for 1 to 6 hours. Thereafter the media was removed, 1 ml warm HBSS was added, and rat neonatal microglia were stimulated with PMA [1 μM] for 120 min and O2- production was assayed as described below.
Experimental protocol to determine the effect of domoic acid and LPS on rat neonatal microglia TNF-α expression: RNA isolation and semiquantitative RT-PCR analysis
Total rat neonatal microglia RNA was isolated with TRI reagent purchased from Molecular Research Center, Inc. (Cincinnati, OH). The RT-PCR were performed in the same sample tube using gene-specific primers obtained from IDT (Coralville, IA), as described below, and the Superscript one-step kit obtained from Gibco-BRL (Grand Island, NY). Briefly, first strand cDNA was made using 0.25 μg of RNA, Superscript II H-reverse transcriptase, antisense gene-specific primers and conditions of 30 min at 50°C. Samples were denatured at 94°C for 2 min, and PCR was performed for various cycle numbers to ensure linear amplification. The PCR cycling conditions consisted of a denaturation step at 94°C for 30 s, an annealing step at 54°C (S12) or 60°C (TNF-α), an extension step at 72°C for 1 min, and a final extension step at 72°C for 10 min. The 50 μl samples contained 0.2 mM of each dNTP, 1.2 mM magnesium sulfate. Platinum® taq DNA polymerase, and 0.2 μM of each gene specific primer. The primers for rat TNF-α, were designed using the DNASTAR Lasergene software (Madison, WI) and the rat TNF-α cDNA sequence (GenBank accession number NM 012675), and are sense (bp 127 – 150):5'-GGG GCC ACC ACG CTC TTC TGT CTA-3' and antisense (bp 285 – 307): 5'-CCT CCG CTT GGT TTG CTA CG-3', and generate a 181 bp cDNA product. The primers for rat constitutively expressed ribosomal S12, were sense:5'-ACG TCA ACA CTG CTC TAC A-3' and antisense:5'-CTT TGC CAT AGT CCT TAA C-3' and generate a 303 bp cDNA product as previously described [63]. Controls for RT-PCR included samples without the enzyme reverse transcriptase and samples without template. The PCR reactions were analyzed by electrophoresis using 1.5 % agarose gels, visualized with SYBR® Gold nucleic acid staining (Molecular Probes; Eugene, OR), photographed, scanned and digitized with the UN-SCAN-IT™ gel automated digitizing system from Silk Scientific (Orem, UT). The relative amounts of TNF-α expression as determined by RT-PCR were normalized to S12 levels using methods similar to those previously described by others [64].
Assays for TNF-α and O2-. TNF-α
Immunoreactive TNF-α in cell-free media supernates was determined using an ultrasensitive rat-specific ELISA for TNF-α purchased from Biosource International (Camarillo, CA) with a detection limit of 0.7 pg/ml. Results are expressed as pg/ml. O2- : O2- generation was determined by the SOD-inhibitable reduction of FCC [7]. Briefly, O2- release from microglia was measured in the presence of FCC (50 μM) and HBSS, with or without SOD (700 Units). All experimental treatments were run in triplicate and in a final volume of 1 ml. Changes in FCC absorbance were measured at 550 nm using a Beckman DU-650 spectrophotometer and O2- generation expressed in nmol by employing the molecular extinction coefficient of 21.0 × 103 M-1 cm-1.
SDS-PAGE gelatinase zymography for MMP-2 and MMP-9 analysis
Following incubation with LPS or domoic acid, MMP expression was analyzed in the harvested media of cultured rat neonatal microglia. As the rat neonatal microglia cultures were normalized for cell number, equal volumes of harvested media obtained from each condition were analyzed. Briefly, 10 μl of each sample were electrophoresed at 4°C and under non-denaturing conditions using a 10% polyacrylamide gel containing 0.05% gelatin. The gels were incubated for 1 hour in a 2.5% Triton X-100 solution and then washed twice with water (20 min each). The gels were then incubated for 24 hours at 37°C in 50 mM Tris-HCl buffer, pH 7.4, containing 5 mM CaCl2. A duplicate negative control gel was incubated as described above but in 50 mM Tris-HCl, pH 7.4 containing 10 mM EDTA instead of CaCl2. The gels were fixed for 1 hour in 40% methanol /7% acetic acid and then stained in Coomassie Blue Solution (Sigma), followed by destaining in 10% methanol, 7% acetic acid. MMP activity was visualized as clear bands against a blue background. Gelatin-containing zymograms are typically used to detect MMP-2 (72 kDa) and MMP-9 (92 kDa) and their identification is based on molecular weight. Relative clearing of each sample was quantitated by determining the inverse optical density units using the NIH Image software package (version 1.60). Values are represented as the mean ± SE and are presented as inverse optical density. The value corresponding to background, a region of equal area as that used to measure the cleared bands and of a lane in which no sample was loaded, was subtracted from each sample value.
