Abstract
Background
The aim of this study was to examine whether there was an association between tardive dyskinesia (TD) and number of functional CYP2D6 genes.
Methods
A Caucasian sample of 70 patients was recruited in 1996–1997 from South London and Maudsley National Health Service (NHS) Foundation Trust, UK. Subjects had a DSM-IIIR diagnosis of schizophrenia and were treated with typical antipsychotics at doses equivalent to at least 100 mg chlorpromazine daily for at least 12 months prior to assessment. All patients were genotyped for CYP2D6 alleles*3–5, *41, and for amplifications of the gene.
Results
There were 13 patients with TD. The mean (standard deviation (SD)) years of duration of antipsychotic treatment in TD-positive was 15.8 (7.9) vs TD-negative 11.1 (7.4) (p=0.04). Increased odds of experiencing TD were associated with increased ability to metabolize CYP2D6, as measured by genotypic category (odds ratio (OR)=4.2), increasing duration in treatment (OR=1.0), and having drug-induced Parkinsonism (OR=9.7).
Discussion
We found a significant association between CYP2D6 genotypic category and TD with the direction of effect being an increase in the number of functional CYP2D6 genes being associated with an increased risk of TD. This is the first study to examine the association between TD and CYP2D6 in Caucasians with this number of genotypic categories. In the future, metabolomics may be utilized in the discovery of biomarkers and novel drug targets.
Keywords: Cytochrome P450, genotype, antipsychotic, tardive dyskinesia, Parkinsonism
Introduction
Tardive dyskinesia (TD) is an abnormal involuntary movement disorder associated with sustained exposure to antipsychotics, characterized by orofacial dyskinesia and choreoathetoid movements of the trunk and limbs. TD is a particularly concerning adverse drug reaction of antipsychotic medication, as it may be irreversible, and hence may impact on the long-term quality of life of patients with schizophrenia (Kane, 1999). The occurrence of TD is thought to be influenced by genetic susceptibility (Morgenstern and Glazer, 1993), as well as by factors such as increasing age (Quinn et al., 2001), female gender (Smith and Baldessarini, 1980), duration of treatment with antipsychotic medication (Jeste and Kelsoe, 1997), and drug-induced Parkinsonism (DIP) (Muscettola et al., 1999). DIP resembles idiopathic Parkinson’s disease, which is characterized by the triad of bradykinesia, rigidity and tremor, except that asymmetrical distribution and the classical pill-rolling tremor are less common in the drug-induced form. The prevalence of TD from antipsychotic treatment TD is approximately 20% (Kane and Smith, 1982). Other studies have shown point-prevalence of 5–45% in psychiatric inpatients and 30% among psychiatric outpatients (Jeste and Wyatt, 1981).
CYP2D6 is involved in the metabolism of most typical antipsychotic drugs, including haloperidol (Llerena et al., 1992), fluphenazine (Shin et al., 1999), and zuclopenthixol (Jaanson et al., 2002). It is encoded by the CYP2D6 gene, which is located on chromosome 22 (22q13). There are over 100 allelic variants of the enzyme (see www.cypalleles.ki.se), resulting in four different phenotypes with respect to CYP2D6 metabolizer status. Poor metabolizers (PMs) have two alleles that do not encode active CYP2D6 enzyme (non-functional or null alleles), intermediate metabolizers (IMs) have one reduced activity allele and one non-functional allele or two reduced activity allele, extensive metabolizers (EMs) have at least one functional allele, and ultrarapid metabolizers (UMs) possess excess enzymatic activity due to multiple copies of functional alleles (3–13) resulting from gene amplification (Ingelman-Sundberg, 2005). The distribution of CYP2D6 functionality shows ethnic differences; for example, up to 10% of European Caucasians are PMs, in comparison to only 1% of Asians (Aitchison et al., 2000).
