Abstract
Triple-negative breast cancer (TNBC) is a long-lasting orphan disease in terms of little therapeutic progress during the past several decades and still the standard of care remains chemotherapy. Experimental discovery of molecular signatures including the ‘BRCAness’ highlighted the innate heterogeneity of TNBC, generating the diversity of TNBC phenotypes. As it contributes to enhancing genomic instability, it has widened the therapeutic spectrum of TNBC. In particular, unusual sensitivity to DNA damaging agents was denoted in patients with BRCA deficiency, suggesting therapeutic benefit from platinum and poly(ADP-ribose) polymerase inhibitors. However, regardless of enriched chemosensitivity and immunogenicity, majority of patients with TNBC still suffer from dismal clinical outcomes including early relapse and metastatic spread. Therefore, efforts into more precise and personalised treatment are critical at this point. Accordingly, the advance of multiomics has revealed novel actionable targets including PI3K-Akt-mTOR and epidermal growth factor receptor signalling pathways, which might actively participate in modulating the chemosensitivity and immune system. Also, TNBC has long been considered a potential protagonist of immunotherapy in breast cancer, supported by abundant tumour-infiltrating lymphocytes and heterogeneous tumour microenvironment. Despite that, earlier studies showed somewhat unsatisfactory results of monotherapy with immune-checkpoint inhibitors, consistently durable responses in responders were noteworthy. Based on these results, further combinatorial trials either with other chemotherapy or targeted agents are underway. Incorporating immune-molecular targets into combination as well as refining the standard chemotherapy might be the key to unlock the future of TNBC. In this review, we share the current and upcoming treatment options of TNBC in the framework of scientific and clinical data, especially focusing on early stage of TNBC.
Keywords: triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) is immunohistochemically defined by the lack of oestrogen receptor (ER), progesterone receptor and human epidermal growth factor receptor 2 (HER2) expression.1 2 Although it is generally more chemosensitive than other types of breast cancer, it is also characterised to harbour the most aggressive behaviour with the front-loaded risk of relapse within the first 3–5 years after completion of adjuvant chemotherapy and its prevalence in younger women.3–6 Once metastasised, TNBC has a high predisposition to involve the critical visceral organs such as lung, liver and brain, eventually leading to a significantly shorter median overall survival than in other subtypes.7 8 Therefore, developing optimal therapeutic strategies for the treatment of early TNBC is crucial to alleviate the burden of TNBC. Accordingly, in the last decade, extensive efforts were undertaken to unravel newer therapeutic targets of TNBC based on its molecular landscape.9–15 But unfortunately, there has been little clinical success of targeted therapy, making most of patients with TNBC still mainly dependent on conventional chemotherapy. With the advances of multiomics,9–12 14–16 however, recent experimental investigations revealed a variety of potentially actionable targets including the immune signatures, which are actively engaged in and communicating with the tumour microenvironment.17 These give us a new insight on the molecular heterogeneity of TNBC and ultimately herald a new therapeutic horizon for TNBC beyond conventional chemotherapy.8 In parallel with these efforts, chemotherapeutic strategies have also been scientifically redefined. In this review, we describe recent scientific and therapeutic progress in TNBC, particularly focusing on the current standard and upcoming systemic treatment options in early stages of disease.
Elucidating the heterogeneity of TNBC
Attempts of molecular classification in TNBC
The first established molecular classification for TNBC was suggested by Lehmann et al with six subtypes based on their distinct gene expression profiles,9 which were the basal-like (BL1 and BL2), mesenchymal (M) or mesenchymal stem-like (MSL), immunomodulatory (IM) or luminal androgen receptor (LAR)-enriched tumours. The BL subtypes represented BL-breast cancer (BLBC)-like phenotypes, with expression of genes involved in cell cycle and DNA damage repair (DDR) in the BL1 subtype and growth factor signalling pathways in the BL2 subtype. The two mesenchymal-related subtypes were closely associated with epithelial-mesenchymal transition and relative chemoresistance. Immune-related signatures were abundantly found in the IM subtype and the LAR subtype highly expressed the androgen receptors (ARs) with luminal-like gene expression signature. Interestingly, while phenotype of the LAR subtype resembled that of luminal-like ER-positive breast cancer, it was mainly categorised as HER2-enriched or luminal B subtype by the PAM50 algorithm. In a correlative analysis comparing PAM50 and Lehmann’s classifications, the majority of TNBC subtypes other than MSL and LAR were classified as BLBC.18 Recently, Lehmann et al revised their previous subclassification to a more concise system consisting of only four subtypes (TNBCtype-4): BL1, BL2, M and LAR.10 A noticeable remark from this study was that the IM phenotype was not an isolated molecular subtype, but can exist within all molecular subtypes to varying degrees. Burstein et al similarly classified TNBC into four subtypes: LAR, mesenchymal (MES), basal-like immunosuppressed (BLIS) and basal-like immune-activated (BLIA).11 These substantially overlapped with Lehmann’s classifications, but they uniquely incorporated immune signatures to further divide BL-related subtypes. As expected, BLIS and BLIA behaved clinically in disparate ways; BLIA showed a better prognosis, which was mainly attributable to its more favourable immunological milieu. Ten integrative clusters (IntClust) were identified by combined transcriptomics and genomics approach, and IntClust4 and 10 were suggested as two major subgroups comprising 80% of BLBCs. However, they clinically behaved in different manners due to their distinct molecular features; IntClust4 had a strong immune-related signature with a paucity of copy-number aberrations (CNA-devoid subgroups), whereas IntClust10 largely depended on the genomic instability of major chromosomal aberrations.15 Recently, a new classification which comprehensively incorporated the tumour microenvironment was proposed. With a hypothesis of possible intersection between immunological and metabolic signatures in tumour microenvironment, they suggested a subtype with enriched tumour-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression and activated glycolytic pathways.19 Despite their inherent immune-molecular divergence, an important commonality exists between the different molecular classifications of TNBC, which are represented by basal-likeness, abundant luminal/AR expression, mesenchymal potency and immune signatures. Regarding the clinical relevance of the molecular classifications, a few retrospective studies suggested the potential predictive role of certain molecular subtypes in patients treated with neoadjuvant chemotherapy.10 20 However, it still remains unclear whether the molecular classification itself could be a firm predictive biomarker for patients with TNBC.
BRCAness, a unique molecular trait of TNBC
The term ‘BRCAness’ describes a spectrum of phenotypes derived from the panoply of genotypes that share the biological features of BRCA-deficient tumours, typically with germline BRCA1/2 mutations.21 Although it is yet unclear whether non-canonical alterations such as promoter methylation, somatic BRCA mutations and copy-number variations result in exactly the same functional deficiency as germline BRCA1/2 mutations, these alterations were experimentally suggested to interact with BRCA-related molecules and induce the loss of function of BRCA proteins.22 BRCA1 signalling plays a critical role in prompting higher fidelity of DDR at the point of double-strand breaks (DSBs), mainly through the process of homologous recombination and also by activating other DNA repair pathways. It is referred to transcription-coupled repair,21 23–25 because BRCA1 interacts with other proteins of DNA repair including RAD5126 27 and regulates the whole transcriptional machinery including cell cycle checkpoints, chromatin remodelling and apoptosis.28–31 While 10%–20% of patients carry germline BRCA1/2 mutations in TNBC that largely overlap with the phenotype of BLBC,12 14 most BLBCs do not carry BRCA1/2 mutations. In addition, aberrant expression of BRCA-related proteins was more frequently observed in BLBC except BRCA1. These heterogeneities underlie the complexity of BRCAness and suggest the existence of intricate networks between TNBC and BLBC and BRCAness.32 33 But, at the same time, it enriches the innate genomic instability of TNBC and contributes to therapeutic benefit by enhanced immunogenicity and chemosensitivity to DNA-damaging agents including platinums and poly(ADP-ribose) polymerase (PARP) inhibitors (PARPi).21 34 35
Current treatment options in early TNBC
The decision of treatment for early TNBC faces a great challenge due to the significantly higher risk of early relapse4 7 36–38 coupled with the lack of available treatment options beyond conventional chemotherapy. In this context, efforts should be made to optimise treatment efficacy in patients harbouring greater risk of relapse or high tumour burden, based on the tailoring of standard systemic treatment and further personalization strategy.
