Abstract
Objectives
Antiretroviral therapy (ART) has facilitated the transition of HIV infection into a chronic disease, where adherence to medications is required along with keeping a healthy lifestyle. Therefore, an increase in physical activity has been recommended for patients with HIV in order to maintain their health status. This study looked to determine the physical activity level and its associated factors among patients with HIV receiving ART treatment.
Settings
Eight outpatient clinic sites across different levels of the health systems in both rural and urban settings in Hanoi and Nam Dinh, Vietnam.
Study design and participants
A cross-sectional study was performed among 1133 patients with HIV receiving ART treatment from January to August 2013.
Primary and secondary outcome measures
Physical activity level was measured using the International Physical Activity Questionnaire (IPAQ). Socioeconomic, health-related quality of life, ART adherence and ART-related characteristics were self-reported.
Results
16% of participants were inactive, and 68% were reported active via health-enhancing physical activity. Rural participants reported a higher level of physical activity compared with urban participants. Participants having a longer duration of ART were less likely to be physically active. Participants who were female and self-employed, who had higher CD4 cell count, higherEuroQol - 5 dimensions - 5levels (EQ-5D-5L) index/EQ-Visual Analogue Scale, and shared their health status with their peers were more likely to have a higher IPAQ score or be physically active. A lower IPAQ score was associated with participants living in urban areas and being at the symptomatic stage. Participants having poor adherence and longer duration of ART were more likely to be physically inactive.
Conclusion
The majority of participants who received ART were physically active. There is a need for interventions to promote physical activity among patients with HIV in urban areas and in the later ART treatment phases. Other potential interventions to increase the level of physical activity include peer support and job guidance.
Keywords: physical activity, antiretroviral therapy, HIV/AIDS, Vietnam, PLWH
Strengths and limitations of this study.
This study included a large sample size of HIV-positive patients who received antiretroviral therapy treatment across different levels of the health systems in both rural and urban areas of Vietnam.
The study employed a number of validated international instruments to ensure comparability between our results and other studies elsewhere.
The International Physical Activity Questionnaire was a subjectively self-reported measure that may underestimate or overestimate the actual physical activity of people living with HIV.
A convenience sampling technique was used and this may limit the generalisability of the findings as well as an accurate representation of HIV/AIDS population.
The causal inference between the level of physical activity and the number of CD4 cells, and the level of physical activity and the quality of life, could not be established due to the study’s cross-sectional design.
Introduction
The implementation of highly active antiretroviral therapy (ART) has led to significant milestones in the reduction of HIV/AIDS-related morbidity and mortality. According to a UNAIDS report in 2016, among 36.7 million people living with HIV (PLWH), an estimated 19.5 million patients had access to life-saving antiretroviral medicines and the global coverage of ART has reached 53%.1 PLWH who take a potent combination of antiretroviral drug regimen enjoy longer and healthier lives.1 This improvement has also transformed HIV/AIDS into a chronic disease.2 However, one of the most common adverse health effects of ART among PLWH is lipodystrophy syndrome,3 including morphological (peripheral lipoatrophy and central fat accumulation) and metabolic (hypertriglyceridaemia, hypercholesterolaemia, insulin resistance, type 2 diabetes, lactic acidaemia) symptoms.4 5 This contributes to an increase in risks of cardiovascular and other non-communicable diseases as a result of changes in adipose tissue distribution and metabolism.6 7 These side effects were also found to be the main reasons for patients’ non-adherence to antiretroviral medication and discontinuation of therapy.8 9
Since ART is a requisite for viral suppression and recovery of the immune system, it is important for health providers to identify and for patients to adopt strategies to prevent these adverse effects and achieve optimisation of treatment.10 11 Physical activity was recommended as an alternative and non-pharmacological treatment intervention to improve patients’ health status.12 Physical activity is defined as the movement of the body that works the skeletal muscles and expends more energy than the resting state.13 There are several subgroups of physical activities, such as occupational activities, conditioning exercises, sports, household tasks (eg, cradling baby, cleaning) and other activities. There are also different levels of intensity, which include light, moderate or heavy.13 Physical activity has been shown to be able to increase the functional capacity, muscle strength, joint flexibility, endurance and energy among PLWH.14 15 Engaging in physical activity has been found to reduce incidences of certain chronic diseases such as cardiovascular diseases by lowering blood pressure in patients with hypertension and improving lipid-lipoprotein profiles16–18; or improving glucose homeostasis19 in people with diabetes; and breast cancer.20 21 Physical activity can also reverse the metabolic and body composition change of patients by lowering the visceral and subcutaneous fat in the centre of the body and increasing the diameter of the peripheral parts, therefore preventing lipodystrophy.22 23
Thus, engaging in physical activities is a very important health-related behaviour for PLWH, because they are found to be less likely to do physical activity compared with uninfected patients.24 A typical physical activity guideline for normal adults recommends a minimum of 150 min of moderate-intensity physical activity or 75 min of vigorous-intensity physical activity each week or an equivalent combination of moderate-intensity and vigorous-intensity activities.25 The Centers for Disease Control and Prevention suggests that PLWH should comply with at least one type of physical activity in this guideline to keep healthy.26 However, in many low-income/middle-income countries, the rates of physical inactivity have been shown to be more than 50% among PLWH27 28 in comparison with about a quarter in the USA and Australia.29 30 The difference depends on various factors such as gender, age groups, occupations, level of CD4 cell count and quality of life.27 28 30
In Vietnam, most previous studies have focused on the physical activity levels among Vietnamese adolescents31 32 and adults with chronic diseases and metabolic syndromes.33 34 A previous study by Bui et al35 found that 70% of the population met the WHO recommendations for physical activity level for adults aged 18–64 years old. Additionally, the socioeconomic and geographical differences were considered as factors that shape attitude and behaviour towards physical activity. Prior studies also revealed that physical activity level was greater in men compared with women35 and among people who had lower educational attainment.36 Furthermore, the level of physical activity in rural areas was significantly higher than those in urban settings perhaps due to more access to modern transportation systems and sedentary behaviours in the urban setting.35 37 A national representative survey of more than 14 700 participants in 2010 indicated that provinces with higher urban population proportions had a lesser proportion of active people.38 Nonetheless, the rate of urbanisation in Vietnam has been quickly on the rise in recent years, from 21% in 2008 to 32% in 2013, and is projected to reach 45% in 2020.39 These changes raise the need for understanding the potential variability of the prevalence of physical activity or inactivity between rural and urban settings, especially among people with chronic illnesses such as HIV/AIDS and other non-communicable diseases.