A meta-analysis of 12 studies reported on the impact of CYP2D6 variants on TD risk in various ethnicities, and concluded that lack of CYP2D6 functionality may predispose patients with schizophrenia treated with antipsychotics to TD (Patsopoulos et al., 2005). A non-significant association between the CYP2D6 PM phenotype and TD was shown in 516 severely mentally ill patients in North America (De Leon et al., 2005). Further studies on Chinese patients with schizophrenia rated for TD concluded that the CYP2D6*10 IM allele may be associated with TD (Liou et al., 2004); whereas a study on Slovenian Caucasians found no association between TD and other antipsychotic-induced extrapyramidal disorders with CYP2D6 genotype (Plesnicar et al., 2006). Finally, in 110 TD-positive and 92 TD-negative Korean patients with schizophrenia, males with ≥1 PM or IM CYP2D6 allele had greater odds of TD than males with wild-type alleles (OR=2.10, 95% CI 0.93–4.74, p=0.07) (Nikoloff et al., 2002). In view of these conflicting results, we examined the association between all four categories of CYP2D6 functionality and TD in an English Caucasian sample from South London, UK.
Methods
Subjects
This was a cross sectional study of all consecutive outpatients from a clinic in a particular catchment area of Bethlem, South London and Maudsley NHS Foundation Trust, UK. Recruitment included some current patients but was mostly from records and contact by letter, via the National Schizophrenia Fellowship through letters to branches, talks at meetings and contact with patients directly or through parents. Seventy patients were enrolled over a one-year period (1996–1997). Subjects had a DSM-IIIR diagnosis (American Psychiatric Association, 1987) of schizophrenia determined by the Structured Clinical Interview for DSM Disorders (Spitzer et al., 1990). They were treated with typical antipsychotics at doses equivalent to at least 100 mg chlorpromazine daily for at least 12 months prior to assessment. No changes in treatment regimen were made for at least three months prior to assessment. The study was approved by the local Research Ethics Committee. Of 70 patients, 68 had information on antipsychotics: 23 (32.9%) were on haloperidol decanoate, 16 (22.9%) on flupentixol decanoate, 16 (22.9%) on fluphenazine decanoate, 6 (8.6%) on thioridazine, 5 (7.1%) on chlorpromazine, 1 (1.4%) on zuclopenthixol decanoate and oral pimozide and another person (1.4%) was on fluphenazine decanoate and oral sulpiride. All 70 patients were examined for the presence of TD by the Abnormal Involuntary Movements Scale (AIMS) (US Department of Health, Education and Welfare, 1974), and the Schooler and Kane criteria were employed to make a research diagnostic criteria (RDC) diagnosis of probable TD (Schooler and Kane, 1982). Our sample consisted of English Caucasian patients treated with typical antipsychotics with antipsychotic-induced adverse reactions including movement disorders. This involved a history of at least three months’ total cumulative antipsychotic exposure, the presence of at least ‘moderate’ abnormal, involuntary movements in one or more body areas or at least ‘mild’ movements in two or more body areas (face, lips, jaw, tongue, upper extremities, lower extremities, trunk), and absence of other conditions that might produce abnormal involuntary movements. For the 66 patients with DSM-IIIR schizophrenia, a further assessment using the AIMS was made between two and six weeks after the initial assessment, and persistent TD was confirmed if the above criteria were still fulfilled. These sixty-six subjects were also examined for the presence of DIP by Webster’s Rating Scale for Parkinsonism (WRSP) (Webster, 1968).
CYP2D6 genotyping
DNA was extracted from blood collected in EDTA tubes using the Nucleon II kit (then supplied by Nucleon Biosciences, UK; current kit equivalent the Nucleon BACC2 kit, GE Health Care Europe, Amersham, UK). All genotyping was performed blind to the patients’ clinical status. Genotyping for all the functional allelic variants common in Caucasians was undertaken. These comprised the following: CYP2D6*41 (IM), CYP2D6*3 (PM), CYP2D6*4 (PM), CYP2D6*5 (PM), and gene amplification (UM if a functional variant is amplified). Genotyping for the CYP2D6*2 allele with promoter variant was undertaken by KBiosciences Ltd (Hoddesdon, UK), providing the company with the sequence surrounding the 2850C>T (rs16947) and −1584C>G single-nucleotide polymorphisms (SNPs). The three non-functional alleles (*3, *4, and *5) comprise the large majority of the mutant alleles responsible for the PM phenotype (Zanger et al., 2004) in Caucasians, and these and CYP2D6 gene amplifications were performed as previously described (Aitchison et al., 1999). In addition, for cases positive on both the CYP2D6 gene amplification assay and the CYP2D6*4 polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay, a further nested PCR followed by restriction enzyme digestion was employed, also as previously described (Aitchison et al., 1999).
