Abstract
Background
Acute liver injury in the setting of hepatic fibrosis is an intriguing and still unsettled issue. We previously have demonstrated the protective effects conferred by M2-like macrophages in the fibrotic liver. In the present work, we further decipher the cellular mechanisms governing this hepatoprotection.
Material/Methods
Macrophages were isolated from control mice (M0 macrophages), then polarized into M1 or M2 phenotype using IFN-γ or IL-4, respectively. Conditioned media (CM) from M0, M1, and M2 macrophages were harvested and applied to M1 macrophages. Cell apoptosis was evaluated by immunostaining and real-time PCR. Similarly, human monocyte-derived macrophages were isolated and polarized, then M0, M1, and M2 CM were applied to HL-7702 or HepG2 cells followed by apoptosis induction. Cell apoptosis was assessed by flow cytometry.
Results
For the mouse conditioned medium experiment, stronger expression of cleaved caspase 3 and higher Bax/Bcl-2 mRNA ratio were found in M1 macrophages pretreated with M2 CM compared to those in M1 macrophages pretreated with M0 or M1 CM. Similarly, exposure of HL-7702 and HepG2 cells to either M0 or M1 CM had no significant effect on cell apoptosis. Nevertheless, the frequency of hepatocyte apoptosis was substantially reduced in HL-7702 (from 32.23±2.99 to 15.37±0.69 for Annexin V+/PI+ staining, p<0.01) and HepG2 cells (from 36.1±7.26 to 15.2±1.2 for Annexin V+/PI+ staining, p<0.01) with M2 CM pretreatment.
Conclusions
M2-like macrophages exert their hepatoprotective effect by promoting M1-like macrophage apoptosis but protecting against hepatocyte apoptosis.
MeSH Keywords: Apoptosis, Cytoprotection, Defense Mechanisms, Macrophage Activation
Background
Acute liver failure (ALF) is a rare but life-threatening critical illness that occurs most often in patients who do not have pre-existing liver disease [1,2]. Acute-on-chronic liver failure (ACLF) is defined as the acute deterioration of pre-existing chronic liver disease, usually related to a precipitating event [3,4]. Compared with ALF, ACLF has a relatively lower mortality [5]. Moreover, there is a crucial ‘golden window’ period preceding sepsis development and organ failure in ACLF, which provides valuable opportunity for reversing progressive liver failure through therapeutic interventions [6,7]. Furthermore, patients without previous decompensation have higher short-term mortality than those with prior hepatic decompensation [5,7]. This is an intriguing and important finding, suggesting previous decompensation may be considered as a beneficial response instead of a deleterious event. These interesting facts motivate us to probe why and how ACLF exhibits favorable protection against acute insult compared with ALF.
We and others have investigated this issue using mouse models of acute insult in the setting of liver fibrosis. The hepatic fibrosis induced by carbon tetrachloride (CCl4), bile duct ligation (BDL), and thioacetamide (TAA) is demonstrated to exert hepatoprotective effects against various acute insults, including D-galactosamine/lipopolysaccharide (D-GalN/LPS) [8], acetaminophen (APAP) [9], CCl4 [10], and tumor necrosis factor alpha (TNF-α)/Fas-induced apoptosis [11–13]. In view of the broad spectrum of injury resistance, we speculated that innate immunity may be the major contributor to injury resistance in the fibrosis setting. Macrophages are innate immune cells with central roles in host defense, immune regulation, tissue repair, and liver regeneration [14,15]. In addition, macrophages have been reported to be critically involved in the pathogenesis of acute or chronic liver diseases [16,17]. In this regard, we performed a loss-of-function experiment (depletion of macrophages), and showed that macrophages assume important but divergent roles in acute injury and hepatic fibrosis. The dichotomous functions of macrophages can be ascribed to their phenotypic heterogeneity of M1/M2 activation: M1-like macrophages in acutely injured mice promote hepatic injury, whereas M2-like macrophages in the fibrotic liver give mice greater resistance against insult. The latter has been confirmed through a gain-of-function experiment (adoptive transfer of M2-like macrophages) [10].
In the present study, we further decipher the cellular mechanisms governing the hepatoprotection conferred by M2-like macrophages. For this purpose, conditioned medium experiments were conducted to analyze the effects of M2-like macrophages on hepatocyte and macrophage apoptosis in vitro. We here report that M2-like macrophages exert beneficial hepatoprotection through promoting M1-like macrophage apoptosis but preventing hepatocyte apoptosis.