Current standard of systemic treatment in early TNBC
Despite the molecular heterogeneity, the standard of systemic treatment for TNBC follows the same general principle with other types of breast cancer.8 39 Thus, neoadjuvant or adjuvant chemotherapy remains a key component of systemic treatment in early TNBC, which is determined primarily by its clinical or pathologic stage.40
Neoadjuvant and adjuvant chemotherapy
Neoadjuvant and adjuvant chemotherapy are the standard systemic treatment for early TNBC, and anthracycline and taxane-based chemotherapy regimens comprise the current standard of care.41 42 In previous pivotal neoadjuvant trials, patients with TNBC showed significantly higher response rates to anthracycline and taxane-based chemotherapy than those of other subtypes, achieving pathologic complete response (pCR) rates of approximately 40%.42–44 Moreover, as pCR showed a significant correlation with therapeutic benefits, it has been established a robust surrogate biomarker for long-term survival outcomes.7 44–47 Although the guideline for adjuvant chemotherapy is generally similar for each breast cancer subtype, adjuvant chemotherapy in TNBC is recommended for primary tumours larger than 0.5 cm due to their aggressive behaviour.48
Regarding the paradox that TNBC carries both higher chemosensitivity and the risk of early relapse,49 efforts have been continued to develop more effective chemotherapeutic regimens for both responders and non-responders. Clinical trials of newer combinations and dose-determination studies that evaluated metronomic or dose-dense regimens suggested the feasibility of refining conventional chemotherapy.43 50–53 More recently, patients with an initially high tumour burden or residual disease after neoadjuvant chemotherapy were identified as compelling candidates for intensive systemic treatment, as they carry higher chance of relapse and metastatic spread. The CREATE-X trial demonstrated the potential survival benefit of adding capecitabine to the standard adjuvant chemotherapy regimen in early TNBC with a residual tumour burden after neoadjuvant treatment.54 A meta-analysis also provided a rationale for adding capecitabine to either neoadjuvant or adjuvant standard chemotherapy in patients with TNBC.55 Despite its substantial toxicity, additional capecitabine might be a reasonable option for patients with TNBC carrying a higher risk of relapse. Furthermore, in these patients, postneoadjuvant trials based on actionable molecular targets identified from residual tumour tissues should also be considered. Molecular profiling of residual TNBC might be helpful to identify genetic alterations involved in drug resistance in the neoadjuvant setting and to further guide adjuvant targeted therapy to eradicate the chance of clinically silent micrometastases at the time of surgery. The additive benefit of platinum-based agents, which have been consolidated in the neoadjuvant and adjuvant setting in the subset of patients with TNBC which had BRCAness, will be discussed more in the next section.
The role of platinum-based chemotherapy in early TNBC
In many preclinical studies, an unusual sensitivity to platinum-based agents was suggested in certain subset of TNBC, mainly due to genomic instability from DDR impairment.31 56 Subsequent clinical trials of metastatic TNBC showed a modest efficacy of platinum-based monotherapy,57–60 consistently suggesting a greater benefit in BRCA1/2 mutation carriers. In early TNBC, patients with stage II–III treated with neoadjuvant cisplatin alone showed a 22% pCR rate in a small retrospective study, in which only 7% of patients carried germline BRCA mutations. Further phase II studies exclusively for patients with BRCA1 mutation demonstrated markedly higher pCR rates from 61% to 90% after neoadjuvant cisplatin monotherapy, which validated the significance of BRCAness in predicting platinum sensitivity in TNBC.61–63 In another neoadjuvant study of carboplatin combined with eribulin, the homologous recombination deficiency (HRD) score was suggested as a potential predictive biomarker.64 As these earlier studies supported the rationale of adding platinum to the standard neoadjuvant chemotherapy in TNBC, two landmark phase II trials evaluated combination of platinum with anthracycline/taxane-based regimens, alone or with other targeted agents; In the CALGB40603 trial,65 the pCR rate was significantly improved from 41% to 54%, in patients who received neoadjuvant chemotherapy combining carboplatin and/or bevacizumab with paclitaxel followed by dose-dense doxorubicin and cyclophosphamide (ddAC). The GeparSixto trial66 similarly showed significantly enhanced pCR rates from 36.9% to 53.2% by adding carboplatin to combinations of ddAC and taxane-based chemotherapy with bevacizumab. In their recent secondary analysis, treatment benefit was consistently maintained even in patients without BRCA1/2 mutations.67 In another phase II PreECOG 0105 study, however, patients with BRCA1/2 mutations achieved the highest pCR rate (56%) after neoadjuvant combination chemotherapy with gemcitabine, carboplatin and the PARPi iniparib, while high score of HRD-loss of heterozygosity (LOH) showed a significant correlation with objective response rates (ORR) in patients without BRCA1/2 mutations. HRD-LOH score, as suggested in the study, has been often regarded a powerful estimation tool of DNA repair capacity, thus correlating with BRCAness. However, the predictive value of the HRD-LOH assay for platinum sensitivity still remains controversial when taking account of discordance from prior metastatic TNBC trials including the TBCR009 and the Triple Negative Breast Cancer Trial.57 60 In later phase II neoadjuvant trials, paclitaxel combined either with cisplatin or carboplatin yielded encouraging efficacy outcomes with improved pCR and survival outcomes, which also suggested a strong relationship between clinical benefit and genetic alterations of DDR pathway.68 69 These results altogether seemed to support the notion of adding platinum to conventional neoadjuvant chemotherapy in the subset of patients with early TNBC, which might be optimised in the context of BRCAness. However, we should give a particular concern about the absence of statistically valid survival benefits in previous studies, for pCR might not always translate into a long-term survival benefit. Because the CALGB40603 and GeparSixto trials were somewhat underpowered studies to draw robust conclusions about the long-term survival benefit beyond pCR improvement, platinum-based combination chemotherapy has been hampered as a new standard neoadjuvant treatment.42 Safety concern with combination is another important issue that should not be overlooked. Therefore, platinum-based combination chemotherapy should be selectively applied in the optimal candidates carrying the BRCAness or high risk of relapse with extensive tumour burden or in a younger age, who necessitate enriched locoregional control. Currently, several neoadjuvant trials of platinum-based combination chemotherapies are underway, including the PEARLY trial (NCT02441933), which is evaluating neoadjuvant taxane with or without carboplatin after doxorubicin/cyclophosphamide (AC) chemotherapy. In an adjuvant setting, a phase III trial of carboplatin monotherapy is about to begin for patients with residual TNBC after conventional neoadjuvant chemotherapy (NCT01752686), and trials adding cisplatin or carboplatin to taxanes with or without doxorubicin-based combination chemotherapy are underway. Completed and ongoing clinical trials in early TNBC are summarised in table 1.
Table 1.
Key completed and ongoing clinical trials of platinum-based chemotherapy for early-stage TNBC, including both monotherapy and combination therapies
| Phase | NCT ID number | Defined breast cancer subtype | Setting | Stage | Experimental drugs | Control | Primary endpoint | |
| Platinum monotherapy | ||||||||
| Completed | II | NCT00148694 | TNBC | Neoadjuvant | NA | Cisplatin (4C) | NA | pCR: 22% |
| II | NCT01630226 | LABC with BRCA1mt | Neoadjuvant | I–III | Cisplatin (4C) | NA | pCR: 61% (in TNBC) | |
| Ongoing | III | Post-NAC study (NCT01752686) (not recruiting yet) |
TNBC Residual after NAC |
Adjuvant | NA | Carboplatin (6C) | Observation as per guideline | DFS |
| Platinum-based combination | ||||||||
| Completed | II | NCT02934828 | Operable TNBC or HER2(+) | Neoadjuvant | I–III | Carboplatin+Eribulin (4C) | NA | pCR: 43.3%*gBRCAm 66.7% |
| II | SHPD001 (NCT02199418) |
LABC, including TNBC | Neoadjuvant | II–III | Cisplatin (4C)+wPaclitaxel (16 weeks) | NA | pCR: 64.7% in TNBC | |
| II | PreECOG 0105 (NCT00813956) |
TNBC or BRCA1/2mt | Neoadjuvant | I–IIIA | Gemcitabine+Carboplatin+Iniparib | NA | pCR: 36% (all) vs 56% (gBRCAmt) | |
| IIB | CALGB40603 (NCT00861705) |
Hormone Receptor (–) and HER2(–) |
Neoadjuvant | IIA-IIIA |
|
Standard NAC | pCR: 62.4% vs 22.3% | |
| IIB | GeparSixto (NCT01426880) |
TNBC or HER2(+) | Neoadjuvant | II, III | Carboplatin+Bevacizumab+standard NAC | Bevacizumab+standard NAC | pCR: 53.2% vs 36.9% | |
| IIB | NCT00930930 | TNBC | Neoadjuvant | II–III | Weekly Cisplatin+wPaclitaxel+Everolimus | wCisplatin+wPaclitaxel | pCR: 36% vs 49% | |
| IIB |
NCT01276769 (unknown) |
Locally advanced TNBC | Neoadjuvant | NA | Paclitaxel+Carboplatin | Paclitaxel+Epirubicin | pCR: 38.6% vs 14.0% (0.014) 5-year RFS: 77.6% vs 56.2 (p=0.043) |
|
| Ongoing | II | NCT02934828 | TNBC or HER2(+) | Neoadjuvant | II, III |
|
NA | DFS |
| II | NCT00148694 | TNBC | Neoadjuvant | II–III | Carboplatin+Docetaxel | Observation as per guideline | Predictors of pCR | |
| II | NCT02315196 | TNBC | Neoadjuvant | II, III |
|
NA | pCR | |
| II/III | SHPD002 (NCT02221999) |
TNBC (and ER+) | Neoadjuvant | NA | Weekly Paclitaxel+Cisplatin (4C) | NA | pCR | |
| III | NCT03168880 | Large, operable TNBC | Neoadjuvant | NA |
|
Paclitaxel | pCR: 62.4% vs 22.3% | |
| III | PEARLY Trial (NCT02441933) |
Operable TNBC | (Neo)adjuvant | II–III | AC → Taxane+Carboplatin (4C) | AC → Taxane | 5-year EFS | |
| III | NCT02455141 | TNBC | Adjuvant | NA | EC → Taxane+Carboplatin (4C) | EC → Taxane | DFS | |
| III | NCT02488967 | High-risk N(–) or N(+) TNBC | Adjuvant | IB–III | AC → Paclitaxel+Carboplatin | AC → Paclitaxel | pCR: 56% in gBRCAmt | |
| III | NCT02879513 | LABC, pathologic PR after NAC | Adjuvant | NA | Paclitaxel+Cisplatin (2C) | CEF (4C) | ||
| II |
NCT03201861 (not recruiting yet) |
High-risk HER2(–) EBC, including TNBC | Adjuvant | NA |
|
EC (4C) → Taxane |
DFS | |
AC, doxorubicin and cyclophosphamide; CEF, cyclophosphamide, epirubicin, and 5-fluorouracil; DFS, disease-free survival; EBC, early breast cancer; EC, epirubicin and cyclophosphamide; EFS, event-free survival; RFS, relapse-free survival; gBRCAmt, germline BRCA mutation; HER2, human epidermal growth factor receptor 2; LABC, locally advanced breast cancer; NA, not available; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; PR, progesterone receptor; TNBC, triple-negative breast cancer.