In the eight cases where, following this genotyping, it was not possible to resolve calls between allelic variants (CYP2D6*35, CYP2D6*41, or CYP2D6*2A), the AmpliChip CYP450 Test (Roche Molecular Systems, Alameda, California, USA) was also employed. The AmpliChip CYP450 Test uses Affymetrix technology, with the original Affymetrix CYP450 array having been made more comprehensive (e.g. including CYP2D6*5 and gene amplifications) by work conducted by Roche Molecular Systems, Inc. It detects 33 polymorphisms and mutations in CYP2D6, using multiple assays for the same variant, thereby generating a high specificity and sensitivity. The assay was conducted according to the manufacturer’s instructions (Roche Molecular Systems, Inc., 2005). Data analysis was performed by the GeneChip Operating Software (GCOS) and the AmpliChip CYP450 Data Analysis Software was used to generate a report summarizing the genotype call and listing the corresponding identified alleles. The report was checked by hand versus the output detailing the call for each variant detectable by the array, in order to generate calls for subtypes of alleles such as CYP2D6*35.
Statistical analysis
All the analyses were performed using STATA 12.0 and SPSS version 13.0 for Windows. Independent t-tests were used to test differences in age and duration of antipsychotic treatment between those with TD (TD-positive) and those without (TD-negative), while the Chi-square test was utilized to test differences in proportions between groups for sex and DIP. A logistic regression tested the association between TD and genotypic category controlling for age, gender, duration of antipsychotic treatment, and DIP. The distributions were skewed to the left, yet both groups had similar medians and variances, thus we performed the more appropriate Wilcoxon rank-sum (Mann-Whitney) test, which found a statistically significant difference in the underlying distribution of duration of treatment between persons who had TD and those that did not (z= −2.075, p=0.0379). The group of patients that had TD had higher ranks of duration of treatment. Genotypic category was treated as a continuous variable with the following levels: PM/PM=0, PM/IM=0.5, EM/PM=1, EM/IM=1.5, EM/EM=2, UM/IM=2.5, UM/EM=3, as the ability to metabolize CYP2D6 is hypothesized to incrementally increase across these allelic combinations. A p value of 0.05 was interpreted as significant for all analyses.
Results
Patients with TD did not differ from those without TD in terms of sex or age; however, they did have a significantly longer duration of antipsychotic treatment (t=−2.05, df=68, p=0.04), and also had a greater proportion with DIP (X2=7.65, df=1, p=0.006). Thirtyfive out of 70 patients (50.0%) met the criteria for DIP, and 13 of 70 (18.6%) met the criteria for TD. These results are summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics.
| Clinical characteristic | TD positive, n=13 | TD negative, n=57 | Test statistic for difference between groups |
|---|---|---|---|
| Sex | X2=0.34, df=1, p=0.561 | ||
| Males, n (%) | 8 (61.5%) | 30 (52.6%) | |
| Females, n (%) | 5 (38.5%) | 27 (47.4%) | |
| Age, years, mean (SD) | 39.6 (11.6) | 42.0 (18.5) | t=0.45, df=68, p=0.653 |
| Duration of antipsychotic treatment, | 15.8 (7.9) | 11.1 (7.4) | z= −2.075, p=0.0379 |
| years, mean (SD) | |||
| Drug-induced Parkinsonism, n (%) | 11 (84.6%) | 24 (42.1%) | X2=7.65, df=1, p=0.006 |
TD: tardive dyskinesia; SD: standard deviation.
The main predictor of interest was CYP2D6 genotypic category, with alleles being classified according to the Human Cytochrome P450 (CYP) Allele Nomenclature Committee (www.cypalleles.ki.se). Seven genotypic categories were created, each representing ascending levels of functional alleles. The frequencies of the CYP2D6*4, CYP2D6*5, CYP2D6*3 (PMs) CYP2D6*2 (varies) and CYP2D6*41 (IM) alleles were 0.221, 0.014, 0.021, 0.229, and 0.20 respectively. All of the calls made by the CYP450 Test were consistent with the presence of a CYP2D6*2 variant as determined by the prior genotyping. Three out of 70 (4.3%) of cases were UMs, of which none was positive for the null alleles tested.
After adjusting for known predictors of TD, each level increase in genetic category (ability to metabolize CYP2D6) increased the odds of experiencing tardive dyskinesia by 4.04 (95% confidence interval (CI): 1.09–14.95). As hypothesized, duration of antipsychotic treatment (odds ratio (OR)=1.248, 95% CI: 1.033–1.507) and DIP (OR=9.71, 95% CI: 1.38–68.37) were also significantly associated with TD. These results are summarized in Table 2. The association between CYP2D6 genotypic category and TD is shown in Figure 1.