Material and Methods
Animals
Male BALB/c mice (6–8 weeks old) were obtained from Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China. Mice were housed in a specific pathogen-free (SPF) environment at 22–24°C with 12-h light-dark cycles. Animals were fed standard laboratory chow with free access to water. All animal care and experimental procedures performed in this study were in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at Beijing YouAn Hospital affiliated to Capital Medical University.
Reverse transcription (RT) and SYBR green real-time quantitative PCR (qPCR) [8]
Total RNA was extracted from isolated macrophages using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Reverse transcription of the purified RNA (2 μg) was performed using random primers and AMV retrotranscriptase system (TakaRa, Dalian, Liaoning, China) according to the manufacturer’s protocol. SYBR Green real-time PCR was carried out using the ABI StepOne Plus (Applied Biosystems, Foster City, CA, USA). All reactions were performed in triplicate. The primers used were designed with Primer 3.0 software and are listed in Table 1. The relative expression of target genes was calculated and normalized to the expression of GAPDH, a housekeeping gene.
Table 1.
The primers used in real-time PCR.
| Gene | Sequence |
|---|---|
| Bax | F: 5′-TGC AGA GGA TGA TTG CTG AC-3′ |
| R: 5′-GAT CAG CTC GGG CAC TTT AG-3′ | |
| Bcl-2 | F: 5′-CTG GCA TCT TCT CCT TCC AG-3′ |
| R: 5′-GAC GGT AGC GAC GAG AGA AG-3′ | |
| GAPDH | F: 5′-AAC TTT GGC ATT GTG GAA GG-3′ |
| R: 5′-ACA CAT TGG GGG TAG GAA CA-3′ |
Isolation and in vitro polarization of primary mouse macrophages
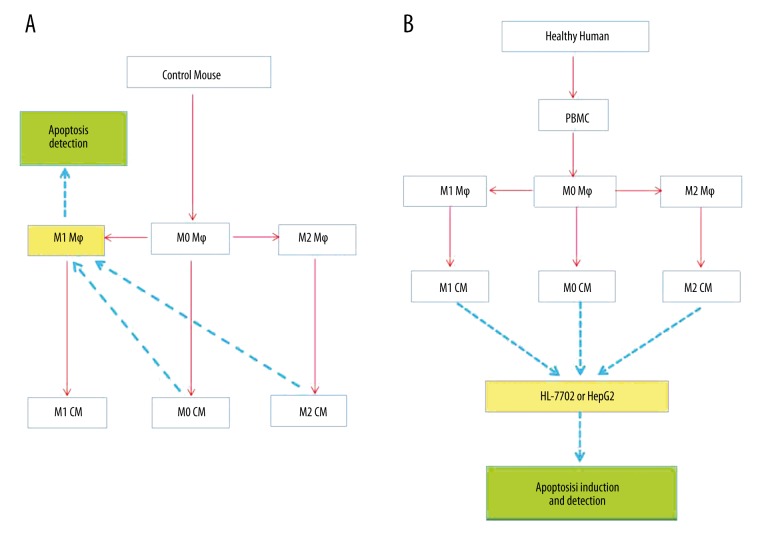
Macrophages were isolated from mice by pronase (Roche Diagnostics GmbH, Mannheim, Germany) and collagenase (Sigma-Aldrich, St. Louis, MO, USA) digestion followed by differential centrifugation using our previously reported method [10]. Isolated macrophages (non-polarized M0 macrophages) were stimulated with mouse recombinant interferon gamma (IFN-γ, 100 U/ml, PeproTech, Rocky Hill, USA) for 48 h for M1 induction or interleukin (IL)-4/IL-13 (10 ng/ml each, PeproTech) for M2 induction. The phenotype of the subsets was identified through qRT-PCR analysis for gene signatures of representative markers [10,18]. Then, M0, M1, and M2 mouse macrophages were used in the conditioned medium experiment and apoptosis detection (Figure 1A).
Figure 1.
(A, B) A flow chart of the study design.