Currently, Vietnam is still in a concentrated HIV epidemic stage and the proportion of ART coverage is estimated approximately at 42% of the country’s PLWH population in 2015.40 Half of this number has been undergoing ART for a long time. Engaging in physical activity to reduce the risk of chronic diseases and enhance the effectiveness of the ART treatment is a potential intervention strategy to be considered by healthcare providers. However, little attention has been paid to determining the degree of physical activity as an intervention that could enhance the level of physical activity among individuals with HIV infection in Vietnam, particularly between urban and rural areas. According to the Vietnamese Constitution in 2013, an urban area is defined as a settlement with a high population density and built environment, whereas a rural area refers to a place that has a low population density and is located outside the urban setting, where people mostly work in the agriculture sector.41 Based on this definition, we hypothesised that PLWH in an urban area would have a lower level of physical activity compared with their counterparts in the rural area. This study looked to assess the level of physical activity across different settings and examine the factors associated with the level of physical activity among patients with HIV receiving ART treatment across different levels of the health systems and in both rural and urban settings.
Methods
Study setting and subjects
A cross-sectional study was performed from January to August 2013 in Hanoi and Nam Dinh, two Vietnamese epicentres providing HIV/AIDS surveillance and treatment services in the northern region of Vietnam. The study was performed at eight outpatient clinics: five in Hanoi and three in Nam Dinh. The locations included one national hospital (Bach Mai Hospital), one provincial hospital (Nam Dinh provincial hospital), one provincial centre (Nam Dinh Provincial AIDS Control Centre) and five district health centres (Hoang Mai, Long Bien, Dong Anh, Ha Dong and Xuan Truong). The eligibility criteria for selecting outpatient clinic sites in Hanoi and Nam Dinh included (1) being representative of the public health system in Vietnam, which has central, provincial and district levels); and (2) being able to afford ART service implemented following the official guidelines of the Vietnamese Ministry of Health.42
Participants were recruited using convenience sampling technique if they met the following eligibility criteria: (1) being 18 years old or above; (2) receiving ART treatment from those clinics mentioned above; (3) having an HIV-positive confirmatory test; (4) agreeing to participate in the study; and (5) being able to communicate with the data collector. The exclusion criteria included those who suffered from serious illness during the recruitment process. We approached participants when they visited clinics for medication or to receive counselling. We identified eligible participants for the study based on the health staff’s feedback. These participants were invited into a small counselling room by well-trained health workers. They were introduced to the purpose of the study, the benefits and drawbacks of participating, and were then asked to join the study. If they agreed, participants would sign a written informed consent. We ensured the participants of the confidentiality of their participation in the study. The consenting process took place in a comfortable room with restricted access, which allowed participants to have some privacy as they decide whether or not to join the project.
Measures and instruments
Participants were invited to participate in 20 min face-to-face interviews. Data were ascertained through interview-administered questionnaires conducted by data collectors who were medical students at Hanoi Medical University and experts in HIV-related fields. We did not involve health staff in collecting data to avoid social desirability bias. We developed a structured questionnaire with the following information:
Socioeconomic characteristics
We asked participants to report their socioeconomic information, which included gender (male/female), education attainment (illiterate/elementary/secondary/high school/vocational training/university), age, marital status (single/live with spouse/live with partner/divorced/widow), religion (cult of ancestors/Buddhism/Catholic/Protestant) and employment status (unemployed/self-employed/white-collar/blue-collar or farmer/others).
ART-related characteristics
We asked each participant for a self-report of their latest CD4 cell count, HIV stages (any asymptomatic/symptomatic condition defined as ‘HIV-infected patients having clinical symptoms but not including AIDS-Indicator Conditions that meet at least one of the eligible criteria/AIDS Indicator Conditions’)43 and ART treatment duration. Data on viral load were not collected due to their unavailability at the time of the study. Non-adherence to ART was measured by self-report items. First, ART adherence of the last month was determined by a Visual Analogue Scale (VAS) where 0 point showed complete non-adherence and 100 points showed complete adherence.44 Participants were asked whether they forget to take ART medicine in the last 4 days. This approach has been applied successfully in a previous study.45 We also asked the participants about whether they received peer support during ART treatment (yes/no) and whether they shared their health status with their peers (yes/no).
Health-related quality of life
Health-related quality of life was measured using the EuroQol-5 dimensions - 5levels (EQ-5D-5L) instrument in the Vietnamese version, which was validated elsewhere.46 This tool measures five dimensions of health-related quality of life including mobility, self-care, usual activities, pain/discomfort and anxiety/depression with five response levels: no problems, slight problems, moderate problems, severe problems and extreme problems. The combination of responses gives 3125 position health statuses with an aggregate single index weighting.46 The Cronbach’s alpha of this instrument was 0.85 with a good convergent validity.46 Furthermore, we also used the EQ-VAS, which measures the self-rated health on a 20 cm vertical scale, with the endpoint ranging from 0 to 100 points, labelled ‘the worst health you can imagine’ and ‘the best health you can imagine’.47
Physical activity
To assess the level of physical activity of the participants, we used the International Physical Activity Questionnaire (IPAQ). The IPAQ was developed for use in adults aged 15–69 years old.48 Several questions were used to assess three specific types of activity: vigorous activity, moderate activity and walking activity. Examples for each type of activity in our research context are listed below:
Vigorous activity: heavy lifting, hoeing, weight lifting, fast-paced cycling and others.
Moderate activity: playing badminton, slow-paced cycling, cradling baby, selling and others.
Walking activity: going to work, going to school, going elsewhere, going jogging and others.
Each activity was scored separately by frequency (measured by days per week) and by duration (measured by times per day). The volume of each activity was also measured by its energy requirement determined in METs (Metabolic Equivalent of Task and METs are a multiple of the resting metabolic rate).48
The total IPAQ score was used as a continuous variable, which was calculated by adding the MET minute per week of the three types of activity. We also evaluated the IPAQ score as a categorical variable, which divided the physical activity into three levels: inactive, minimally active and health-enhancing physical activity (HEPA) active.
Participants were categorised into (1) HEPA-active group if they did the following:
Vigorous activity for at least 3 days and obtained a total physical activity of at least 1500 MET-min/week.
Or 7 or more days of combination physical activities of walking, moderate-intensity or vigorous activities and obtained a total physical activity of at least 3000 MET-min/week.
Participants were in the (2) minimally active group if they had the following:
3 or more days of vigorous activity of at least 20 min per day (800 MET-min/week).