Table 2.
Association between tardive dyskinesia and clinical variables using logistic regression.
| Variables | OR (95% CI) | p value |
|---|---|---|
| Genotypic category | 4.2 (1.1–15.7) | 0.032 |
| Females | 0.5 (0.08–2.6) | 0. 371 |
| Age | 0.9 (0.8–1.0) | 0.141 |
| Duration of treatment | 1.248 (1.033–1.507) | 0.022 |
| Drug-induced Parkinsonism | 9.7 (1.4–68.4) | 0.022 |
| constant | 0.04 (0.0007–2.7) | 0.135 |
CI: confidence interval; OR: odds ratio.
Figure 1.
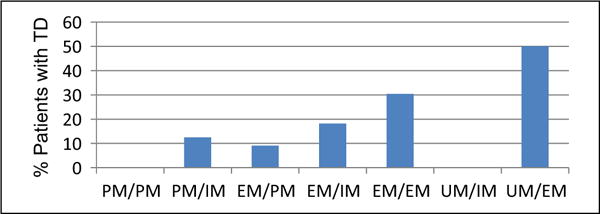
Association between CYP2D6 genotypic category and tardive dyskinesia.
Genotypic category was treated as a continuous variable with the following levels: PM/PM=0, PM/IM=0.5, EM/PM=1, EM/IM=1.5, EM/EM=2, UM/IM=2.5, UM/EM=3, as the ability to metabolize CYP2D6 is hypothesized to incrementally increase across these allelic combinations. The risk of tardive dyskinesia (TD) increases with increasing CYP2D6 metabolizer status. Of those with UM alleles, 0/1 with UM/IM and 1/2 with UM/EM developed TD. EM: extensive metabolizer; IM: intermediate metabolizer; PM: poor metabolizer; UM: ultrarapid metabolizer.
Discussion
We found a significant association between greater ability to metabolize CYP2D6, as measured by increasing number of functional alleles, and tardive dyskinesia. This is the first study to examine the association between TD and CYP2D6 in Caucasians when genotyped comprehensively enough to generate the genotypic category as described. The allele frequencies found in our sample were consistent with those reported previously for European Caucasians (Sachse et al., 1997). The prevalence of TD in our sample is 19% (13/70) which is also consistent with other studies (Jeste and Wyatt, 1981; Kane and Smith, 1982).
Consistent with our study, there are studies which found an association between CYP2D6 and TD (Fu et al., 2006). In contrast to our study, there are other studies which have shown no association between CYP2D6 and TD (De Leon et al., 2005). Poor metabolizers were 1.6 times more likely to suffer from TD compared to other patients with schizophrenia, although it was not significant in a meta-analysis of 12 studies (Patsopoulos et al., 2005). One possible interpretation of our findings is that metabolism of typical antipsychotics by CYP2D6 produces toxic metabolites that are associated with increased risk of TD, such that individuals with a greater number of functional CYP2D6 genes produce more such metabolites. This hypothesis is only a speculation. The pharmacodynamic effects of most metabolites of typical antipsychotics have not been established; however, in the case of thioridazine, it is known that CYP2D6 catalyzes the formation of mesoridazine, which has D2 blocking activity. Furthermore, in addition to generating active metabolites of antipsychotic drugs, CYP2D6 also generates toxic metabolites. For example, with haloperidol, CYP2D6 is involved in the production of haloperidol pyridinium, a highly neurotoxic metabolic product (Fang et al., 1995) which has been proposed to play a role in the emergence of haloperidol-induced extrapyramidal side-effects (Fang et al., 1997). Of note, the most commonly prescribed antipsychotic in our study was haloperidol. Moreover, CYP2D6 is found in the nigrostriatal pathway. It is therefore possible that increased oxidative metabolism resulting from higher CYP2D6 activity may be responsible for higher plasma or local neuronal levels of neurotoxic antipsychotic drug metabolites, and hence, increased risk for the development of TD and there is evidence of CYP2D6 activity in various brain regions in the rat (Aitchison et al., 2010). Authors who have not found an association between TD and CYP2D6 PM status have suggested that this may be explained by CYP2D6 PMs having a low level of lifetime exposure to antipsychotics metabolized by CYP2D6 due to severe intolerance (Kapitany et al., 1998). Similarly, it is possible that an association with UM status has been previously missed owing to CYP2D6 UM individuals not remaining on antipsychotics associated with tardive dyskinesia.