Statistical analysis of the data
Data were analyzed with the PrismR software package purchased from GraphPad (San Diego, CA.). One way analysis of variance followed by Dunnett's test was performed on all sets of data. LPS or domoic acid-treated groups were compared with the vehicle-treated group, shown as 0 or control in the corresponding figures. Differences were considered statistically significant at p < 0.05 and reported in each figure legend.
Abbreviations
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; DMEM, Dulbecco's modified Eagle medium; FBS, fetal bovine serum certified; FCC, ferricytochrome c type III; HBSS, Hank's balanced salt solution; LPS, lipopolysaccharide; MMP-9, matrix metalloproteinase-9; O2-, superoxide; P, penicillin; PBS, phosphate buffered saline; PMA, phorbol 12-myristate 13-acetate ; RT-PCR, reverse transcriptase polymerase chain reaction; S, streptomycin; SOD, superoxide dismutase; TNF-α, tumor necrosis factor.
Acknowledgments
Acknowledgments
This publication was made possible by grant number R03 ES10138–01 from the National Institute of Environmental Health Sciences, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIEHS, NIH. Valuable support by Midwestern University's animal facility and library staff as well as the excellent secretarial assistance of Mrs. Victoria Sears is gratefully acknowledged.
Contributor Information
Alejandro MS Mayer, Email: amayer@midwestern.edu.
Mary Hall, Email: mhallx@midwestern.edu.
Michael J Fay, Email: mfayxx@midwestern.edu.
Peter Lamar, Email: plamar@midwestern.edu.
Celeste Pearson, Email: cpears@midwestern.edu.
Walter C Prozialeck, Email: wprozi@midwestern.edu.
Virginia KB Lehmann, Email: v-bowden@scs.uiuc.edu.
Peer B Jacobson, Email: peer.b.jacobson@abbott.com.
Anne M Romanic, Email: Anng_Romanic-1@sbphrd.com.
Tolga Uz, Email: uz@psych.uic.edu.
Hari Manev, Email: hmanev@psych.uic.edu.
References
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. GLIA. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-H. [DOI] [PubMed] [Google Scholar]
- Mayer AM. Therapeutic implications of microglia activation by lipopolysaccharide and reactive oxygen species generation in septic shock and central nervous system pathologies: a review. Medicina (B Aires) 1998;58:377–385. [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Flavin MP, Zhao G, Ho LT. Microglial tissue plasminogen activator (tPA) triggers neuronal apoptosis in vitro. GLIA. 2000;29:347–354. doi: 10.1002/(SICI)1098-1136(20000215)29:4<347::AID-GLIA5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS, Oh S, Ramsey KH, Jacobson PB, Glaser KB, Romanic AM. Escherichia Coli Lipopolysaccharide potentiation and inhibition of rat neonatal microglia superoxide anion generation: correlation with prior lactic dehydrogenase, nitric oxide, tumor necrosis factor-α, thromboxane B2, and metalloprotease release. SHOCK. 1999;11:180–186. [PubMed] [Google Scholar]
- Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res. 1995;42:335–342. doi: 10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. GLIA. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, et al. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels [see comments]. N Engl J Med. 1990;322:1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J. Toxicology and seafood toxins: domoic acid. Nat Toxins. 1994;2:334–339. doi: 10.1002/nt.2620020514. [DOI] [PubMed] [Google Scholar]
- Xi D, Peng YG, Ramsdell JS. Domoic acid is a potent neurotoxin to neonatal rats. Nat Toxins. 1997;5:74–79. doi: 10.1002/(SICI)(1997)5:2<74::AID-NT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Doucette TA, Strain SM, Alien GV, Ryan CL, Tasker RA. Comparative behavioural toxicity of domoic acid and kainic acid in neonatal rats. Neurotoxicol Teratol. 2000;22:863–869. doi: 10.1016/S0892-0362(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Schmued LC, Andrews AM, Scallet AC, Slikker W, Jr, Binienda Z. Systemic administration of domoic acid-induced spinal cord lesions in neonatal rats. J Spinal Cord Med. 2000;23:31–39. doi: 10.1080/10790268.2000.11753506. [DOI] [PubMed] [Google Scholar]