Or 5 or more days of moderate activity or walking of at least 30 min per day.
Or 5 or more days of walking combining with moderate-intensity or vigorous-intensity activities and obtained at least 600 MET-min/week.
Participants were (3) inactive or were determined as insufficiently active48 if they did not meet the requirements for category 2 or 3.
Statistical analysis
Data were analysed by STATA V.12. A χ2 test and a Mann-Whitney test were used to analyse the demographic characteristics of the participants, as well as the health-related quality of life, ART status and sexual behaviours. Multivariate linear regression was employed to identify factors associated with IPAQ score. Because the IPAQ score had a non-normal distribution, we performed log transformation for this variable in order to meet the requirement of the regression model. We also applied multivariate logistic regression to identify factors associated with whether the participants were active in physical activity or not. We applied a forward stepwise selection strategy to remove non-significant factors; the p value of log-likelihood ratio test was set as less than 0.1, and this was the threshold to include a variable. A p value <0.05 was considered statistically significant.
Patient and public involvement
We conducted a pilot survey of 50 participants of different ages, genders and occupations, and only minor changes to the wording were made in order to meet patients’ preferences and culture. After revision, we performed data collection using a structured questionnaire. Participants did not take part in the recruitment as well as in the conduct of the study. We aim to present our study results at national and international scientific meetings, as well as publish our study in open-access journals. Therefore, our study is widely available to be disseminated to study participants and other interested international researchers.
Results
A total of 1200 patients were invited to participate in the study and 1133 patients agreed to enrol. The reasons for refusal included insufficient health, discomfort or having busy work schedules.
The demographic and socioeconomic characteristics of participants living in rural and urban are given in table 1. Out of 1133 ART patients, approximately 60% was male. The majority had secondary and high school education (36.9% and 32%, respectively); participants in urban areas had higher education than people in rural area (p=0.04). Marital status and employment were also significantly different between the rural and urban groups (p<0.01). There was no difference in age group between rural and urban participants.
Table 1.
Socioeconomic characteristic of PLWH in the study (n=1133)
| Rural | Urban | Total | P values | ||||
| n | % | n | % | n | % | ||
| Gender | |||||||
| Male | 145 | 56.2 | 520 | 59.4 | 665 | 58.7 | 0.36* |
| Female | 113 | 43.8 | 355 | 40.6 | 468 | 41.3 | |
| Education | |||||||
| Illiterate | 5 | 1.9 | 7 | 0.8 | 12 | 1.1 | 0.04* |
| Elementary school | 48 | 18.6 | 172 | 19.7 | 220 | 19.4 | |
| Secondary school | 109 | 42.3 | 309 | 35.3 | 418 | 36.9 | |
| High school | 80 | 31 | 282 | 32.2 | 362 | 32 | |
| Vocational training | 6 | 2.3 | 48 | 5.5 | 54 | 4.8 | |
| University | 10 | 3.9 | 57 | 6.5 | 67 | 5.9 | |
| Marital status | |||||||
| Single | 26 | 10.1 | 143 | 16.3 | 169 | 14.9 | 0.01* |
| Live with spouse | 178 | 69 | 507 | 57.9 | 685 | 60.5 | |
| Live with partner | 0 | 0 | 8 | 0.9 | 8 | 0.7 | |
| Divorced | 22 | 8.5 | 66 | 7.5 | 88 | 7.8 | |
| Widow | 32 | 12.4 | 151 | 17.3 | 183 | 16.2 | |
| Religion | |||||||
| Cult of ancestors | 222 | 86.1 | 779 | 89 | 1001 | 88.4 | 0.03* |
| Buddhism | 12 | 4.7 | 43 | 4.9 | 55 | 4.9 | |
| Catholic | 24 | 9.3 | 44 | 5 | 68 | 6 | |
| Protestant | 0 | 0 | 9 | 1 | 9 | 0.8 | |
| Employment | |||||||
| Unemployed | 47 | 18.2 | 184 | 21 | 231 | 20.4 | <0.01* |
| Self-employed | 98 | 38 | 371 | 42.4 | 469 | 41.4 | |
| White-collar worker | 13 | 5 | 67 | 7.7 | 80 | 7.1 | |
| Blue-collar worker or farmer | 94 | 36.4 | 188 | 21.5 | 282 | 24.9 | |
| Others | 6 | 2.3 | 65 | 7.4 | 71 | 6.3 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 35.6 | 6.6 | 35.5 | 7.0 | 35.5 | 6.9 | 0.6† |
Significance level was p<0.05.
*Χ2 test.
†Mann-Whitney test.
PLWH, people living with HIV.
ART status
About half of the participants were asymptomatic, and the percentage of the rural participants who were unaware of their stage of HIV infection was significantly higher than the urban participants (52.1% vs 24.7%) (table 2). The mean number of CD4 measurements and duration of ART treatment were 294.7 cells×10^9/L (SD=215.2) and 3.5 years (SD=2.2), respectively. About 50% of the participants shared health status with their peers and only one-third received peer support.
Table 2.
Antiretroviral therapy status of participants (n=1133)
| Rural | Urban | Total | P values | ||||
| n | % | n | % | n | % | ||
| HIV period | |||||||
| Asymptomatic | 67 | 28.4 | 389 | 46 | 456 | 42.1 | <0.01* |
| Symptomatic | 32 | 13.6 | 161 | 19 | 193 | 17.8 | |
| AIDS | 14 | 5.9 | 87 | 10.3 | 101 | 9.3 | |
| Unknown | 123 | 52.1 | 209 | 24.7 | 332 | 30.7 | |
| ART duration (year) | |||||||
| 1 year | 47 | 19.0 | 213 | 26.9 | 260 | 25.0 | <0.01* |
| 2–4 years | 91 | 36.9 | 354 | 44.7 | 445 | 42.8 | |
| More than 4 years | 109 | 44.1 | 225 | 28.4 | 334 | 32.2 | |
| Share health status with peers | |||||||
| Yes | 110 | 43.3 | 446 | 52.7 | 556 | 50.6 | 0.01* |
| No | 144 | 56.7 | 400 | 47.3 | 544 | 49.5 | |
| Receiving peer support | |||||||
| Yes | 77 | 29.8 | 314 | 35.9 | 391 | 34.5 | 0.07* |
| No | 181 | 70.2 | 561 | 64.1 | 742 | 65.5 | |
| Forgetting to take medicine in the last 4 days | |||||||
| No | 243 | 98 | 780 | 97.7 | 1023 | 97.8 | 0.82* |
| Yes | 5 | 2 | 18 | 2.3 | 23 | 2.2 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| ART duration (year) | 4.0 | 2.4 | 3.3 | 2.1 | 3.5 | 2.2 | <0.01† |
| CD4 cell count (×10^9/L) | 312.5 | 220.6 | 289.2 | 213.4 | 294.7 | 215.2 | 0.08† |
| ART adherence (VAS) | 94.8 | 8.2 | 93.9 | 11 | 94.1 | 10.4 | 0.55† |
Significance level was p<0.05.