Other cytochrome P450 enzymes such as CYP1A2 (Basile et al., 2000) or CYP3A5 (De Leon et al., 2005) are also involved in the metabolism of typical antipsychotics, and have been implicated in TD. However, subsequent studies investigating the putative association between CYP1A2 and TD were negative (Schulze et al., 2001). In a published abstract there was no association between the CYP1A2 C–164A polymorphism and TD (Tsapakis et al., 2002). Other factors, e.g. the Ser9Gly SNP in the dopamine D3 receptor (Bakker et al., 2006), and polymorphisms in the serotonin (5-HT2A) and 5-HT2C receptors may also contribute to a vulnerability to TD, possibly in interaction with pharmacokinetic factors such as CYP2D6, CYP1A2, and CYP17 (Segman et al., 2002). Movement disorders may be part of the pathophysiology of schizophrenia (Owens et al., 1982; Waddington et al., 1995) and hence one might expect overlap between genes associated with schizophrenia and genes associated with TD. The associations found with the DRD3 (Bakker et al., 2006; Ebstein et al., 1997; Segman et al., 1999; Williams et al., 1998) are an example of such overlap. Drug availability may also be influenced by other factors such as plasma protein binding, drug transporters, nutrition, hormones and inflammatory cytokines (Morgan et al., 1998).
In agreement with previous findings, duration of treatment with antipsychotic medication differed between TD-positive and TD-negative groups (Smith and Baldessarini, 1980; Woerner et al., 1998), and DIP was significantly associated with TD (Kane et al., 1986; Woerner et al., 1998), but there was no apparent effect of age and sex on the presence of persistent TD in this sample. This may be attributable to the short duration of follow-up (six weeks). In the multicenter GENDEP pharmacogenomics trial (n=790), CYP2D6 UM genotype was associated with a significantly lower dose of escitalopram, and a higher rate of a specific adverse drug reaction (nausea) on escitalopram (Keers et al., 2010).
In HIV-infected patients, oral metabolites have been found to be useful biomarkers for monitoring immune status (Ghannoum et al., 2013). The advantages include, but are not limited to, this being an easy to collect, noninvasive technique, and safer than blood to handle. Such methodology may well be useful to explore in patients with psychiatric diagnoses and treatment.
The limitations of this study include the following. First and foremost, the relatively small number affected with TD means that our finding may be false positive. Secondly, the fact that the sample was collected in 1996–1997, and clinical notes from many of the patients are no longer available to us, means that for these patients we are unable to establish the exact doses of the antipsychotics, nor other relevant clinical parameters, such as use of anticholinergic medications. With 13 patients with TD on seven different typical antipsychotics and seven genotypic categories, we do not have the power to test for a difference. The one patient with UM/IM was prescribed chlorpromazine, while one of the two patients with UM/EM was on flupentixol decanoate while the other was on haloperidol decanoate. Finally, patients were heterogeneous in terms of medication received (seven different antipsychotics being in the dataset). There were no data on the following: drug plasma concentrations, concomitant medication which could have increased the risk for TD either pharmacokinetically and/or pharmacodynamically (e.g. selective serotonin reuptake inhibitor, inhibitors of CYP2D6), and previous treatment with other antipsychotics. None of the patients was a ‘true’ PM. Those categorized as PM were actually heterozygotes as shown in Figure 1. There may be important overlap in the final expression of alleles. And part of the reason for analyzing like this was the small size. However, there are papers that have analyzed the data in the same way we did (for example, Huezo-Diaz et al., 2012). The strength of this study is that it is the first to examine the association of CYP2D6 genotype with tardive dyskinesia so comprehensively in Caucasians, including the relatively common IM and UM alleles.
In conclusion, in our sample, the number of functional CYP2D6 genes was positively associated with an increased risk of TD, and we suggest that this could be due to the generation of neurotoxic metabolites. However, this hypothesis is only a speculation and requires replication, ideally with a larger prospective sample collection, in which clinical data collection includes complete drug histories and plasma levels of antipsychotic medications, and in which genotyping is performed in a similar, comprehensive manner.