Isolation, culture, and polarization of human monocyte-derived macrophages
Peripheral blood mononuclear cells (PBMC) were isolated from the whole blood of healthy volunteers using a Ficoll (Hao Yang Biological Manufacturing Co., LTD, Tian Jin, China) density gradient, and cultured in DMEM supplemented with 10% FBS. Two hours later, non-adherent cells were removed [19], and the resultant adherent cells (monocytes) were cultured in DMEM supplemented with human recombinant granulocyte macrophage colony-stimulating factor (GM-CSF, 50 ng/ml, PeproTech) or macrophage colony-stimulating factor (M-CSF, 50 ng/ml, PeproTech) for 6 days. Differentiated macrophages were polarized with human recombinant IFN-γ (50 ng/ml, Peprotech) for M1 induction or IL-4 (50 ng/ml, Peprotech) for M2 activation. Then, human M0, M1, and M2 macrophages were used in the conditioned medium experiment and subjected to apoptosis induction (Figure 1B).
Conditioned medium (CM) experiments and apoptosis detection (Figure 1)
Conditioned medium from human and mouse M0 macrophages (M0 CM), M1 macrophages (M1 CM) or M2 macrophages (M2 CM) were collected and centrifuged to remove cell debris. For mice, M0, M1, and M2 CMs were incubated with M1-like macrophages for 6 h, and then cell apoptosis was analyzed by immunostaining for cleaved caspase-3 (Abcam, Cambridge, MA, USA) and real-time PCR. For humans, M0, M1, and M2 CMs were incubated with HL-7702 or HepG2 cells for 6 h, and then hepatocyte apoptosis was induced by TNF-α (50 μg/ml, Peprotech)/D-GalN (100 mg/ml, Sigma-Aldrich) for 12 h. To evaluate quantitatively hepatocyte apoptosis, HL-7702 or HepG2 cells were stained with PI/Annexin V (FITC Annexin V Apoptosis Detection Kit II, BD Pharmingen, San Jose, CA, USA) and detected by flow cytometry according to the manufacturer’s protocol. The data were processed using FlowJo v10 software (TreeStar Inc., Ashland, OR, USA).
Cell culture
Human liver cell lines (HL-7702 and HepG2) as well as isolated mouse and human macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco) in a 37°C incubator.
Immunofluorescence staining
Macrophages were fixed with 4% paraformaldehyde for 30 min at room temperature, then were treated with 0.2% Triton X-100 for 5 min. After that, macrophages were incubated with Tris-buffered saline (TBS) buffer containing 5% FBS for 30 min. Immunofluorescence staining was carried out with primary antibody against cleaved caspase-3 (Abcam, Cambridge, MA, USA) and Alexa Fluor 594 goat anti-rabbit IgG fluorescent secondary antibody (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China). We used a Nikon Inverted Fluorescence Microscope ECLIPSE Ti and NIS-Elements F 3.0 Software (Nikon Corporation, Tokyo, Japan) for image capture.
Statistical analysis