*χ2 test.
†Mann-Whitney test.
ART, antiretroviral therapy; VAS, Visual Analogue Scale.
Self-reported health status
The percentage of urban participants who reported having any problem in mobility, self-care and doing usual activities was significantly higher than those of rural participants (p<0.01) (table 3). About 40% of the participants reported suffering from anxiety or depression, and about half of the participants reported suffering from pain or discomfort, with no significant difference between rural and urban participants. The mean EQ-5D-5L index was 0.8 (SD=0.2) and the perceived EQ-VAS score among rural participants was statistically significantly higher than those of urban participants (p<0.01).
Table 3.
Health status among participants (n=1133)
| Rural | Urban | Total | P values | ||||
| n | % | n | % | n | % | ||
| Self-reported health problems | |||||||
| Mobility | 33 | 12.8 | 199 | 22.7 | 232 | 20.5 | <0.01* |
| Self-care | 12 | 4.7 | 98 | 11.2 | 110 | 9.7 | <0.01* |
| Usual activities | 26 | 10.1 | 162 | 18.5 | 188 | 16.6 | <0.01* |
| Pain or discomfort | 85 | 33.0 | 342 | 39.1 | 427 | 37.7 | 0.07* |
| Anxiety or depression | 104 | 40.3 | 405 | 46.3 | 509 | 44.9 | 0.09* |
| Complications and concurrent disease | 76 | 29.5 | 329 | 37.6 | 405 | 35.8 | 0.02* |
| Mean | SD | Mean | SD | Mean | SD | ||
| EQ-5D-5L index | 0.8 | 0.2 | 0.8 | 0.3 | 0.8 | 0.2 | 0.18† |
| EQ-VAS | 70.1 | 16.0 | 68.4 | 17.6 | 68.8 | 17.3 | <0.01† |
Significance level was p<0.05.
*χ2 test.
†Mann-Whitney test.
VAS, Visual Analogue Scale; EQ-5D-5L, EuroQol - 5 dimensions - 5levels.
Physical activity level
Table 4 shows that 16% of the participants were inactive and 68% were HEPA-active using the IPAQ. Rural participants reported a statistically higher level of physical activity and IPAQ score compared with urban participants (p=0.03 and p<0.01, respectively). In terms of moderate activity, the number of days per week and the mean value of MET scores from participants living in rural areas were higher than those in urban areas (p<0.01 and p=0.01, respectively). However, the mean MET scores of vigorous activity and walking activity were similar between the two groups.
Table 4.
Physical activity levels of participants
| Rural | Urban | Total | P values | ||||
| n | % | n | % | n | % | ||
| Level of physical activity | |||||||
| Inactive | 29 | 11.3 | 152 | 17.4 | 181 | 16.0 | 0.03* |
| Minimally active | 37 | 14.3 | 144 | 16.4 | 181 | 16.0 | |
| HEPA-active | 192 | 74.4 | 579 | 66.2 | 771 | 68.0 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Vigorous activity | |||||||
| Days per week | 1.5 | 0.2 | 1.2 | 0.1 | 1.3 | 2.5 | 0.09† |
| Minutes per day | 95.4 | 11.2 | 81.1 | 6 | 84.4 | 177.6 | 0.15† |
| MET score | 4360.9 | 555.5 | 3444.4 | 282.5 | 3651.9 | 8465.9 | 0.11† |
| Moderate activity | |||||||
| Days per week | 5.4 | 0.2 | 4.8 | 0.1 | 4.9 | 2.8 | <0.01† |
| Minutes per day | 170.2 | 5.7 | 155.9 | 3.3 | 159.1 | 96 | 0.04† |
| MET score | 4211.3 | 165.7 | 3737.9 | 92.2 | 3845.9 | 2711.1 | 0.01† |
| Walking activity | |||||||
| Days per week | 3.4 | 0.2 | 3.3 | 0.1 | 3.4 | 3.3 | 0.67† |
| Minutes per day | 18.6 | 1.8 | 21.8 | 1.3 | 21.1 | 36.8 | 0.99† |
| MET score | 377.1 | 38.3 | 451.5 | 29.3 | 434.6 | 816.3 | 0.87† |
| IPAQ score | 8977.9 | 535.5 | 7613.5 | 280.5 | 7922.9 | 8333.8 | <0.01† |
Number of total participants, n=1133.
Significance level was p<0.05.
*χ2 test.
†Mann-Whitney test.
HEPA, health-enhancing physical activity; IPAQ, International Physical Activity Questionnaire; MET, Metabolic Equivalent of Task.
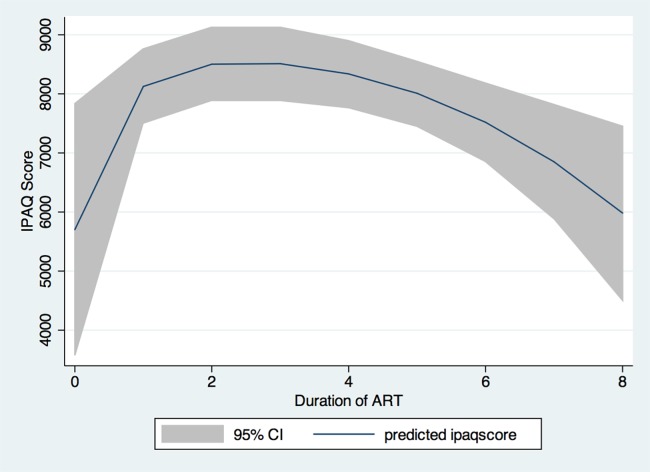
The IPAQ score was also found to be differentially associated with ART treatment duration (figure 1). A higher IPAQ score was associated with a shorter ART duration, and longer ART treatment was associated with a lower IPAQ score. Specifically, the score increased within the first year of ART, plateaued during 2–4 years of treatment, and then decreased.
Figure 1.
Physical activity among antiretroviral therapy (ART) patients in terms of ART duration. IPAQ, International Physical Activity Questionnaire.