Acknowledgments
MM Koola and E Tsapakis contributed equally to the article and are joint first authors. KL Nugent and KJ Aitchison are joint senior authors. The authors wish to thank Jing H Zhao for previous relevant statistical advice. A poster was presented by KJ Aitchison at the XXth World Congress of Psychiatric Genetics, 14–18 October 2012, Hamburg, Germany. MM Koola presented a poster at the 12th Annual Pharmacogenetics in Psychiatry Meeting 31 May–1 June 2013, Hollywood, Florida, USA.
Funding
KJ Aitchison was previously funded by the Wellcome Trust (UK) as a Wellcome Mental Health Research Training Fellow, grant 045968, during which period she undertook much of the sample genotyping and is now an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta (Canada). Roche Molecular Systems provided the AmpliChip CYP450 Test and associated research support, and Johnson and Johnson Pharmaceuticals Research and Development provided salary support to E Tsapakis whilst undertaking the remainder of the genotyping. The manuscript preparation by MM Koola and statistical analyses by KL Nugent were supported by National Institute of Mental Health T32 grant MH06753307 (Carpenter, PI) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (Koola). MM Koola received a scholarship to present a poster at the 53rd Annual Short Course on Medical and Experimental Mammalian Genetics, 15–27 July 2012, the Jackson Laboratory, Bar Harbor, Maine, USA.
Footnotes
Conflicts of interest
KJ Aitchison has received consultancy fees from companies including Johnson and Johnson, Roche Diagnostics and Roche Molecular Systems, and has previously sat on advisory boards for various companies including Johnson and Johnson. All other authors declare no conflict of interest.
References
- Aitchison K, Datla K, Rooprai H, et al. Regional distribution of clomipramine and desmethylclomipramine in rat brain and peripheral organs on chronic clomipramine administration. J Psychopharmacol. 2010;24:1261–1268. doi: 10.1177/0269881109105789. [DOI] [PubMed] [Google Scholar]
- Aitchison KJ, Gonzalez FJ, Quattrochi LC, et al. Identification of novel polymorphisms in the 5′ flanking region of CYP1A2, characterization of interethnic variability, and investigation of their functional significance. Pharmacogenetics. 2000;10:695–704. doi: 10.1097/00008571-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Aitchison KJ, Munro J, Wright P, et al. Failure to respond to treatment with typical antipsychotics is not associated with CYP2D6 ultrarapid hydroxylation. Br J Clin Pharmacol. 1999;48:388–394. doi: 10.1046/j.1365-2125.1999.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders. 3rd. Washington, DC: American Psychiatric Press, Inc; 1987. [Google Scholar]
- Bakker PR, Van Harten PN, Van Os J. Antipsychotic-induced tardive dyskinesia and the Ser9gly polymorphism in the Drd3 gene: A meta analysis. Schizophr Res. 2006;83:185–192. doi: 10.1016/j.schres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Basile VS, Ozdemir V, Masellis M, et al. A functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene: Association with tardive dyskinesia in schizophrenia. Mol Psychiatry. 2000;5:410–417. doi: 10.1038/sj.mp.4000736. [DOI] [PubMed] [Google Scholar]
- De Leon J, Susce MT, Pan RM, et al. Polymorphic variations in Gstm1, Gstt1, Pgp, CYP2D6, CYP3A5, and dopamine D2 and D3 receptors and their association with tardive dyskinesia in severe mental illness. J Clin Psychopharmacol. 2005;25:448–456. doi: 10.1097/01.jcp.0000177546.34799.af. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Macciardi F, Heresco-Levi U, et al. Evidence for an association between the dopamine D3 receptor gene Drd3 and schizophrenia. Hum Hered. 1997;47:6–16. doi: 10.1159/000154382. [DOI] [PubMed] [Google Scholar]
- Fang J, Baker GB, Silverstone PH, et al. Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol. 1997;17:227–233. doi: 10.1023/a:1026317929335. [DOI] [PubMed] [Google Scholar]