Upcoming treatment options beyond conventional chemotherapy
The panoply of old and new targets
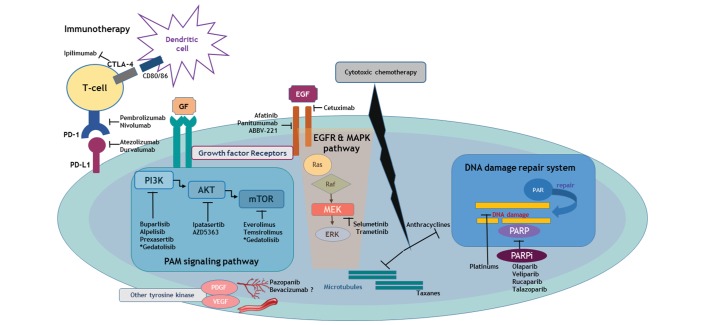
The advance of multiomics has introduced a vast range of actionable targets and accelerated the new horizon of targeted therapies, which could be particularly efficacious in TNBC subsets that are expected to be relatively chemoresistant (figure 1). However, widening the road of treatment spectrum does not always guarantee consequent therapeutic benefit. Therefore, we should recognise that molecular heterogeneity is a double-edged sword, which could be the hope or the hype for TNBC.
Figure 1.
Signalling pathways and involved entities that are unravelling experimental therapeutic targets for TNBC. Depicted molecular landscape of TNBC confers an insight of novel and investigational targeted therapeutic strategy which are directly unlocking its heterogeneous biology. In the context of its intrinsic genetic instability which derives an immunogenic microenvironment, blockade of the immune-checkpoint targeting PD-1 and PD-L1 as well as CTLA-4 can boost the adaptive immune reaction. PAM signalling pathways are actively participating in cell cycle regulation, which are in the tight network with various growth factors including EGF and MAPK signalling. Platinum-based agents and PARPi is a master regulator of DNA damage repair and can induce synergistic inhibitory effect in TNBC harbouring BRCAness. Other multikinase inhibitors involving angiogenesis or developmental process are also a potential therapeutic entity of current interest. All these investigational but key targets are consistently interacting with cytotoxic effect of conventional chemotherapy. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular signal-related kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; PAM, PI3K-Akt-mTOR; PARP, poly(ADP-ribose) polymerase; PARPi, PARP inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TNBC, triple-negative breast cancer.
Targets that light the BRCAness of TNBC
PARPi
Because of their critical role in the process of DDR, PARPi have been thought to be a potential game changer for TNBC. PARPs, specifically PARP1 and 2, are enzymes that facilitate DDR at sites of single-strand breaks by activation of various intracellular signalling pathways through auto-poly(ADP)-ribosylation.70–72 A meaningful link between PARP inhibition and BRCAness is demonstrated by the concept of synthetic lethality. As BRCA1/2 deficiency leads to HRD, a dysfunction of the cell’s intrinsic DNA repair mechanism, repair of DNA damage solely depends on the action of PARP1 in BRCA-deficient breast cancer.73 74 Therefore, inhibiting PARP1 in patients with BRCAness might induce accumulation of DSBs and eventually result in synthetic lethality, which could in turn enhance the sensitivity to PARPi.73–75 Based on this scientific evidence, earlier clinical data suggested that a subset of patients with TNBC having BRCA deficiency might benefit from PARPi treatment.76 In the pivotal phase III OlympiAD trial of metastatic breast cancer,77 investigators compared the efficacy of monotherapy with olaparib with that of standard single chemotherapy at the discretion of physician in metastatic HER2-negative breast cancer with germline BRCA1/2 mutations. The subgroup of patients treated with olaparib had nearly double the response rate (59.5%) as well as a longer median progression-free survival (PFS; 7.0 months) and a better toxicity profile. Based on these dramatic treatment outcomes, PARPi have been recently approved for breast cancer with BRCA1/2 mutations, which mainly constitutes TNBC. Other PARPi than olaparib have been also vigorously investigated initially in metastatic breast cancer, which include veliparib, talazoparib and rucaparib. Once a phase I study of talazoparib showed favourable efficacy and safety profiles in advanced solid tumours with deleterious BRCA1/2 mutations including breast cancer (NCT01945775),78 feasibility trials of veliparib in combination with other alkylating agents are also undergoing in advanced or metastatic TNBC.79–83 And recently, a phase III trial EMBRACA comparing talazoparib and treatment by physician’s choice in metastatic TNBC revealed significant benefit of talazoparib with better PFS and ORRs.84
In early TNBC, the phase III OlympiA trial (NCT02032823) is currently ongoing to evaluate adjuvant olaparib monotherapy for a year after standard neoadjuvant chemotherapy and local treatment in high-risk TNBC with germline BRCA1/2 mutations. PARTNER, another phase II/III trial of neoadjuvant olaparib in combination with carboplatin followed by the standard chemotherapy, is also under investigation in patients with breast cancer with TNBC or germline BRCA mutations (NCT03150576).85 The I-SPY 2 trial evaluated neoadjuvant veliparib and carboplatin (VC) in addition to the standard chemotherapy in patients with high-risk breast cancer and TNBC seemed to gain the most significant benefit from this combination therapy (pCR rates: 52% vs 24%).86 In a recent biomarker analysis of the I-SPY2, a genomics-based signature of BRCA1ness was suggested as a significant predictive biomarker of response to neoadjuvant VC.87 However, the true benefit from adding PARPi to platinum-based chemotherapy is still controversial, for platinum itself already showed its efficacy either as monotherapy or in combination.65 66 Another phase II neoadjuvant trial in high-risk, residual TNBC after standard neoadjuvant chemotherapy failed to show a significant therapeutic benefit from the combination of low-dose rucaparib and cisplatin compared with cisplatin alone, in terms of both toxicity and survival outcomes, although the applied dose of rucaparib has been considered therapeutically insufficient (NCT01074970).88 Therefore, the plausibility of combining these two chemosensitisers of germline BRCA-mutant TNBC remains questionable at this point. Currently, the efficacy of neoadjuvant veliparib with radiation therapy is under exploration in a phase I study for node-positive, residual breast cancer after neoadjuvant chemotherapy (NCT01618357), and another phase I trial of neoadjuvant monotherapy with the novel PARPi niraparib is about to be initiated (NCT03329937) (table 2). Last, strategies for restoring BRCAness in order to prevent or counteract acquired resistance to PARPi are now actively underway.
Table 2.
Ongoing clinical trials of PARPi for patients with early-stage TNBC
| Phase | NCT ID number | Defined breast cancer subtype | Setting | Stage | Experimental drugs | Control | Primary endpoint |
| PARPi monotherapy | |||||||
| I | NCT01618357 | Node (+) BC, Residual after NAC | Neoadjuvant | NA |
|
Standard NAC | MTD |
| I |
NCT03329937 (not recruiting yet) |
HER2(–), BRCA1/2mt | Neoadjuvant | NA | Niraparib | NA | Preliminary antitumour activity Measured by breast MRI |
| II | NCT02282345 | Invasive BC and deleterious BRCAmt | Neoadjuvant | I–III | Talazoparib | Observation as per guideline | IDFS |
| III | OlympiA (NCT02032823) |
gBRCA1/2mt High-risk HER2(–) BC including TNBC |
Adjuvant (after NAC) | Olaparib up to maximum 1 year | Placebo | IDFS | |
| PARPi-based combination | |||||||
| II/III | PARTNER (NCT03150576) |
TNBC or gBRCAmt | Neoadjuvant | II, III |
|
Standard NAC | pCR |
| II | I-SPY 2 (NCT01042379) *Neoadjuvant, personalised adaptive trial with novel agents |
Locally advanced TNBC | Neoadjuvant | II, III |
|
Standard NAC | pCR: 52% vs 24% (*preliminary result) |
| II | NCT01074970 | gBRCA1/2 mt or TNBC Residual disease after NAC |
Neoadjuvant | I–III |
|
Cisplatin after neoadjuvant A or T | 2-year DFS |
AC, doxorubicin and cyclophosphamide; DFS, disease-free survival; gBRCAmt, germline BRCA mutation; HER2, human epidermal growth factor receptor 2; IDFS, invasive DFS; MTD, maximal tolerated dose; NA, not available; NAC, neoadjuvant chemotherapy; PARPi, poly(ADP-ribose) polymerase inhibitors; pCR, pathologic complete response; TNBC, triple-negative breast cancer.