Results are expressed as mean ±SEM. Group comparisons were performed using one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. Statistics and graphs were generated using Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
M2-like macrophages promote the apoptosis of M1-like macrophages in mice
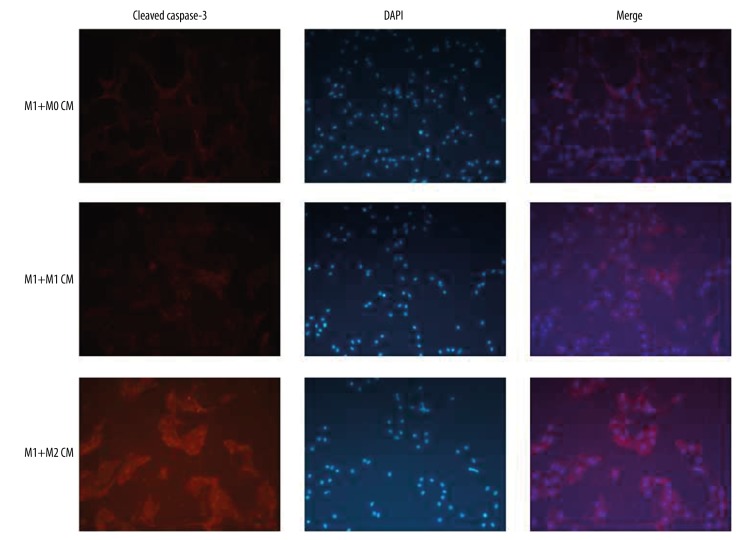
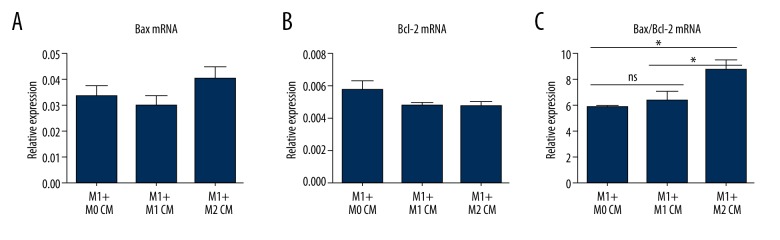
In previous work, we have demonstrated the beneficial hepatoprotection conferred by M2-like macrophages in the fibrotic liver [10]. In the present study, we probed the cellular mechanisms underlying the hepatoprotection against lethal insults. Macrophages were isolated from the livers of healthy mice (M0 macrophages), and then polarized into M1-like or M2-like macrophages with mouse interferon-γ or interleukin-4, respectively. Conditioned media (CM) from M0, M1, and M2 macrophages were applied to M1 macrophages. Stronger expression of cleaved caspase 3, the convergence of intrinsic and extrinsic apoptotic pathways and the main executor of apoptosis, was noticed in M1 macrophages pretreated with M2 CM compared to that in M1 macrophages pretreated with M0 or M1 CM (Figure 2). Similarly, higher mRNA levels of pro-apoptotic Bax (Figure 3A) and lower levels of anti-apoptotic Bcl-2 (Figure 3B), especially higher Bax/Bcl-2 ratio (Figure 3C), were detected in M1 macrophages pretreated with M2 CM, but not in M1 macrophages pretreated with M0 or M1 CM. Therefore, M2-like CM promotes M1-like macrophage apoptosis in mice.
Figure 2.
M2-like macrophages promote the apoptotic activity in M1-like macrophages. Macrophages were isolated from control mice (M0 macrophages), then polarized into M1 or M2 phenotype using mouse IFN-γ or IL-4, respectively. Conditioned media (CM) from M0, M1, M2 macrophages were harvested and applied to M1 macrophages. Cell apoptosis was evaluated by immunostaining for cleaved caspase-3.
Figure 3.
(A–C) M2-like macrophages tilt the balance of pro- and anti-apoptotic genes toward apoptosis in M1-like macrophages. Macrophages were isolated from control mice (M0 macrophages), then polarized into M1 or M2 phenotype using mouse IFN-γ or IL-4, respectively. Conditioned media (CM) from M0, M1, M2 macrophages were harvested and applied to M1 macrophages. Cell apoptosis was evaluated by the gene expression of pro-apoptotic and anti-apoptotic proteins. * P<0.05, ** P<0.01, *** P<0.001.
M2-like macrophages confer apoptosis resistance to hepatocytes in humans
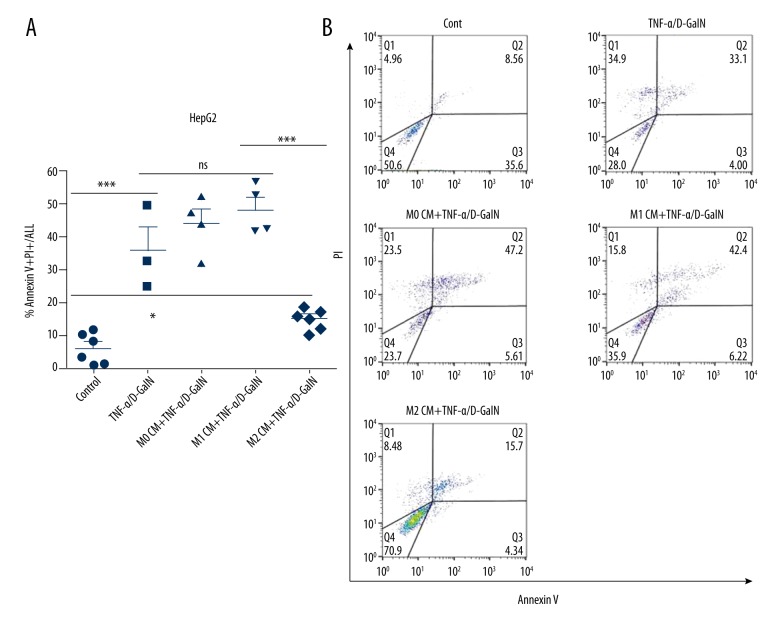
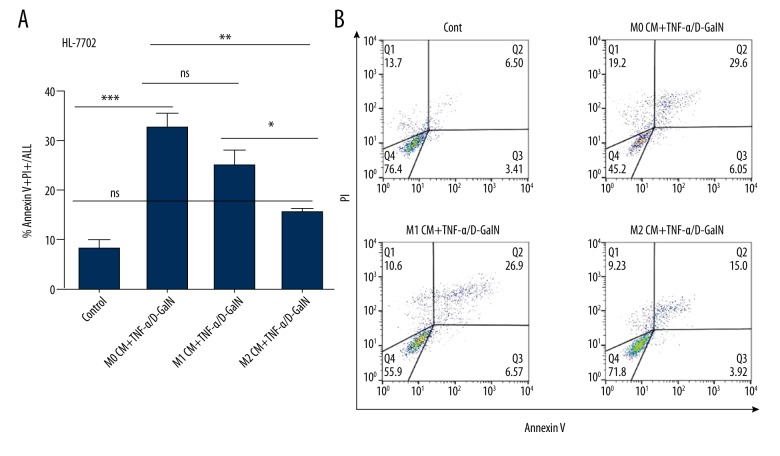