- Peng YG, Ramsdell JS. Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundam AppI Toxicol. 1996;31:162–168. doi: 10.1006/faat.1996.0087. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Binienda Z, Caputo FA, Hall S, Paule MG, Rountree RL, et al. Domoic acid-treated cynomolgus monkeys (M. fascicularis): effects of dose on hippocampal neuronal and terminal degeneration. Brain Res. 1993;627:307–313. doi: 10.1016/0006-8993(93)90335-K. [DOI] [PubMed] [Google Scholar]
- Ross IA, Johnson W, Sapienza PP, Kim CS. Effects of the seafood toxin domoic acid on glutamate uptake by rat astrocytes. Food Chem Toxicol. 2000;38:1005–1011. doi: 10.1016/S0278-6915(00)00083-1. [DOI] [PubMed] [Google Scholar]
- Mayer AM. The marine toxin domoic acid may affect the developing brain by activation of neonatal brain microglia and subsequent neurotoxic mediator generation. Med Hypotheses. 2000;54:837–841. doi: 10.1054/mehy.1999.0962. [DOI] [PubMed] [Google Scholar]
- Grewal RP, Yoshida T, Finch CE, Morgan TE. Scavenger receptor mRNAs in rat brain microglia are induced by kainic acid lesioning and by cytokines. Neuroreport. 1997;8:1077–1081. doi: 10.1097/00001756-199703240-00003. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol. 1997;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Hampson DR, Huang XP, Wells JW, Walter JA, Wright JL. Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur J Pharmacol. 1992;218:1–8. doi: 10.1016/0014-2999(92)90140-Y. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.neuro.17.1.31. [DOI] [PubMed] [Google Scholar]
- Lerma J. Kainate receptors: an interplay between excitatory and inhibitory synapses. FEBS Lett. 1998;430:100–104. doi: 10.1016/S0014-5793(98)00462-1. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Evans RH, Headley PM, Martin M, Watkins JC. Domoic and quisqualic acids as potent amino acid excitants of frog and rat spinal neurones. Nature. 1975;255:166–167. doi: 10.1038/255166a0. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Zorumski CF, Price MT, Olney JW. Domoic acid: a dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp Neurol. 1990;110:127–138. doi: 10.1016/0014-4886(90)90057-y. [DOI] [PubMed] [Google Scholar]
- Novelli A, Kispert J, Reilly A, Zitko V. Excitatory amino acids toxicity in cerebellar granule cells in primary culture. Can Dis Wkly Rep. 1990;16 Suppl 1E:83–88. [PubMed] [Google Scholar]
- Novelli A, Kispert J, Fernandez-Sanchez MT, Torreblanca A, Zitko V. Domoic acid-containing toxic mussels produce neurotoxicity in neuronal cultures through a synergism between excitatory amino acids. Brain Res. 1992;577:41–48. doi: 10.1016/0006-8993(92)90535-H. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez MT, Novelli A. Basic fibroblast growth factor protects cerebellar neurons in primary culture from NMDA and non-NMDA receptor mediated neurotoxicity. FEBS Lett. 1993;335:124–131. doi: 10.1016/0014-5793(93)80453-2. [DOI] [PubMed] [Google Scholar]
- Polischuk TM, Jarvis CR, Andrew RD. Intrinsic optical signaling denoting neuronal damage in response to acute excitotoxic insult by domoic acid in the hippocampal slice. Neurobiol Dis. 1998;4:423–437. doi: 10.1006/nbdi.1998.0172. [DOI] [PubMed] [Google Scholar]
- Al-Sarraf H, Preston JE, Segal MB. Changes in the kinetics of the acidic amino acid brain and CSF uptake during development in the rat. Brain Res Dev Brain Res. 1997;102:127–134. doi: 10.1016/S0165-3806(97)00089-8. [DOI] [PubMed] [Google Scholar]
- Bose R, Pinsky C, Glavin GB. Sensitive murine model and putative antidotes for behaviorial toxicosis from contaminated mussel extracts. Can Dis Wkly Rep. 1990;16 Suppl 1E:91–98. [PubMed] [Google Scholar]