Revisiting the PI3K-Akt-mTOR (PAM) pathway in TNBC
Data from the Cancer Genome Atlas (TCGA) revealed that the major genetic aberrations observed in TNBC occurred within the PI3K-Akt-mTOR (PAM) pathway,12 and accumulating data from next-generation sequencing (NGS) studies further confirmed the PAM pathway as an appealing actionable target in TNBC.89 90 PIK3CA hotspot mutations and aberrations in phosphatase and tensin homolog (PTEN) are the two most frequent but mutually exclusive alterations, accounting for approximately 10% and 35%–50% of TNBC, respectively.8 As in other subtypes of breast cancer, targeting the PAM pathway has been heavily investigated in TNBC and expanded to other molecules that are in a circuit of cross-talk with the PAM signalling, including the epidermal growth factor receptor (EGFR) and mitogen-activated protein kinase (MAPK) pathways.
Inhibitors of the PAM pathway: from preclinical to clinical data
The PAM pathway comprehensively controls the cell cycle from survival to apoptosis and is therefore vitally involved in tumourigenesis and its progression. PAM signalling is primarily modulated by the key molecule PI3K, but is also regulated by active communication with other growth factor tyrosine kinase receptors, including EGFR and insulin-like growth factor 1 receptor (IGF1R). Activation of the PAM pathway often confers chemoresistance in breast cancer. In TNBC, PI3K was capable of enhancing the effects of BRCA1/2 mutations by interacting with the homologous recombination machinery, stabilising DNA DSBs. Thus, inhibition of the PI3K pathway with buparlisib, an oral pan-PI3K inhibitor, produced promising antitumour cytotoxicity in TNBC cell lines.91 Experimentally, it also enhanced sensitivity to PARPi in both BRCA1/2-deficient and BRCA1/2-sufficient TNBC cell lines by activation of extracellular signal-related kinase (ERK) and MAPK kinase (MEK1), which induced downregulation of BRCA1/2.92–94 Regardless of these encouraging preclinical studies, subsequent clinical trials showed somewhat disappointing results as in the recent BELL-4 trial, which failed to prove a benefit from combination treatment with buparlisib and paclitaxel in advanced HER2-negative breast cancer.95 Given that efficacy of cotargeting is strongly supported by recent preclinical data,96 97 combining another targeted therapy along with PI3K inhibitors might be a compelling overcoming strategy. Accordingly, a phase I study with olaparib and an oral pan-PI3K inhibitor, buparlisib, is ongoing in recurrent TNBC and high-grade ovarian cancer (NCT01623349).
AKT, which is activated by PI3K, serves as another central node in the PAM signalling pathway. Once preclinical studies demonstrated the antitumour activity of the AKT inhibitors in TNBC,98–100 many clinical trials were initiated including the phase II PAKT trial evaluating the combination of AZD5363 and paclitaxel. The latest phase II trial, LOTUS,101 evaluated the combination of paclitaxel and ipatasertib, a highly selective ATP-competitive AKT inhibitor and showed its efficacy in patients with advanced TNBC, demonstrating significantly improved PFS compared with paclitaxel alone. This trial was the first study showing a significant PFS benefit of anti-PAM pathway targeted agents in IHC-defined TNBC population, although it was not maintained in the PTEN-low subset of patients, which was assumed as an appealing candidate for the drug. However, when PIK3CA/AKT1/PTEN alterations were refined based on NGS, the benefit in the subset was greater, suggesting that NGS-derived genomic biomarkers might better define the target population. Clever selection of targets might be another critical point of the LOTUS data, which newly highlighted AKT as a powerful druggable target of the PAM signalling pathway. Theoretically, AKT could be a preferable target to PI3K from the perspective of selectivity or it might act as a more direct functional regulator of PAM signalling, interacting with intertwining feedback loops across messenger molecules. There is a paucity of data regarding PAM inhibitors in early TNBC except for a previous phase II neoadjuvant trial combining mTOR inhibitors with conventional FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) chemotherapy, which unfortunately did not show clinical benefit.102 However, in a recent phase I study, temsirolimus or everolimus in addition to liposomal doxorubicin and bevacizumab showed notable efficacy in the mesenchymal subset of TNBC, predominantly in patients with an aberrant PI3K pathway (NCT00761644).103 Summarised clinical trials targeting the PAM pathway, which are currently ongoing, are mainly in the metastatic setting (online supplementary table 1).
esmoopen-2018-000357supp001.pdf (233.7KB, pdf)
EGFR inhibitors and the PI3K pathway
Although amplification of EGFR is rare among patients with breast cancer in general, the frequency of EGFR overexpression is markedly higher in TNBC ranging from 13% to 76%, which is considered a poor prognostic factor for TNBC.104 Based on the dynamic molecular network between EGFR and PAM signalling,105 preclinical studies revealed a therapeutic synergism between anti-EGFR targeted treatment and DNA damaging agents such as platinum or PARPi, by increasing chemosensitivity in TNBC cell-lines with BRCA1 deficiency but intact PTEN.105–107 Unfortunately, subsequent clinical trials of EGFR tyrosine kinase inhibitors, alone or in combination, were disappointing and actually showed a more favourable response in non-TNBC subtypes.104 However, in two previous phase II trials of metastatic TNBC, adding cetuximab to cisplatin or carboplatin improved clinical outcomes compared with platinum-based monotherapy regardless of statistical insignificance.108 109 Cetuximab in combination with ixabepilone or irinotecan also showed a slightly higher response rate, but this did not translate to a survival benefit.110 111 Another recent phase II trial also failed to prove a significant benefit of adding panitumumab to the combination of gemcitabine and carboplatin in patients with metastatic TNBC, but the triple combination seemed feasible in the patient cohort.112 Given that these studies precluded biomarker-driven selection of target populations, further studies of EGFR inhibitors, particularly anti-EGFR mAbs, should be performed in a molecularly predefined subset of TNBC cases. In this context, several trials of metastatic TNBC are ongoing in selected cohorts harbouring alterations of the EGFR and/or PAM pathways, including the afatinib arm of the NCI-MATCH (NCT02465060) trial (online supplementary table 2).
In early TNBC, combination neoadjuvant trials of cetuximab and panitumumab with docetaxel after FEC showed modest efficacy. In these trials, the number of TILs could predict the response to anti-EGFR mAbs, suggesting an important association between the EGFR signalling pathway and T cell-mediated immunity.113 114 Currently, a phase II study of neoadjuvant panitumumab, carboplatin and paclitaxel (PaCT) is underway in patients with chemoresistance to standard treatment or inflammatory breast cancer (NCT02593175, NCT02876107). A phase II trial of neoadjuvant afatinib, alone and in combination with paclitaxel, is also recruiting patients (NCT02511847).
Significance of antagonising MEK activation
The mechanism of MAPK activation in TNBC and BLBC was initially investigated in vitro. Although these cell lines seldom harbour activating mutations of canonical oncoproteins such as Ras or c-Myc, they frequently harbour copy-number variations in these oncogenes or show overexpression of other growth factor receptors such as EGFR, IGF1R, vascular endothelial growth factor receptor (VEGFR) or fibroblast growth factor receptor 1. Dual-specificity phosphatase 4, a negative regulator of ERK1/2 and c-Jun N-terminal kinase 1/2, might also contribute to activation of MAPK signalling in TNBC, in that its loss or downregulation, either by genetic or epigenetic mechanisms, is associated with chemoresistance. Based on these molecular characteristics, MEK inhibitors were suggested as an attractive therapeutic option in TNBC, particularly in BLBC with intact PTEN, and PTEN itself was suggested as a potential negative predictor of response.115 However, in the presence of abundant signalling cross-talk, MEK inhibitors alone could not efficiently suppress activation of the MAPK pathway. Accordingly, combination treatment with MEK inhibitors and other targeted therapies or chemotherapeutic agents have been proposed to overcome the consequent chemoresistance including a phase II COLET trial evaluating upfront combination of cobimetinib and paclitaxel. The COLET showed a promising activity to control the resistance against taxane chemotherapy in the early data.116 117 Another recent data reported a therapeutic synergy when combining the MEK inhibitor selumetinib with either buparlisib or the platelet-derived growth factor receptor inhibitor pazopanib, which was even effective for brain metastases of TNBC.118 Among numerous combination trials of MEK inhibitors currently ongoing, a phase Ib trial combining the MEK inhibitor trametinib with gemcitabine in advanced solid tumours showed a case of complete response in the patient with metastatic TNBC.119 On the other hand, phase I trials evaluating combination treatment of MEK inhibitors either with mTOR or PI3K inhibitors (NCT01476137, NCT0095573) revealed unacceptable fatal toxicities, prohibiting subsequent phase II studies.120 121 They showed the potential lethality of coblockade of master signalling pathways, including MAPK and PAM, which should be taken into consideration when designing future combinatorial trials with targeted agents.