Factors associated with level of activity
Table 5 illustrates that participants were more likely to have a higher IPAQ score and classified as physically active if they were female, self-employed, and blue-collar workers or farmers. Participants who had a higher CD4 cell count, who shared their health status with their peers and who reported a higher EQ-5D-5L index/EQ-VAS were also more likely to have higher IPAQ score or be physically active. By contrast, a participant with a lower IPAQ score was associated with living in an urban area and being at the symptomatic stage. In addition, participants who had poor adherence and longer duration of ART were more likely to be physically inactive.
Table 5.
Factors associated with levels of physical activity among antiretroviral therapy patients in Vietnam in 2013
| IPAQ score | Active | |||
| Coefficient | 95% CI | OR | 95% CI | |
| Gender (female vs male) | 0.25* | 0.11 to 0.39 | 2.53* | 1.58 to 4.07 |
| Living location (urban vs rural) | −0.17* | −0.34 to −0.01 | 0.60 | 0.3 to 1.05 |
| Education attainment (vs illiterate) | ||||
| High school | 1.52 | 0.94 to 2.46 | ||
| Religion (vs cults of ancestor) | ||||
| Buddhism | 2.78 | 0.73 to 10.57 | ||
| Occupation (vs unemployed) | ||||
| Self-employed | 0.60* | 0.41 to 0.79 | 2.98* | 1.78 to 4.99 |
| White-collar workers | 0.29 | −0.02 to 0.59 | 2.43 | 0.87 to 6.79 |
| Blue-collar worker/farmers | 0.73* | 0.52 to 0.94 | 2.24* | 1.27 to 3.95 |
| Others | 0.53* | 0.22 to 0.84 | 3.28* | 1.07 to 10.05 |
| EQ-5D index | 4.49* | 1.68 to 12.02 | ||
| EQ-VAS | 0.01* | 0.00 to 0.01 | 1.02* | 1.00 to 1.03 |
| Current CD4 cell count | 0.01* | 0.00 to 0.01 | 1.02* | 1.01 to 1.03 |
| ART duration | 0.91* | 0.82 to 0.98 | ||
| HIV stages (vs asymptomatic) | ||||
| Symptomatic | −0.20* | −0.38 to −0.01 | ||
| AIDS | 1.81 | 0.87 to 3.79 | ||
| Forgetting to take medicine in the last 4 days (yes vs no) | 0.26* | 0.09 to 0.80 | ||
| Share health status with peers (yes vs no) | 0.26* | 0.12 to 0.40 | 1.86* | 1.21 to 2.84 |
*P<0.05.
ART, antiretroviral therapy; IPAQ, International Physical Activity Questionnaire; VAS, Visual Analogue Scale; EQ-5D-5L, EuroQol - 5 dimensions - 5levels.
Discussion
Findings of this study suggest that PLWH in urban areas reported a lower level of physical activity compared with their peer in rural settings. In our study, a majority of ART patients achieved HEPA-active status in physical activity. By using multivariate regression models, we found a number of sociodemographic, clinical and social factors that are associated with the level of physical activity among PLWH in Vietnam. These results could contribute significantly to the development of interventions aimed to boost the level of physical activity among this population in the future.
Compared with previous studies on ART patients using the IPAQ system, the percentage of inactive (16%) or minimally active (16%) participants in our study was much lower.12 27 29 30 Most participants reported the highest frequency of moderate activity (playing sport, cycling, cradling baby and others), which was different from previous studies’ findings that walking was the most preferred physical activity among PLWH.14 30 These activities can have a positive effect on PLWH by decreasing the side effects associated with HIV/AIDS and cardiometabolic complications accompanied with ART treatment.15 49 However, a number of participants in our study reported having difficulty in mobility, which limited their ability to engage in healthy physical activities regardless of living location. With this information, health staff could provide alternative methods of exercising such as passive motion exercise, hydrotherapy or stationary cycling, which are found to be just as effective for enhancing functional fitness as active exercise practice.50
The total physical activity score in rural areas was higher than the score in urban areas in our study, which is consistent with the physical activity level of the general Vietnamese population.35 There are certain environmental factors in rural areas such as the condition of sidewalks, the availability of exercise equipment and the sedentary behaviour in urbanised setting, which may affect individuals’ willingness to participate in physical activity.37 51 At the same time, in Vietnam, the main occupations in rural areas are farming and blue-collar works, in which the rural participants often consider working on the farm or in heavy working conditions as vigorous and moderate physical activities. However, the MET score obtained by walking activity indicated that this was the least performed physical activity and this did not differ between rural and urban areas. This can be due to factors such as low residential density and long distances between destinations in rural areas that may discourage people from walking.52 Similarly, in urban settings, walking intensity could be influenced by the presence or lack of pedestrian infrastructure such as sidewalks, as well as safety concerns from crime or traffic flow.53
Our study also found that participants who were unemployed were less likely to be physically active, which concurs with prior findings.54 55 Unemployed patients are less likely to obtain adequate physical activity because an occupational activity is likely to be included as a component of daily physical activity in adulthood.56–58 In addition, our study found that female participants were more likely to get a higher level of physical activity. This can be explained by the fact that in the traditional Vietnamese culture, women still have many responsibilities requiring physical movements such as taking care of their children and all household activities, which were mainly classified as moderate physical activity in our study.59 60
Interestingly, we found that people who reported a longer duration of ART were less likely to be physically active. Notably, this association was found in a non-linear relationship. This is likely because these patients may have had to adapt to a rigorous adherence at the initiation of their treatment, which might have promoted their willingness to involve in physical activities.45 61 62 However, they might become more complacent when their health status recovered in the latter stages and less motivated to comply to any strict physical regimen.45 61 Findings from another study emphasised the role of age in the relationships between the advanced stages of HIV disease and worse physical function.24 As prolonged ART treatment has been positively associated with an accelerated ageing process, age-related comorbidity may reduce patients’ level of physical activity intensity.24 However, in this study, the influence of age on the level of physical activity was not statistically significant since it was dropped out of multivariate regression model. We also observed that non-adherence to ART was associated with a physically inactive status. Other studies also revealed that a low level of physical activity has been found to be significantly related to a low antiretroviral medication adherence.61 63 This finding can be explained by the fact that engaging in physical activity can reduce depressive symptomatology, which may lead to a more optimal ART adherence.63
In this study, we combined both EQ-5D-5L and EQ-VAS instruments due to the variation of health utility scores based on different instruments.64 EQ-VAS is a self-reported instrument that directly assesses the perceived health status of patients in the short term. Meanwhile, the EQ-5D index is composited by five domains that indirectly measure the quality of life in the long term.65 In the short term, patients’ hope for improving their health condition might influence their perceived health status. On the other hand, in the long term, because of the acclimation with their health status, patients tend to report quality of life more accurately.64 In terms of health status, participants who reported a higher quality of life and a higher number of CD4 cell count also had a higher IPAQ score, while participants at the symptomatic stage had a lower IPAQ score compared with those at the asymptomatic stage. We supposed that engaging in physical activity would help patients improve and maintain their strength and quality of life. Two systematic reviews by O’Brien et al66 67 in 2016 and 2017 found that exercises including aerobic and resistive or a combination of both performed at least three times per week for at least 5 weeks may lead to improvements in cardiopulmonary fitness, strength, weight and body composition, and quality of life among PLWH who are medically stable.