- Fang J, Yu PH, Gorrod JW, et al. Inhibition of monoamine oxidases by haloperidol and its metabolites: Pharmacological implications for the chemotherapy of schizophrenia. Psychopharmacology (Berl) 1995;118:206–212. doi: 10.1007/BF02245841. [DOI] [PubMed] [Google Scholar]
- Fu Y, Fan CH, Deng HH, et al. Association of CYP2A6 and CYP1A2 gene polymorphism with tardive dyskinesia in Chinese schizophrenic patients. Acta Pharmacol Sin. 2006;27:328–332. doi: 10.1111/j.1745-7254.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA, Mukherjee PK, Jurevic RJ, et al. Metabolomics reveals differential levels of oral metabolites in HIV-infected patients: toward novel diagnostic targets. OMICS. 2013;17:5–15. doi: 10.1089/omi.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huezo-Diaz P, Perroud N, Spencer EP, et al. CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. J Psychopharmacol. 2012;26:398–407. doi: 10.1177/0269881111414451. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- Jaanson P, Marandi T, Kiivet RA, et al. Maintenance therapy with zuclopenthixol decanoate: Associations between plasma concentrations, neurological side effects and CYP2D6 genotype. Psychopharmacology (Berl) 2002;162:67–73. doi: 10.1007/s00213-002-1059-5. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Kelsoe JR. Schizophrenia, tardive dyskinesia, and a D3 receptor gene variant: A new twist on dyskinesias? Mol Psychiatry. 1997;2:86–88. doi: 10.1038/sj.mp.4000262. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wyatt RJ. Changing epidemiology of tardive dyskinesia: An overview. Am J Psychiatry. 1981;138:297–309. doi: 10.1176/ajp.138.3.297. [DOI] [PubMed] [Google Scholar]
- Kane JM. Tardive dyskinesia in affective disorders. J Clin Psychiatry. 1999;60:S43–S47. discussion 48–49. [PubMed] [Google Scholar]
- Kane JM, Smith JM. Tardive dyskinesia: Prevalence and risk factors, 1959 to 1979. Arch Gen Psychiatry. 1982;39:473–481. doi: 10.1001/archpsyc.1982.04290040069010. [DOI] [PubMed] [Google Scholar]
- Kane JM, Woerner M, Borenstein M, et al. Integrating incidence and prevalence of tardive dyskinesia. Psychopharmacol Bull. 1986;22:254–258. [PubMed] [Google Scholar]
- Kapitany T, Meszaros K, Lenzinger E, et al. Genetic polymorphisms for drug metabolism (CYP2D6) and tardive dyskinesia in schizophrenia. Schizophr Res. 1998;32:101–106. doi: 10.1016/s0920-9964(98)00038-3. [DOI] [PubMed] [Google Scholar]
- Keers R, Uher R, Gupta B, et al. Stressful life events, cognitive symptoms of depression and response to antidepressants in Gendep. J Affect Disord. 2010;127:337–342. doi: 10.1016/j.jad.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Wang YC, Bai YM, et al. Cytochrome P-450 2D6*10 C188t polymorphism is associated with antipsychotic-induced persistent tardive dyskinesia in chinese schizophrenic patients. Neuropsychobiology. 2004;49:167–173. doi: 10.1159/000077360. [DOI] [PubMed] [Google Scholar]
- Llerena A, Dahl ML, Ekqvist B, et al. Genetic factors in the metabolism of haloperidol. Clin Neuropharmacol. 1992;15:84A–85A. doi: 10.1097/00002826-199201001-00045. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Sewer MB, Iber H, et al. Physiological and pathophysiological regulation of cytochrome P450. Drug Metab Dispos. 1998;26:1232–1240. [PubMed] [Google Scholar]
- Morgenstern H, Glazer WM. Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiatry. 1993;50:723–733. doi: 10.1001/archpsyc.1993.01820210057007. [DOI] [PubMed] [Google Scholar]
- Muscettola G, Barbato G, Pampallona S, et al. Extrapyramidal syndromes in neuroleptic-treated patients: Prevalence, risk factors, and association with tardive dyskinesia. J Clin Psychopharmacol. 1999;19:203–208. doi: 10.1097/00004714-199906000-00002. [DOI] [PubMed] [Google Scholar]
- Nikoloff D, Shim JC, Fairchild M, et al. Association between CYP2D6 genotype and tardive dyskinesia in Korean schizophrenics. Pharmacogenomics J. 2002;2:400–407. doi: 10.1038/sj.tpj.6500138. [DOI] [PubMed] [Google Scholar]