Immunotherapy for TNBC
The immune microenvironment as a component of TNBC heterogeneity
The immune system plays a dual role in breast cancer, that is, the tumour initially induces innate immunity, but later suppresses adaptive immunity, ultimately resulting in disease progression. Due to its genomic instability and high mutational burden, tumour microenvironment of TNBC is considered to be ‘hot’ with abundant infiltrating immune cells, which are actively engaged in the process of ‘immunoediting’.8 122 TILs, mainly the CD8 +T cells, are the most famous immune-related player in breast cancer.123–125 In TNBC treated with neoadjuvant treatment, TILs was identified as a robust predictive biomarker of long-term survival and its significance in remnant disease was subsequently validated,126–132 revealing an active communication between immune system and cytotoxic agents.124 126 129 131 133–136 Although relatively unexplored, the significance of TILs in the adjuvant setting has also been suggested in recent years.127 129–131 135 Of note, the latest study showed the prognostic significance of TILs even in systemically untreated early TNBC, suggesting that the presence of TILs may refine the candidates for adjuvant chemotherapy or immunotherapy.137 Schmid and colleagues recently showed in metastatic TNBC that response rate and overall survival after atezolizumab treatment significantly correlated with the level of TILs.138 In addition, a lower level of TILs was significantly associated with aberrant activation of Ras-MAPK signalling, which can promote immune evasion in TNBC.139 Correlation between TILs and programmed cell death 1/ligand 1 (PD-1/PD-L1) expression was suggested in recent experiments, which assumed the existence of a feedback loop regulating PD-L1 expression as a means of immune homeostasis. Several retrospective studies of early TNBC demonstrated significantly worse survival outcomes in patients harbouring high PD-L1 expression and a low number of TILs or a high ratio of PD-L1/CD8 expression.140–142 By contrast, immunogenic factors potentially involved in the expression of neoantigens positively correlated with higher TILs and a more favourable prognosis.141 143 144 Taken together, TILs play a significant role in orchestrating the immune microenvironment and vigorously interact with cytotoxic signals including both chemotherapy and immunotherapy.145 146 Taken together, TILs could serve as a currently available predictive and prognostic immune biomarker, and at the same time, immune molecules could be another appealing therapeutic target of TNBC.
Efficacy outcomes of monotherapy with immune checkpoint inhibitors
Although TNBC has been assumed the potential protagonist of immunotherapy in breast cancer,122 147 no immunotherapy has yet been officially approved for breast cancer. However, earlier efficacy data on cytotoxic T-lymphocyte-associated protein 4 inhibitors148 149 and accumulating recent clinical evidences demonstrated a durable response in a small subset of patients with metastatic TNBC, raising the hope of success with immunotherapy.
In earlier phase I trial of atezolizumab in advanced solid cancers including heavily pretreated TNBC, the response was strikingly durable in responders although only 10% of patients responded; the survival rate of patients who achieved an ORRs was 100% at over 2 years.150 Phase I (KEYNOTE-012) and II (KEYNOTE-086) studies of pembrolizumab in metastatic TNBC showed modest ORRs of approximately 20% with several complete responses, while the median duration of the response was not reached at the time.151–153 Phase II trial was performed in two different cohorts (A and B) according to the disease setting and PD-L1 expression; cohort A enrolled patients with any level of PD-L1 expression who would receive pembrolizumab as salvage therapy, while cohort B only included patients with positive PD-L1 expression treated with first-line pembrolizumab. Although PD-L1 expression did not significantly affect the treatment outcomes in cohort A, patients in cohort B showed a higher response rate than cohort A. These studies with atezolizumab and pembrolizumab altogether suggest that the therapeutic benefit could be maximised when given as upfront treatment and/or possibly in patients with predefined PD-L1 expression. Also, in the phase I JAVELIN trial, patients with heavily pretreated metastatic TNBC treated with the PD-L1 antibody avelumab showed encouraging efficacy outcomes with a 31% disease control rate and PD-L1 expression were closely associated with response.154 Currently, a phase II trial of pembrolizumab monotherapy for BRCA-mutated breast cancer is underway (NCT03025035) (table 3).
Table 3.
Ongoing clinical trials of immune checkpoint inhibitors for patients with early-stage TNBC
| Phase | NCT ID | Defined breast cancer subtype | Setting | Stage | Experimental drugs | Control | Primary endpoint |
| IO monotherapy | |||||||
| III | SWOG1418 (NCT02954874) |
Residual TNBC (ypT>1 cm or ypN+) |
Adjuvant after NAC | NA | Pembrolizumab for 1 year | Observation as per guideline | Invasive DFS (IDFS) |
| III | NCT02926196 | High-risk TNBC | Adjuvant or post-NAC | NA | Avelumab for 1 year | Observation as per guideline |
|
| IO-based combination | |||||||
| II | I-SPY 2 (NCT01042379) *Neoadjuvant, personalised adaptive trial with novel agents |
LABC including TNBC | Neoadjuvant | II, III |
|
Standard NAC | pCR: 62.4% vs 22.3% |
| IB | KEYNOTE-173 (NCT02622074) |
Locally advanced TNBC | Neoadjuvant | II, III | (Arm A)
|
NA | pCR (Arm A vs B): 60% vs 90% |
| III | KEYNOTE-522 (NCT03036488) |
TNBC | Neo/adjuvant | NA | (Neoadjuvant)
|
Placebo rather than Pembrolizumab | pCR, EFS |
| I/II | NCT02489448 | TNBC | Neoadjuvant | I–III |
|
NA | pCR |
| II | Triple-negative first-line study (NCT02530489) | TNBC | (Neo)adjuvant | NA |
|
NA | pCR |
| III | NeoTRIPaPDL1 (NCT02620280) |
Locally advanced TNBC | Neoadjuvant | NA | Atezolizumab+Nab-paclitaxel+Carboplatin | Nab-paclitaxel+Carboplatin | EFS |
| Ib | NCT02826434 | TNBC | Adjuvant | II/III |
|
NA | DLT of PVX-410 in combination with Durvalumab |
(dd)AC, (dose-dense) doxorubicin and cyclophosphamide; DFS, disease-free survival; DLT, dose-limiting toxicity; EFS, event-free survival; IDFS, invasive DFS; LABC, locally advanced breast cancer; NA, not available; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; PD-L1, programmed death ligand 1; TNBC, triple-negative breast cancer.
Finding new combinatorial partners
Monotherapy with immune-checkpoint inhibitor revealed disappointing results in the metastatic trials with ORRs less than 10%. Hence, current efforts are concerted on developing combination strategies with immune checkpoint inhibitors, based on the remarkably durable responses in the subset of responders shown in previous studies (table 3).
Immune-based chemotherapy
Early data of nab-paclitaxel combined with atezolizumab showed a 40% ORR in metastatic TNBC, which hastened a phase III trial currently underway (NCT02425891). The combination of eribulin and pembrolizumab is being tested in an ongoing trial for heavily pretreated metastatic TNBC, and the interim analysis revealed a 41.2% ORR to first-line treatment and a 27.3% ORR to later-line treatment.155 These trials involving eribulin and paclitaxel, which are regarded modulators of immune priming, have once again suggested the importance of early application of immunotherapy in systemic treatment for metastatic TNBC. However, PD-L1 status failed to predict treatment response to either combination. Currently, KEYNOTE-355, a phase III trial evaluating the combination of pembrolizumab plus conventional chemotherapy compared with chemotherapy alone as the first-line treatment, is running in metastatic TNBC (NCT02819518). The combination of durvalumab and nab-paclitaxel followed by dose-dense conventional chemotherapy as well as the combination of avelumab and an antibody to another immune modulator, 41BB, is under investigation in advanced solid tumours, including TNBC (NCT02489448).
In early TNBC, preliminary results from the neoadjuvant I-SPY 2 trial demonstrated that pCR rates increased from 22.3% to 62.4% by adding neoadjuvant pembrolizumab to paclitaxel followed by anthracycline-based chemotherapy, which represents an approximately 40% improvement in pCR compared with standard chemotherapy alone.156 The KEYNOTE-173 trial also showed a remarkably increased pCR rate from 60% to 90% in high-risk patients by combining pembrolizumab with paclitaxel or conventional chemotherapy according to the physician’s discretion.157 In the adjuvant setting, the SWOG1418 phase III trial is evaluating adjuvant monotherapy with pembrolizumab after neoadjuvant chemotherapy followed by curative surgery. Another phase III trial for high-risk patients with early TNBC is investigating the addition of avelumab for a year after standard curative treatment including (neo)adjuvant chemotherapy (NCT02926196). A phase I study for the feasibility of adjuvant durvalumab with a peptide vaccine is underway for patients with stage II and III TNBC after completion of standard adjuvant therapy (NCT02826434).
Combining targeted agents with immune checkpoint inhibitors
In recent experimental study, PARPi modulated cancer-associated immunosuppression by upregulating PD-L1 in breast cancer cell lines, suggesting that blockade of PD-L1 could restore their sensitivity to PARPi. Followed xenograft study of combining PARPi to a PD-L1 inhibitor revealed significant synergistic effect compared with either agent alone.158 Accordingly, the feasibility of combination treatment with durvalumab with either a PARPi or a VEGFR inhibitor is under exploration in current phase II trial.159 As MAPK signalling pathway is highly activated and involved in regulating the level of TILs in TNBC,8 TNBC and BLBC cell lines showed broad sensitivity to MEK inhibition, and the generation of effector T cells was enriched by the treatment. These results suggested the MEK inhibitor as another weapon to harness immune surveillance, and more importantly, potential synergy with immune checkpoint inhibitors is suggested.139 On the basis of these experimental results, a phase Ib trial of MEK inhibitor in combination with a PD-L1 antibody is currently recruiting patients with advanced adenocarcinoma, including TNBC (NCT02900664).