Our current study suggested that participants who shared their health status with their peers were more likely to have higher IPAQ scores. The association between peer support and physical activity was investigated by Jerome et al,68 who found that peer support was an important determinant to assist patients in adhering to exercise programmes. Additionally, WHO, President’s Emergency Plan for AIDS Relief and Global Fund have proposed that social support should be considered as an effective adjunct in improving physical health among PLWH receiving ART treatment.69 70 As PLWH were more vulnerable and withdrawn from social situations,71 peer support can have a positive effect on patients’ health through sharing relevant personal experiences to acquire knowledge, reduce stigma and discrimination, improve physical functioning, and enhance retention in ART treatment.72–74
Several implications can be drawn from this study. First, providing different physical activity strategies based on rural and urban settings is necessary to improve the health status of PLWH in these areas. For example, health staff in urban clinics may organise some outdoor activities via peer educators/groups to engage urban patients in physical activity. Second, job opportunities and vocational training should be prioritised to promote physical activity among ART patients.54 55 Third, the reduction in IPAQ score observed in prolonged ART duration suggests that physical health assessments and appropriate physical activity programmes such as resistance training and aerobic exercises should be in place. Additionally, passive motion exercises should be considered for immobilised patients or those who had difficulty in mobility or physical impairments.50 Fourth, given the association between the level of physical activity and ART treatment status from our findings, integrative intervention including physical activity, ART medication adherence and health-related quality of life may prove to be efficient and cost-effective.63 Finally, programmes promoting social support, especially among peers, should be prioritised to enable patients with HIV to share their experiences that motivate others to be involved in physical activity.68 Furthermore, peer support groups integration into assigned health facilities would be useful to patients who are at the initial ART medication stage.
The strengths of this study included the large sample size of HIV-infected patients receiving ART treatment, which increased the statistical power of the result. Additionally, we recruited participants from different levels of the health systems (central, provincial and district ART clinics) in both rural and urban areas, which made the sample more representative of the general Vietnamese population. We also employed international instruments such as IPAQ and EQ-5D-5L, which would help increase comparability between our results and other global studies.
However, several limitations should be acknowledged. First, a convenience sampling technique was used, which may limit the generalisability of our findings to other settings and patient populations. Second, because the collected data were based on self-reported information, it is susceptible to be influenced by interviewers, social desirability and recall bias. To minimise these biases, interviewers affiliated with selected health centres were excluded from the study and patients were given clear instructions on the benefits and drawbacks of the study. Third, the causal inferences could not be established due to the cross-sectional design. Finally, some barriers to physical activity such as social factors (stigma, discrimination), family support or clinical settings (healthcare providers) were not fully addressed in this study, warranting further research to elucidate these gaps.
Conclusion
In conclusion, findings from this study provided many suggestions for potential health behaviour interventions to improve the level of physical activity for patients with HIV receiving ART in rural and urban Vietnam. Healthcare providers should consider developing peer support and job guidance programmes for PLWH as they have great potentials to increase PLWH’s level of physical activity, quality of life and overall health status. Furthermore, future studies of a similar population in different settings (coastal, mountainside and others) are needed to confirm the positive association between the high level of physical activity and ART adherence.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support from the Vietnam Authority of HIV/AIDS Control and Hanoi Provincial AIDS Centers for the acceptance to implement the study.
Footnotes
Contributors: AKD, LHN, AQN, BXT, TTT, CAL, MWBZ, RCMH conceived of the study, participated in its design and implementation, and wrote the manuscript. AKD, LHN, BXT analysed the data. All authors read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study protocol was reviewed and granted ethics approval by the Vietnam Authority of HIV/AIDS Control’s Scientific Research Committee. Participants confidently participated in the study and signed a written informed consent after receiving clear information on the benefits and drawbacks of the study. The consent process took place in a room with restricted access. Participants can withdraw from the interview at any time and this did not affect their current treatment.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data that support the findings of this study are available from the Vietnam Authority of HIV/AIDS Control, but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from the Vietnam Authority of HIV/AIDS Control.
References
- 1.UNAIDS. Global AIDS Update 2016. 2016.