We also assessed the effects of polarized human macrophages on hepatocyte apoptosis. Conditioned media from human unstimulated and polarized monocyte-derived macrophages (i.e., M0 CM, M1 CM, and M2 CM) were harvested. Next, HepG2 and HL-7702 cells were pretreated with M0, M1, or M2 CM. Then, hepatocyte apoptosis was induced by human TNF-α/D-GalN. Exposure of HepG2 cells to either M0 CM or M1 CM had no significant effect on cell apoptosis. Nevertheless, the frequency of hepatocyte apoptosis was substantially reduced (from 36.1±7.26 to 15.2±1.2 for Annexin V+/PI+ staining, p<0.01) in HepG2 cells with M2 CM pretreatment (Figure 4A, 4B). Similarly, hepatocyte apoptosis was remarkably reduced (from 32.23±2.99 to 15.37±0.69 for Annexin V+/PI+ staining, p<0.01) in HL-7702 cells pretreated with M2 CM (Figure 5A, 5B). Collectively, M2-like macrophages confer apoptosis resistance to hepatocytes in humans.
Figure 4.
M2-like macrophages protect HepG2 cells from apoptosis. Human monocyte-derived macrophages were isolated from PBMC, then polarized into M1 or M2 phenotype using human IFN-γ or IL-4, respectively. M0, M1, M2 CM were incubated with HepG2 cells for 6 hours, then apoptosis was induced by TNF-α/D-GalN for 12 hours. Cell apoptosis was assessed by flow cytometry. * P<0.05, ** P<0.01, *** P<0.001.
Figure 5.
M2-like macrophages protect HL-7702 cells from apoptosis. Human monocyte-derived macrophages were isolated from PBMC, then polarized into M1 or M2 phenotype using human IFN-γ or IL-4, respectively. M0, M1, M2 CM were incubated with HL-7702 cells for 6 hours, then apoptosis was induced by TNF-α/D-GalN for 12 hours. Cell apoptosis was assessed by flow cytometry. * P<0.05, ** P<0.01, *** P<0.001.
Discussion
Acute liver injury in the setting of hepatic fibrosis is an intriguing and still unsettled issue. We previously demonstrated the favorable protective effects conferred by M2-like macrophages in the fibrotic liver [10]. In the present study, we further investigated the cellular mechanisms responsible for this hepatoprotection, and focused on the effects of M2-like macrophages on liver cell apoptosis. Here, we provide powerful evidence that M2-like macrophages promote the apoptosis of M1-like macrophages but protect against hepatocyte apoptosis, thus leading to the development of injury resistance.
ACLF is an increasingly recognized disorder that imposes a significant burden on critical care services and health care resources. However, the exact pathophysiology of the development of ACLF remains to be elucidated [3]. Given that there is currently no mouse model of ACLF, researchers utilize mice subjected to acute insult in the context of hepatic fibrosis to simulate the development of ACLF. As mentioned in the introduction, hepatoprotection against a variety of lethal hepatic toxins has been confirmed in fibrotic mice derived from different stimuli. For example, in a mouse model of partial bile duct ligation, ligated lobes (fibrotic) exhibit improved tolerance to TNF-α- and Fas-induced hepatocyte apoptosis, compared with non-ligated lobes [11,12].