Future challenges of TNBC treatment
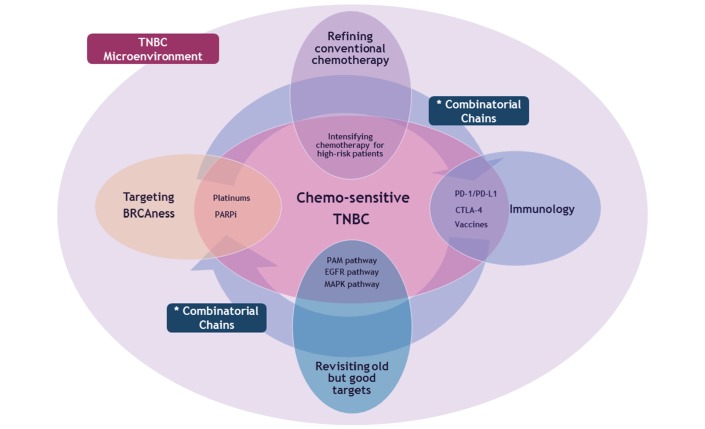
Although a plethora of novel immune-molecular targets have been experimentally validated, the challenge lies in transforming these preclinical and clinical benefits into our daily practice and current standard treatment. To design next-generation targeted therapeutic strategies, we should return to the basics to perform personalised tumour genotyping based on valid actionable and druggable targets. In the era of modern immunotherapy, we need to figure out the optimal application of immunotherapy in TNBC including the disease setting and its combinatorial partners, based on more robust biomarkers. In addition, overcoming strategies for acquired and intrinsic resistance to immunotherapy and salvage treatment after failure of conventional immunotherapy should be defined. Selection of chemosensitive subset and enriching the efficacy of chemotherapy in these patients should not be also overlooked (figure 2).
Figure 2.
Future aspects of therapeutic strategies in patients with TNBC based on its chemosensitivity and immune-molecular heterogeneity. Future challenge in TNBC is fundamentally to enrich the therapeutic efficacy to the optimal level both for chemosensitive and chemoresistant population. In this context, conventional chemotherapy and these four key entities constitute the main domain of upcoming treatment strategies. Targeting the BRCAness, revisiting our old but competent targets including PAM pathway and emerging immunotherapy can be the master molecular regulators of TNBC tumour microenvironment. Smart refining of conventional chemotherapy should be accompanied with these molecular targeting. Finally, combinatorial chains between these four independent domains would be the key of future therapeutics for TNBC. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; PAM, PI3K-Akt-mTOR; PARPi, poly(ADP-ribose) polymerase inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TNBC, triple-negative breast cancer.
Conclusion
TNBC is a unique disease entity with intrinsic molecular and immunological heterogeneity that therefore can manifest as a variety of clinical phenotypes. Extensive investigations to surmount this heterogeneity illustrated several comprehensive classification systems that incorporate immune-molecular signatures of TNBC. An important discovery was the identification of the BRCAness and its molecular synergism with PARPi as well as platinum-based agents. The BRCAness further unleashed inherent immunogenicity of TNBC by fostering dynamic tumour microenvironment, which conferred a rationale for immunotherapy in the subset. Witnessing a huge breakthrough in TNBC treatment, however, these promising scientific progresses have not yet been pertinently incorporated into our daily practice, and patients are still starved of available treatment options. To translate these therapeutic potentials into practical benefit, we must consistently and vigorously pursue the maximal opportunities of clinical trials for patients with TNBC. Customised clinical trials based on individualised genotyping seem also inevitable to set precise personalised medicine against its wide evolutionary mutational spectrum. In parallel, optimal tailoring of conventional chemotherapy in the landscape of immune-molecular heterogeneity should also be continued to establish the most effective regimens. Juggling with these efforts, the next chapter of TNBC treatment will be finally written on the novel combinatorial strategies, necessitating master refinement of target population and molecules.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Zaharia M, Gómez H. [Triple negative breast cancer: a difficult disease to diagnose and treat]. Rev Peru Med Exp Salud Publica 2013;30:649–56. [PubMed] [Google Scholar]
- 2.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol 2014;141:462–77. 10.1309/AJCPQN8GZ8SILKGN [DOI] [PubMed] [Google Scholar]
- 3.Malorni L, Shetty PB, De Angelis C, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 2012;136:795–804. 10.1007/s10549-012-2315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarneri V, Dieci MV, Conte P. Relapsed triple-negative breast cancer: challenges and treatment strategies. Drugs 2013;73:1257–65. 10.1007/s40265-013-0091-6 [DOI] [PubMed] [Google Scholar]
- 5.Billar JA, Dueck AC, Stucky CC, et al. Triple-negative breast cancers: unique clinical presentations and outcomes. Ann Surg Oncol 2010;17(Suppl 3):384–90. 10.1245/s10434-010-1260-4 [DOI] [PubMed] [Google Scholar]
- 6.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med Overseas Ed 2010;363:1938–48. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008;113:2638–45. 10.1002/cncr.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674–90. 10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–67. 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One 2016;11:e0157368 10.1371/journal.pone.0157368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clinical Cancer Research 2015;21:1688–98. 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333–9. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012;7:395–9. 10.1038/nature10933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis C, Shah SP, Chin S-F, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;105:346–52. 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012;486:405–9. 10.1038/nature11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraiva DP, Guadalupe Cabral M, Jacinto A, et al. How many diseases is triple negative breast cancer: the protagonism of the immune microenvironment. ESMO Open 2017;2:e000208 10.1136/esmoopen-2017-000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 2014;232:142–50. 10.1002/path.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams TA, Vail PJ, Ruiz A, et al. Composite analysis of immunological and metabolic markers defines novel subtypes of triple negative breast cancer. Mod Pathol 2018;31 10.1038/modpathol.2017.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clinical Cancer Research 2013;19:5533–40. 10.1158/1078-0432.CCR-13-0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Summa S, Pinto R, Sambiasi D, et al. BRCAness: a deeper insight into basal-like breast tumors. Annals of Oncology 2013;24(suppl 8):viii13–viii21. 10.1093/annonc/mdt306 [DOI] [PubMed] [Google Scholar]
- 22.Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1 / 2 mutation–associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. Journal of Clinical Oncology 2015;33:1895–901. 10.1200/JCO.2014.57.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 2009;459:460–3. 10.1038/nature07955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman A-R, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet 2002;32:180–4. 10.1038/ng953 [DOI] [PubMed] [Google Scholar]
- 25.Greenberg RA, et al. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev 2006;20:34–46. 10.1101/gad.1381306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi T, et al. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 2002;100:2414–20. 10.1182/blood-2002-01-0278 [DOI] [PubMed] [Google Scholar]
- 27.Scully R, Chen J, Plug A, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 1997;88:265–75. 10.1016/S0092-8674(00)81847-4 [DOI] [PubMed] [Google Scholar]
- 28.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002;108:171–82. 10.1016/S0092-8674(02)00615-3 [DOI] [PubMed] [Google Scholar]
- 29.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 2004;4:665–76. 10.1038/nrc1431 [DOI] [PubMed] [Google Scholar]
- 30.Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans 2006;34:633–45. 10.1042/BST0340633 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 2004;95:866–71. 10.1111/j.1349-7006.2004.tb02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulkes WD, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. CancerSpectrum Knowledge Environment 2003;95:1482–5. 10.1093/jnci/djg050 [DOI] [PubMed] [Google Scholar]
- 33.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene 2006;25:5846–53. 10.1038/sj.onc.1209876 [DOI] [PubMed] [Google Scholar]
- 34.Turner N, Tutt A, Ashworth A. Opinion: hallmarks of ’brcaness' in sporadic cancers. Nat Rev Cancer 2004;4:814–9. 10.1038/nrc1457 [DOI] [PubMed] [Google Scholar]
- 35.Venkitaraman AR. Targeting the molecular defect in brca-deficient tumors for Cancer Therapy. Cancer Cell 2009;16:89–90. 10.1016/j.ccr.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 36.Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys 2012;82:1185–91. 10.1016/j.ijrobp.2011.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical Cancer Research 2007;13:4429–34. 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 38.Prat A, Lluch A, Albanell J, et al. Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br J Cancer 2014;111:1532–41. 10.1038/bjc.2014.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran MS. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol 2015;16:e113–e122. 10.1016/S1470-2045(14)71104-0 [DOI] [PubMed] [Google Scholar]