- 2.Grossman HA, Sullivan PS, Wu AW. Quality of life and HIV: current assessment tools and future directions for clinical practice. AIDS Read 2003;13:583–7. [PubMed] [Google Scholar]
- 3.Savès M, Raffi F, Capeau J, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis 2002;34:1396–405. 10.1086/339866 [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. The Lancet 2000;356:1423–30. 10.1016/S0140-6736(00)02854-3 [DOI] [PubMed] [Google Scholar]
- 5.Montessori V, Press N, Harris M, et al. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004;170:229–38. [PMC free article] [PubMed] [Google Scholar]
- 6.Mutimura E, Stewart A, Rheeder P, et al. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2007;46:451–5. 10.1097/QAI.0b013e318158c0a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potthoff A, Brockmeyer NH, Gelbrich G, et al. Lipodystrophy - a sign for metabolic syndrome in patients of the HIV-HEART study. J Dtsch Dermatol Ges 2010;8:92–8. 10.1111/j.1610-0387.2009.07330.x [DOI] [PubMed] [Google Scholar]
- 8.O’Brien ME, Clark RA, Besch CL, et al. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr 2003;34:407–14. 10.1097/00126334-200312010-00008 [DOI] [PubMed] [Google Scholar]
- 9.Gebrezgabher BB, Kebede Y, Kindie M, et al. Determinants to antiretroviral treatment non-adherence among adult HIV/AIDS patients in northern Ethiopia. AIDS Res Ther 2017;14:16 10.1186/s12981-017-0143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev 2013;254:343–54. 10.1111/imr.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley H, Mattson CL, Beer L, et al. Increased antiretroviral therapy prescription and HIV viral suppression among persons receiving clinical care for HIV infection. AIDS 2016;30:2117–24. 10.1097/QAD.0000000000001164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segatto AF, Freitas Junior IF, Santos VR, et al. Lipodystrophy in HIV/AIDS patients with different levels of physical activity while on antiretroviral therapy. Rev Soc Bras Med Trop 2011;44:420–4. 10.1590/S0037-86822011000400004 [DOI] [PubMed] [Google Scholar]
- 13.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Clingerman EM. Participation in physical activity by persons living with HIV disease. J Assoc Nurses AIDS Care 2003;14:59–70. 10.1177/1055329003255284 [DOI] [PubMed] [Google Scholar]
- 15.Hand GA, Lyerly GW, Jaggers JR, et al. Impact of Aerobic and Resistance Exercise on the Health of HIV-Infected Persons. Am J Lifestyle Med 2009;3:489–99. 10.1177/1559827609342198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland TW. The role of physical activity and fitness in children in the prevention of adult cardiovascular disease. Prog Pediatr Cardiol 2001;12:199–203. 10.1016/S1058-9813(00)00074-6 [DOI] [PubMed] [Google Scholar]
- 17.Warburton DE, Glendhill N, Quinney A. The effects of changes in musculoskeletal fitness on health. Can J Appl Physiol 2001;26:161–216. 10.1139/h01-012 [DOI] [PubMed] [Google Scholar]
- 18.Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol 2005;10:229–49. [PMC free article] [PubMed] [Google Scholar]
- 19.Boulé NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286:1218–27. [DOI] [PubMed] [Google Scholar]
- 20.Kruk J. Physical activity in the prevention of the most frequent chronic diseases: an analysis of the recent evidence. Asian Pac J Cancer Prev 2007;8:325–38. [PubMed] [Google Scholar]
- 21.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res 2011;186:13–42. 10.1007/978-3-642-04231-7_2 [DOI] [PubMed] [Google Scholar]
- 22.Mendes EL, Ribeiro Andaki AC, Brito CJ, et al. Beneficial effects of physical activity in an HIV-infected woman with lipodystrophy: a case report. J Med Case Rep 2011;5:430 10.1186/1752-1947-5-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roubenoff R, Schmitz H, Bairos L, et al. Reduction of abdominal obesity in lipodystrophy associated with human immunodeficiency virus infection by means of diet and exercise: case report and proof of principle. Clin Infect Dis 2002;34:390–3. 10.1086/338402 [DOI] [PubMed] [Google Scholar]
- 24.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS 2011;25:13–20. 10.1089/apc.2010.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global recommendations on physical activity for health. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 26.Center for Disease Control and Prevention. Act Against AIDS: Physical Activity Atlanta. The United States of America. 2017. https://www.cdc.gov/actagainstaids/campaigns/hivtreatmentworks/livewell/physical.html (accessed 13 Nov 2017).
- 27.Frantz JM, Murenzi A. The physical activity levels among people living with human immunodeficiency virus/acquired immunodeficiency syndrome receiving high active antiretroviral therapy in Rwanda. Sahara J 2013;10:113–8. 10.1080/17290376.2014.886081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevisol FS, et al. Association of physical activity with lipodystrophy syndrome in HIV-infected patients. J AIDS Clin Res 2012;3:1 10.4172/2155-6113.1000177 [DOI] [Google Scholar]
- 29.Monroe AK, Brown TT, Cox C, et al. Physical Activity and Its Association with Insulin Resistance in Multicenter AIDS Cohort Study Men. AIDS Res Hum Retroviruses 2015;31:1250–6. 10.1089/aid.2015.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fillipas S, Bowtell-Harris CA, Oldmeadow LB, et al. Physical activity uptake in patients with HIV: who does how much? Int J STD AIDS 2008;19:514–8. 10.1258/ijsa.2007.007237 [DOI] [PubMed] [Google Scholar]
- 31.Trang NH, Hong TK, VAN DER Ploeg HP, et al. Longitudinal physical activity changes in adolescents: Ho Chi Minh City Youth Cohort. Med Sci Sports Exerc 2012;44:1481–9. 10.1249/MSS.0b013e31824e50dc [DOI] [PubMed] [Google Scholar]
- 32.Trang NH, Hong TK, Dibley MJ, et al. Factors associated with physical inactivity in adolescents in Ho Chi Minh City, Vietnam. Med Sci Sports Exerc 2009;41:1374–83. 10.1249/MSS.0b013e31819c0dd3 [DOI] [PubMed] [Google Scholar]
- 33.Tran VD, Lee AH, Jancey J, et al. Community-based physical activity and nutrition programme for adults with metabolic syndrome in Vietnam: study protocol for a cluster-randomised controlled trial. BMJ Open 2016;6:e011532 10.1136/bmjopen-2016-011532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TH, Tang HK, Kelly P, et al. Association between physical activity and metabolic syndrome: a cross sectional survey in adolescents in Ho Chi Minh City, Vietnam. BMC Public Health 2010;10:141 10.1186/1471-2458-10-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bui TV, Blizzard CL, Luong KN, et al. Physical Activity in Vietnam: Estimates and Measurement Issues. PLoS One 2015;10:e0140941 10.1371/journal.pone.0140941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees S, Silove D, Chey T, et al. Physical activity and psychological distress amongst Vietnamese living in the Mekong Delta. Aust N Z J Psychiatry 2012;46:966–71. 10.1177/0004867412459568 [DOI] [PubMed] [Google Scholar]
- 37.Minh HV, Byass P, Wall S. Mortality from cardiovascular diseases in Bavi District, Vietnam. Scand J Public Health Suppl 2003;62:26–31. [DOI] [PubMed] [Google Scholar]
- 38.Bui TV, Blizzard CL, Luong KN, et al. National survey of risk factors for non-communicable disease in Vietnam: prevalence estimates and an assessment of their validity. BMC Public Health 2016;16:498 10.1186/s12889-016-3160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Service UFA. Vietnam retails food sector report. Hanoi, Vietnam: USDA Foreign Agriculture Service, 2016. [Google Scholar]
- 40.UNAIDS. Antiretroviral therapy coverage (% of people living with HIV). 2017.
- 41.National Assembly. Constitution of the Socialist Republic of Vietnam: National Assembly, 2013. [Google Scholar]
- 42.Vietnam Ministry of Health. Decision No 3003/QD-BYT on issuing "Guideline for HIV diagnosis and treatment". 2009.
- 43.Centers for Disease Control and Prevention. Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. 1993.