- Owens DG, Johnstone EC, Frith CD. Spontaneous involuntary disorders of movement: Their prevalence, severity, and distribution in chronic schizophrenics with and without treatment with neuroleptics. Arch Gen Psychiatry. 1982;39:452–461. doi: 10.1001/archpsyc.1982.04290040052008. [DOI] [PubMed] [Google Scholar]
- Patsopoulos NA, Ntzani EE, Zintzaras E, et al. CYP2D6 polymorphisms and the risk of tardive dyskinesia in schizophrenia: A meta-analysis. Pharmacogenet Genomics. 2005;15:151–158. doi: 10.1097/01213011-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Plesnicar BK, Zalar B, Breskvar K, et al. The influence of the CYP2D6 polymorphism on psychopathological and extrapyramidal symptoms in the patients on long-term antipsychotic treatment. J Psychopharmacol. 2006;20:829–833. doi: 10.1177/0269881106062894. [DOI] [PubMed] [Google Scholar]
- Quinn J, Meagher D, Murphy P, et al. Vulnerability to involuntary movements over a lifetime trajectory of schizophrenia approaches 100%, in association with executive (frontal) dysfunction. Schizophr Res. 2001;49:79–87. doi: 10.1016/s0920-9964(99)00220-0. [DOI] [PubMed] [Google Scholar]
- Roche Molecular Systems, Inc. AmpliChip CYP450 Test for In Vitro Diagnostic Use. Branchburg, New Jersey: Roche Molecular Systems; 2005. [Google Scholar]
- Sachse C, Brockmoller J, Bauer S, et al. Cytochrome P450 2D6 variants in a Caucasian population: Allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39:486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Schumacher J, Muller DJ, et al. Lack of association between a functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene and tardive dyskinesia in schizophrenia. Am J Med Genet. 2001;105:498–501. doi: 10.1002/ajmg.1472. [DOI] [PubMed] [Google Scholar]
- Segman R, Neeman T, Heresco-Levy U, et al. Genotypic association between the dopamine D3 receptor and tardive dyskinesia in chronic schizophrenia. Mol Psychiatry. 1999;4:247–253. doi: 10.1038/sj.mp.4000511. [DOI] [PubMed] [Google Scholar]
- Segman RH, Heresco-Levy U, Yakir A, et al. Interactive effect of cytochrome P450 17alpha-hydroxylase and dopamine D3 receptor gene polymorphisms on abnormal involuntary movements in chronic schizophrenia. Biol Psychiatry. 2002;51:261–263. doi: 10.1016/s0006-3223(01)01302-6. [DOI] [PubMed] [Google Scholar]
- Shin JG, Soukhova N, Flockhart DA. Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: Preferential inhibition of CYP2D6. Drug Metab Dispos. 1999;27:1078–1084. [PubMed] [Google Scholar]
- Smith JM, Baldessarini RJ. Changes in prevalence, severity, and recovery in tardive dyskinesia with age. Arch Gen Psychiatry. 1980;37:1368–1373. doi: 10.1001/archpsyc.1980.01780250054006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First Michael B. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition,(SCID-P/SCID-NP) Washington, DC: American Psychiatric Press, Inc; 1990. [Google Scholar]
- Tsapakis EM, Meagher D, Quinn J, et al. A genetic association study of the CYP1A2 C164A polymorphism and tardive dyskinesia. Schizophrenia Research. 2002;53(suppl):75. [Google Scholar]
- Waddington JL, O’Callaghan E, Buckley P, et al. Tardive dyskinesia in schizophrenia. relationship to minor physical anomalies, frontal lobe dysfunction and cerebral structure on magnetic resonance imaging. Br J Psychiatry. 1995;167:41–44. doi: 10.1192/bjp.167.1.41. [DOI] [PubMed] [Google Scholar]
- Webster DD. Critical analysis of the disability in Parkinson’s disease. Mod Treat. 1968;5:257–282. [PubMed] [Google Scholar]
- Williams J, Spurlock G, Holmans P, et al. A meta-analysis and transmission disequilibrium study of association between the dopamine D3 receptor gene and schizophrenia. Mol Psychiatry. 1998;3:141–149. doi: 10.1038/sj.mp.4000376. [DOI] [PubMed] [Google Scholar]
- Woerner MG, Alvir JM, Saltz BL, et al. Prospective study of tardive dyskinesia in the elderly: Rates and risk factors. Am J Psychiatry. 1998;155:1521–1528. doi: 10.1176/ajp.155.11.1521. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2d6: Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]