- 40.Moran MS. Should Triple-Negative Breast Cancer (TNBC) subtype affect local-regional therapy decision making? Am Soc Clin Oncol Educ Book 2014;34:e32–e36. 10.14694/EdBook_AM.2014.34.e32 [DOI] [PubMed] [Google Scholar]
- 41.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 43.von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. Journal of Clinical Oncology 2012;30:1796–804. 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 44.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. Journal of Clinical Oncology 2008;26:1275–81. 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 45.von Minckwitz G, Kummel S, Vogel P, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. JNCI Journal of the National Cancer Institute 2008;100:552–62. 10.1093/jnci/djn089 [DOI] [PubMed] [Google Scholar]
- 46.Jones RL, Salter J, A’Hern R, A’Hern R, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009;116:53–68. 10.1007/s10549-008-0081-7 [DOI] [PubMed] [Google Scholar]
- 47.Guarneri V, Piacentini F, Ficarra G, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Annals of Oncology 2009;20:1193–8. 10.1093/annonc/mdn761 [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Shi M, Ling R, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: A prospective randomized controlled multi-center trial. Radiotherapy and Oncology 2011;100:200–4. 10.1016/j.radonc.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 49.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clinical Cancer Research 2007;13:2329–34. 10.1158/1078-0432.CCR-06-1109 [DOI] [PubMed] [Google Scholar]
- 50.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741/Cancer and leukemia group B Trial 9741. Journal of Clinical Oncology 2003;21:1431–9. 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 51.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 2006;295:1658–67. 10.1001/jama.295.14.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gluz O, Nitz UA, Harbeck N, et al. Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Annals of Oncology 2008;19:861–70. 10.1093/annonc/mdm551 [DOI] [PubMed] [Google Scholar]
- 53.Masuda N, Higaki K, Takano T, et al. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer. Cancer Chemother Pharmacol 2014;74:229–38. 10.1007/s00280-014-2492-y [DOI] [PubMed] [Google Scholar]
- 54.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med Overseas Ed 2017;376:2147–59. 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 55.Natori A, Ethier J-L, Amir E, et al. Capecitabine in early breast cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2017;77:40–7. 10.1016/j.ejca.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 56.Kennedy RD, Quinn JE, Mullan PB, et al. The role of brca1 in the cellular response to chemotherapy. JNCI Journal of the National Cancer Institute 2004;96:1659–68. 10.1093/jnci/djh312 [DOI] [PubMed] [Google Scholar]
- 57.Isakoff SJ, Mayer EL, He L, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. Journal of Clinical Oncology 2015;33:1902–9. 10.1200/JCO.2014.57.6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. The Lancet 2017;389:2430–42. 10.1016/S0140-6736(16)32454-0 [DOI] [PubMed] [Google Scholar]
- 59.Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Research 2012;14:R110 10.1186/bcr3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tovey H, Bliss J, Tutt A, et al. Managing non-proportionality of hazards (PH) within TNT: a randomised phase III trial of carboplatin compared to docetaxel for patients with metastatic or recurrent locally advanced triple negative (TN) or brca1/2 breast cancer (BC). Trials 2015;16:P150 10.1186/1745-6215-16-S2-P150 [DOI] [Google Scholar]
- 61.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. Journal of Clinical Oncology 2010;28:1145–53. 10.1200/JCO.2009.22.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 2014;147:401–5. 10.1007/s10549-014-3100-x [DOI] [PubMed] [Google Scholar]
- 63.Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 2009;115:359–63. 10.1007/s10549-008-0128-9 [DOI] [PubMed] [Google Scholar]
- 64.Kaklamani VG, Jeruss JS, Hughes E, et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast Cancer Res Treat 2015;151:629–38. 10.1007/s10549-015-3435-y [DOI] [PubMed] [Google Scholar]
- 65.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). Journal of Clinical Oncology 2015;33:13–21. 10.1200/JCO.2014.57.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747–56. 10.1016/S1470-2045(14)70160-3 [DOI] [PubMed] [Google Scholar]
- 67.Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the geparsixto randomized clinical trial. JAMA Oncol 2017;3:1378–85. 10.1001/jamaoncol.2017.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jovanović B, Mayer IA, Mayer EL, et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III Triple-Negative Breast Cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC Subtype, AR Status, and Ki67. Clinical Cancer Research 2017;23:4035–45. 10.1158/1078-0432.CCR-16-3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang P, Yin Y, Mo H, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 2016;7:60647–56. 10.18632/oncotarget.10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Audebert M, Salles B, Calsou P. Involvement of poly(adp-ribose) polymerase-1 and xrcc1/dna ligase iii in an alternative route for dna double-strand breaks rejoining. Journal of Biological Chemistry 2004;279:55117–26. 10.1074/jbc.M404524200 [DOI] [PubMed] [Google Scholar]
- 71.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res 2000;460:1–15. 10.1016/S0921-8777(00)00016-1 [DOI] [PubMed] [Google Scholar]
- 72.Bürkle A. Poly(APD-ribosyl)ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett 2001;163:1–5. [DOI] [PubMed] [Google Scholar]
- 73.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 74.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 75.Turner NC, Lord CJ, Iorns E, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. Embo J 2008;27:1368–77. 10.1038/emboj.2008.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. The Lancet 2010;376:235–44. 10.1016/S0140-6736(10)60892-6 [DOI] [PubMed] [Google Scholar]
- 77.Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med Overseas Ed 2017;377:523–33. 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 78.de Bono J, Ramanathan RK, Mina L, et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov 2017;7:620–9. 10.1158/2159-8290.CD-16-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isakoff SJ, Puhalla S, Domchek SM, et al. A randomized Phase II study of veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in BRCA1 / 2 metastatic breast cancer: design and rationale. Future Oncol 2017;13:307–20. 10.2217/fon-2016-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Somlo G, Frankel PH, Arun BK, et al. Efficacy of the PARP inhibitor veliparib with carboplatin or as a single agent in patients with germline BRCA1 - or BRCA2 -associated metastatic breast cancer: california cancer consortium trial NCT01149083. Clinical Cancer Research 2017;23:4066–76. 10.1158/1078-0432.CCR-16-2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anampa J, Chen A, Wright J, et al. Phase I trial of veliparib, a poly ADP ribose polymerase inhibitor, plus metronomic cyclophosphamide in metastatic HER2-negative breast cancer. Clin Breast Cancer 2018;18 10.1016/j.clbc.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 82.Rodler ET, Kurland BF, Griffin M, et al. Phase I study of veliparib (ABT-888) combined with cisplatin and vinorelbine in advanced triple-negative breast cancer and/or brca mutation-associated breast cancer. Clinical Cancer Research 2016;22:2855–64. 10.1158/1078-0432.CCR-15-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kummar S, Wade JL, Oza AM, et al. Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Invest New Drugs 2016;34:355–63. 10.1007/s10637-016-0335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Litton JK, Blum JL, Y-h I, et al. A phase 3, open-label, randomized, parallel, 2-arm international study of the oral PARP inhibitor talazoparib (BMN 673) in BRCA mutation subjects with locally advanced and/or metastatic breast cancer (EMBRACA). Journal of Clinical Oncology 2015;33:TPS1107. [Google Scholar]
- 85.Helena Margaret Earl A-LV, Qian W, Grybowicz L, et al. PARTNER: Randomised, phase II/III trial to evaluate the safety and efficacy of the addition of olaparib to platinum-based neoadjuvant chemotherapy in triple negative and/or germline BRCA mutated breast cancer patients. 2017. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.TPS591#.