- 44.Giordano TP, Guzman D, Clark R, et al. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 2004;5:74–9. 10.1310/JFXH-G3X2-EYM6-D6UG [DOI] [PubMed] [Google Scholar]
- 45.Tran BX, Nguyen LT, Nguyen NH, et al. Determinants of antiretroviral treatment adherence among HIV/AIDS patients: a multisite study. Glob Health Action 2013;6:19570 10.3402/gha.v6i0.19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran BX, Ohinmaa A, Nguyen LT. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual Life Outcomes 2012;10:132 10.1186/1477-7525-10-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ternent LMP, Newlands D. Exploring biases in the double bounded dichotomous choice (DBDC) and DBDC with open ended follow-up methods. 2010.
- 48.Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) - Short Form. 2004.
- 49.Grace JM, Semple SJ, Combrink S. Exercise therapy for human immunodeficiency virus/AIDS patients: Guidelines for clinical exercise therapists. J Exerc Sci Fit 2015;13:49–56. 10.1016/j.jesf.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi T, Takeshima N, Rogers NL, et al. Passive and active exercises are similarly effective in elderly nursing home residents. J Phys Ther Sci 2015;27:2895–900. 10.1589/jpts.27.2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerman LM, Barnason S, Schulz P, et al. Rural Versus Urban Comparison: Physical Activity and Functioning Following Coronary Artery Bypass Surgery. Online J Rural Nurs Health Care 2012;12:16–28. [PMC free article] [PubMed] [Google Scholar]
- 52.Yousefian A, Hennessy E, Umstattd MR, et al. Development of the Rural Active Living Assessment Tools: measuring rural environments. Prev Med 2010;50(Suppl 1):S86–92. 10.1016/j.ypmed.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 53.Chrisman M, Nothwehr F, Yang G, et al. Environmental influences on physical activity in rural Midwestern adults: a qualitative approach. Health Promot Pract 2015;16:142–8. 10.1177/1524839914524958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Domelen DR, Koster A, Caserotti P, et al. Employment and physical activity in the U.S. Am J Prev Med 2011;41:136–45. 10.1016/j.amepre.2011.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clingerman E. Physical activity, social support, and health-related quality of life among persons with HIV disease. J Community Health Nurs 2004;21:179–97. 10.1207/s15327655jchn2103_5 [DOI] [PubMed] [Google Scholar]
- 56.Grayson JP, Health GJP. Health, physical activity level, and employment status in Canada. Int J Health Serv 1993;23:743–61. 10.2190/W5NR-A7A4-BX4A-T4F7 [DOI] [PubMed] [Google Scholar]
- 57.Lagerros YT, Lagiou P. Assessment of physical activity and energy expenditure in epidemiological research of chronic diseases. Eur J Epidemiol 2007;22:353–62. 10.1007/s10654-007-9154-x [DOI] [PubMed] [Google Scholar]
- 58.Salmon J, Owen N, Bauman A, et al. Leisure-time, occupational, and household physical activity among professional, skilled, and less-skilled workers and homemakers. Prev Med 2000;30:191–9. 10.1006/pmed.1999.0619 [DOI] [PubMed] [Google Scholar]
- 59.Tran BX, Ohinmaa A, Nguyen LT, et al. Gender differences in quality of life outcomes of HIV/AIDS treatment in the latent feminization of HIV epidemics in Vietnam. AIDS Care 2012;24:1187–96. 10.1080/09540121.2012.658752 [DOI] [PubMed] [Google Scholar]
- 60.Tran BX, Hwang J, Nguyen LH, et al. Impact of socioeconomic inequality on access, adherence, and outcomes of antiretroviral treatment services for people living with HIV/AIDS in Vietnam. PLoS One 2016;11:e0168687 10.1371/journal.pone.0168687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansana V, Sanchaisuriya P, Durham J, et al. Adherence to antiretroviral therapy (ART) among people living with HIV (PLHIV): a cross-sectional survey to measure in Lao PDR. BMC Public Health 2013;13:617 10.1186/1471-2458-13-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andréo C, Bouhnik AD, Soletti J, et al. [Non-compliance in HIV-infected patients, supported by a community association]. Sante Publique 2001;13:249–62. [PubMed] [Google Scholar]
- 63.Blashill AJ, Mayer KH, Crane H, et al. Physical activity and health outcomes among HIV-infected men who have sex with men: a longitudinal mediational analysis. Ann Behav Med 2013;46:149–56. 10.1007/s12160-013-9489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran BX, Nguyen LH, Ohinmaa A, et al. Longitudinal and cross sectional assessments of health utility in adults with HIV/AIDS: a systematic review and meta-analysis. BMC Health Serv Res 2015;15:7 10.1186/s12913-014-0640-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen LH, Nguyen LHT, Boggiano VL, et al. Quality of life and healthcare service utilization among methadone maintenance patients in a mountainous area of Northern Vietnam. Health Qual Life Outcomes 2017;15:77 10.1186/s12955-017-0633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien KK, Tynan AM, Nixon SA, et al. Effectiveness of Progressive Resistive Exercise (PRE) in the context of HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2017;17:268 10.1186/s12879-017-2342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Brien KK, Tynan AM, Nixon SA, et al. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2016;16:182 10.1186/s12879-016-1478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jerome GJ, Dalcin AT, Young DR, et al. Rationale, design and baseline data for the Activating Consumers to Exercise through Peer Support (ACE trial): A randomized controlled trial to increase fitness among adults with mental illness. Ment Health Phys Act 2012;5:166–74. 10.1016/j.mhpa.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harding R, Simms V, Penfold S, et al. Multi-centred mixed-methods PEPFAR HIV care & support public health evaluation: study protocol. BMC Public Health 2010;10:584 10.1186/1471-2458-10-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection: recommendations for a public health approach. 2nd ed Geneva, 2016. [PubMed] [Google Scholar]
- 71.Kalichman SC. Understanding AIDS: a guide for mental health professionals: American Psychological Association, 1995. [Google Scholar]
- 72.Peer Support for Diabetes, Heart Disease and HIV/AIDS: A Review of the Clinical Effectiveness, Cost-effectiveness, and Guidelines. Ottawa 2013. [PubMed] [Google Scholar]
- 73.Peterson JL, Rintamaki LS, Brashers DE, et al. The forms and functions of peer social support for people living with HIV. J Assoc Nurses AIDS Care 2012;23:294–305. 10.1016/j.jana.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care 2008;20:311–7. 10.1080/09540120701594776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.