- Glavin G, Pinsky C, Bose R. Toxicology of mussels contaminated by neuroexcitant domoic acid . Lancet. 1989;1:506–507. doi: 10.1016/S0140-6736(89)91414-1. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J Neurosci. 1993;13:4486–4495. doi: 10.1523/JNEUROSCI.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/S0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Falk M, Seto PF, Walter JA. Solubility of domoic acid in water and in non-aqueous solvents. Canadian Journal of Chemistry. 1991;69:1740–1744. [Google Scholar]
- Johannessen JN. Stability of domoic acid in saline dosing solutions. JAOAC Int. 2000;83:411–412. [PubMed] [Google Scholar]
- Noda M, Nakainshi Ft, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 2000 Jan 1;20 (1):251-8. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Berman FW, Murray TF. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J Neurochem. 1997;69:693–703. doi: 10.1046/j.1471-4159.1997.69020693.x. [DOI] [PubMed] [Google Scholar]
- Super H, Del Rio JA, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW, Raivich G. Inflammation biology of the central nervous system – part 1. Brain Pathol. 1997;7:1231–1234. [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Nakanishi I, Yamashita K, Hayakawa T, Okada Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci. 1993;104 (Pt 4):991–999. doi: 10.1242/jcs.104.4.991. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Weiss F, Samson ME, Bloom FE, Pich EM. Rapid induction of tumor necrosis factor alpha in the cerebrospinal fluid after intracerebroventricular injection of lipopolysaccharide revealed by a sensitive capture immuno-PCR assay. Proc Natl Acad Sci USA. 1995;92:272–275. doi: 10.1073/pnas.92.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock F, Derijard B, Dornand J, Bockaert J, Rondouin G. The neuronal death induced by endotoxic shock but not that induced by excitatory amino acids requires TNF-alpha. Eur J Neurosci. 1998;10:3107–3114. doi: 10.1046/j.1460-9568.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- Lukes A, Mun-Bryce S, Lukes M, Rosenberg GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, et al. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- Partridge CA, Jeffrey JJ, Malik AB. A 96-kDa gelatinase induced by TNF-alpha contributes to increased microvascular endothelial permeability. Am J Physiol. 1993;265:L438–L447. doi: 10.1152/ajplung.1993.265.5.L438. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J EXP MED. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M, Nakajima K, Takei N, Hamanoue M, Kohsaka S. Production of basic fibroblast growth factor in cultured rat brain microglia. Neurosci Lett. 1991;123:229–231. doi: 10.1016/0304-3940(91)90937-O. [DOI] [PubMed] [Google Scholar]
- Elkabes S, Peng L, Black IB. Lipopolysaccharide differentially regulates microglial trk receptor and neurotrophin expression. J Neurosci Res. 1998;54:117–122. doi: 10.1002/(SICI)1097-4547(19981001)54:1<117::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Appel NM, Rapoport SI, O'Callaghan JP, Bell JM, Freed LM. Sequelae of parenteral domoic acid administration in rats: comparison of effects on different metabolic markers in brain. Brain Res. 1997;754:55–64. doi: 10.1016/S0006-8993(97)00042-5. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Meier E, Drejer J, Hertz Preparation of primary cultures of mouse(rat) cerebellar granule cells. A Dissection and Tissue Culture Manual of the Nervous System. (Edited by Shahar A, de Vellis J, Vernadakis A, and Haber B) New York, Alan R. Liss. 1989. pp. 203–206.
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Ren L. Lipopolysaccharide-induced expression of IP-10 mRNA in rat brain and in cultured rat astrocytes and microglia. Brain Res Mol Brain Res. 1998;59:256–263. doi: 10.1016/S0169-328X(98)00170-3. [DOI] [PubMed] [Google Scholar]
- Lyn D, Liu X, Bennett NA, Emmett NL. Gene expression profile in mouse myocardium after ischemia. Physiol Genomics. 2000;2:93–100. doi: 10.1152/physiolgenomics.2000.2.3.93. [DOI] [PubMed] [Google Scholar]