- 86.Rugo HS, Olopade O, DeMichele A, et al. Abstract S5-02: Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: first efficacy results from the I-SPY 2 TRIAL:. Cancer Res 2013;73:S5-02–S05-02. 10.1158/0008-5472.SABCS13-S5-02 [DOI] [Google Scholar]
- 87.Severson TM, Wolf DM, Yau C, et al. The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting. Breast Cancer Research 2017;19:99 10.1186/s13058-017-0861-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller K, Tong Y, Jones DR, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: final efficacy results of hoosier oncology group BRE09-146. Journal of Clinical Oncology 2015;33:1082. [Google Scholar]
- 89.Marotti JD, de Abreu FB, Wells WA, et al. Triple-negative breast cancer: next-generation sequencing for target identification. Am J Pathol 2017;187:2133–8. 10.1016/j.ajpath.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 90.Weisman PS, Ng CKY, Brogi E, et al. Genetic alterations of triple negative breast cancer by targeted next-generation sequencing and correlation with tumor morphology. Modern Pathology 2016;29:476–88. 10.1038/modpathol.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang M, Zhou Y, Hu Y, et al. [Effects of NVP-BKM120 on the triple-negative breast cancer cell]. Zhonghua Yi Xue Za Zhi 2015;95:3308–12. [PubMed] [Google Scholar]
- 92.Ibrahim YH, García-García C, Serra V, et al. PI3K inhibition impairs brca1/2 expression and sensitizes brca-proficient triple-negative breast cancer to parp inhibition. Cancer Discov 2012;2:1036–47. 10.1158/2159-8290.CD-11-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimbung S, Biskup E, Johansson I, et al. Co-targeting of the PI3K pathway improves the response of BRCA1 deficient breast cancer cells to PARP1 inhibition. Cancer Lett 2012;319:232–41. 10.1016/j.canlet.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 94.Yi YW, Park JS, Kwak SJ, et al. Co-treatment with BEZ235 Enhances Sensitivity of BRCA1-negative Breast Cancer Cells to Olaparib. Anticancer Res 2015;35:3829–38. [PubMed] [Google Scholar]
- 95.Martín M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol 2017;28:313–20. 10.1093/annonc/mdw562 [DOI] [PubMed] [Google Scholar]
- 96.de Lint K, Poell JB, Soueidan H, et al. Sensitizing triple-negative breast cancer to PI3K inhibition by cotargeting IGF1R. Mol Cancer Ther 2016;15:1545–56. 10.1158/1535-7163.MCT-15-0865 [DOI] [PubMed] [Google Scholar]
- 97.Solzak JP, Atale RV, Hancock BA, et al. Dual PI3K and Wnt pathway inhibition is a synergistic combination against triple negative breast cancer. NPJ Breast Cancer 2017;3:17 10.1038/s41523-017-0016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sangai T, Akcakanat A, Chen H, et al. Biomarkers of response to akt inhibitor mk-2206 in breast cancer. Clinical Cancer Research 2012;18:5816–28. 10.1158/1078-0432.CCR-12-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hudis C, Swanton C, Janjigian YY, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Research 2013;15:R110 10.1186/bcr3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies BR, Greenwood H, Dudley P, et al. Preclinical pharmacology of azd5363, an inhibitor of akt: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 2012;11:873–87. 10.1158/1535-7163.MCT-11-0824-T [DOI] [PubMed] [Google Scholar]
- 101.Kim S-B, Dent R, Im S-A, Sa I, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18:1360–72. 10.1016/S1470-2045(17)30450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez-Angulo AM, Akcakanat A, Liu S, et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancer†. Ann Oncol 2014;25:1122–7. 10.1093/annonc/mdu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basho RK, Gilcrease M, Murthy RK, et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol 2017;3:509–15. 10.1001/jamaoncol.2016.5281 [DOI] [PubMed] [Google Scholar]
- 104.Nakai K, Hung MC, Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res 2016;6:1609–23. [PMC free article] [PubMed] [Google Scholar]
- 105.El Guerrab A, Bamdad M, Kwiatkowski F, et al. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget 2016;7:73618–37. 10.18632/oncotarget.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El Guerrab A, Bamdad M, Bignon YJ, et al. Anti-EGFR monoclonal antibodies enhance sensitivity to DNA-damaging agents in BRCA1-mutated and PTEN-wild-type triple-negative breast cancer cells. Mol Carcinog 2017;56:1383–94. 10.1002/mc.22596 [DOI] [PubMed] [Google Scholar]
- 107.Al-Ejeh F, Shi W, Miranda M, et al. Treatment of triple-negative breast cancer using anti-EGFR-directed radioimmunotherapy combined with radiosensitizing chemotherapy and PARP inhibitor. J Nucl Med 2013;54:913–21. 10.2967/jnumed.112.111534 [DOI] [PubMed] [Google Scholar]
- 108.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012;30:2615–23. 10.1200/JCO.2010.34.5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baselga J, Gómez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013;31:2586–92. 10.1200/JCO.2012.46.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trédan O, Campone M, Jassem J, et al. Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin Breast Cancer 2015;15:8–15. 10.1016/j.clbc.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 111.Crozier JA, Advani PP, LaPlant B, et al. N0436 (Alliance): a phase II trial of irinotecan with cetuximab in patients with metastatic breast cancer previously exposed to anthracycline and/or taxane-containing therapy. Clin Breast Cancer 2016;16:23–30. 10.1016/j.clbc.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yardley DA, Ward PJ, Daniel BR, et al. Panitumumab, gemcitabine, and carboplatin as treatment for women with metastatic triple-negative breast cancer: a sarah cannon research institute phase II trial. Clin Breast Cancer 2016;16:349–55. 10.1016/j.clbc.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 113.Nabholtz JM, Abrial C, Mouret-Reynier MA, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol 2014;25:1570–7. 10.1093/annonc/mdu183 [DOI] [PubMed] [Google Scholar]
- 114.Nabholtz JM, Chalabi N, Radosevic-Robin N, et al. Multicentric neoadjuvant pilot phase II study of cetuximab combined with docetaxel in operable triple negative breast cancer. Int J Cancer 2016;138:2274–80. 10.1002/ijc.29952 [DOI] [PubMed] [Google Scholar]
- 115.Hoeflich KP, O’Brien C, Boyd Z, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 2009;15:4649–64. 10.1158/1078-0432.CCR-09-0317 [DOI] [PubMed] [Google Scholar]
- 116.Miles D, Kim S-B, McNally VA, et al. COLET (NCT02322814): a multistage, phase 2 study evaluating the safety and efficacy of cobimetinib (C) in combination with paclitaxel (P) as first-line treatment for patients (pts) with metastatic triple-negative breast cancer (TNBC). Journal of Clinical Oncology 2016;34:TPS1100. [Google Scholar]
- 117.Sung-Bae Kim DM, Velu T. José Angel García Saenz. Cobimetinib (COBI) + paclitaxel (PTX) as first-line treatment in patients (pts) with advanced triple-negative breast cancer (TNBC): Interim safety review of the ongoing phase 2 COLET study: Presented at the 10th European Breast Cancer Conference (EBCC-10), 2016:Poster 358. [Google Scholar]
- 118.Van Swearingen AED, Sambade MJ, Siegel MB, et al. Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro Oncol 2017;19:1481–93. 10.1093/neuonc/nox052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Infante JR, Papadopoulos KP, Bendell JC, et al. A phase 1b study of trametinib, an oral Mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. Eur J Cancer 2013;49:2077–85. 10.1016/j.ejca.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 120.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol 2015;75:183–9. 10.1007/s00280-014-2615-5 [DOI] [PubMed] [Google Scholar]
- 121.Tolcher AW, Bendell JC, Papadopoulos KP, et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann Oncol 2015;26:58–64. 10.1093/annonc/mdu482 [DOI] [PubMed] [Google Scholar]
- 122.Emens LA. Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res 2018;24 10.1158/1078-0432.CCR-16-3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949–55. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 124.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24:5373–80. 10.1200/JCO.2006.05.9584 [DOI] [PubMed] [Google Scholar]
- 125.Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 2013;109:2705–13. 10.1038/bjc.2013.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983–91. 10.1200/JCO.2014.58.1967 [DOI] [PubMed] [Google Scholar]
- 127.Gu-Trantien C, Loi S, Garaud S, et al. CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873–92. 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Issa-Nummer Y, Darb-Esfahani S, Loibl S, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One 2013;8:e79775 10.1371/journal.pone.0079775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–66. 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544–50. 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 131.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860–7. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 132.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, et al. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 2014;148:467–76. 10.1007/s10549-014-3185-2 [DOI] [PubMed] [Google Scholar]
- 133.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105–13. 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 134.Oda N, Shimazu K, Naoi Y, et al. Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat 2012;136:107–16. 10.1007/s10549-012-2245-8 [DOI] [PubMed] [Google Scholar]
- 135.Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 2015;26:1698–704. 10.1093/annonc/mdv239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu F, Lang R, Zhao J, et al. CD8⁺ cytotoxic T cell and FOXP3⁺ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 2011;130:645–55. 10.1007/s10549-011-1647-3 [DOI] [PubMed] [Google Scholar]
- 137.Leon-Ferre RA, Polley MY, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 2018;167 10.1007/s10549-017-4499-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Atezolizumab Extends Survival for Breast Cancer. Cancer Discov 2017;7:Of10 10.1158/2159-8290.CD-NB2017-053 [DOI] [PubMed] [Google Scholar]
- 139.Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res 2016;22:1499–509. 10.1158/1078-0432.CCR-15-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer 2018;25 10.1007/s12282-017-0781-0 [DOI] [PubMed] [Google Scholar]
- 141.Mori H, Kubo M, Yamaguchi R, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017;8:15584–92. 10.18632/oncotarget.14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okabe M, Toh U, Iwakuma N, et al. Predictive factors of the tumor immunological microenvironment for long-term follow-up in early stage breast cancer. Cancer Sci 2017;108:81–90. 10.1111/cas.13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Polónia A, Pinto R, Cameselle-Teijeiro JF, et al. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J Clin Pathol 2017;70:860–7. 10.1136/jclinpath-2016-203990 [DOI] [PubMed] [Google Scholar]
- 144.Kim YA, Lee HJ, Heo SH, et al. MxA expression is associated with tumor-infiltrating lymphocytes and is a prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat 2016;156:597–606. 10.1007/s10549-016-3786-z [DOI] [PubMed] [Google Scholar]
- 145.Wein L, Savas P, Luen SJ, et al. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol 2017;7:156 10.3389/fonc.2017.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 2016;27:249–56. 10.1093/annonc/mdv571 [DOI] [PubMed] [Google Scholar]
- 147.Emens LA, Ascierto PA, Darcy PK, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer 2017;81:116–29. 10.1016/j.ejca.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 148.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010;16:3485–94. 10.1158/1078-0432.CCR-10-0505 [DOI] [PubMed] [Google Scholar]
- 149.McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res 2016;22:5729–37. 10.1158/1078-0432.CCR-16-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460–7. 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Adams S, Loi S, Toppmeyer D, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1–positive metastatic triple-negative breast cancer (mTNBC): Preliminary data from KEYNOTE-086 cohort B. Journal of Clinical Oncology 2017;35:1088. [Google Scholar]
- 153.Adams S, Schmid P, Rugo HS, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. Journal of Clinical Oncology 2017;35:1008. [Google Scholar]
- 154.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tolaney S, Savulsky C, Aktan G, et al. Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Eur J Cancer 2017;72:S16 10.1016/S0959-8049(17)30131-4 [DOI] [Google Scholar]
- 156.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. Journal of Clinical Oncology 2017;35:506.28029304 [Google Scholar]
- 157.Schmid P, Park YH, Muñoz-Couselo E, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. Journal of Clinical Oncology 2017;35:556. [Google Scholar]
- 158.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017;23:3711–20. 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (adp-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1-3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J Clin Oncol 2017;35:2193–202. 10.1200/JCO.2016.72.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000357supp001.pdf (233.7KB, pdf)