Maintenance of liver homoeostasis relies on the critical balance between cell growth and cell death. As a major mode of cell death, programmed cell death (apoptosis) has been shown to be significantly enhanced in several acute and chronic liver diseases, such as fulminant hepatic failure, hepatitis originating from various etiologies, fibrosis, and cirrhosis [20]. In our latest report [10], massive apoptosis was found in acutely injured mice, which might mean the balance of cell growth/death has been tilted towards cell death. Conversely, apoptosis was remarkably inhibited in the fibrotic liver, even under challenge, suggesting that this balance has been tilted towards cell growth/proliferation.
Why does the fibrotic liver manifest great resistance against lethal insult? We probed this issue from the viewpoint of the pathophysiology of ACLF. Persistent inflammation and immune dysregulation constitute the main pathophysiological features of ACLF [6]. As a central player in the pathogenesis of liver failure, macrophages drive the initiation, propagation, and resolution of inflammatory immune responses [21]. This diversity of macrophage functions is intimately associated with their great plasticity and remarkable heterogeneity. Macrophages adapt their phenotypes in response to various microenvironmental signals, and exhibit different characteristic markers, gene expression profiles, and functions [21–24]]. They are broadly delineated into 2 categories: M1 and M2 macrophages. M1 macrophages are activated by pathogens or toxins (such as LPS), and secrete pro-inflammatory mediators which induces inflammation and liver damage; conversely, M2 macrophages are activated by IL-4/IL-13, and release anti-inflammatory or pro-resolving mediators which mediates wound repair, tissue remodeling, and fibrosis [22,24,25]. Macrophages in vivo adopt a mixed phenotype between M1- and M2-type macrophages. The M1/M2 balance is regarded as a decisive factor for macrophage function [16,26].
In our latest report [10], we demonstrated that macrophages in the fibrotic liver manifest M2-predominant activation, and M2-like macrophages exert a hepatoprotective effects against lethal insults through conferring apoptosis resistance to hepatocytes. In the present study, we assessed the impact of M2-like macrophages on M1-like macrophage apoptosis by immunostaining (cleaved caspase-3) and real-time PCR (pro- and anti-apoptotic proteins). Both assays confirmed the apoptosis resistance derived from M2-like CM. Our results are in agreement with a previous discovery by Wan et al. [27]. Importantly, we proved the beneficial protection of M2-like CM using human liver cell lines. Both HL-7702 and HepG2 cells incubated with M2-like CM exhibited remarkable resistance to apoptosis induced by TNF-α/D-GalN. Collectively, M2-like macrophages exert protective effects through inducing M1-like macrophage apoptosis and/or preventing hepatocyte apoptosis.
Conclusions
In summary, we confirmed the hepatoprotective effects of M2-like macrophages in mice and human through in vitro experiments. Although it is also important to investigate the effects of M2-like macrophages on other liver cells (e.g., Tregs and NK cells), in view of the complexity of liver immunity, our findings will help advance understanding of the pathogenesis of ACLF and shed light on a novel therapeutic intervention through manipulating macrophage polarization.
Footnotes
Source of support: This study was supported by the National Science and Technology Key Project (grant no. 2017ZX10201201, 2017ZX10203201-005, 2017ZX10202203-006-001 and 2017ZX10302201-004-002), the Beijing Municipal Administration of Hospital’s Ascent Plan (grant no. DFL20151601), the National Natural Science Foundation of China (grant no. 31571165), the Beijing Municipal Science & Technology Commission (grant no. Z151100004015066, Z171100002217070), the YouAn fund for liver diseases and AIDS (grant no. YNKT20160012) and the Basic-Clinical Cooperation Project of Capital Medical University (grant no. 17JL47)
References
- 1.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Lee WM, Wendon J, et al. Acute liver failure: A curable disease by 2024? J Hepatol. 2015;62:S112–20. doi: 10.1016/j.jhep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–48. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet. 2015;386:1576–87. doi: 10.1016/S0140-6736(15)00309-8. [DOI] [PubMed] [Google Scholar]
- 5.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37. 37e1–9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Sarin SK, Choudhury A. Acute-on-chronic liver failure: Terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–49. doi: 10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 7.Hernaez R, Sola E, Moreau R, et al. Acute-on-chronic liver failure: An update. Gut. 2017;66:541–53. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai L, Kong M, Zheng Q, et al. Inhibition of the translocation and extracellular release of high-mobility group box 1 alleviates liver damage in fibrotic mice in response to D-galactosamine/lipopolysaccharide challenge. Mol Med Rep. 2016;13:3835–41. doi: 10.3892/mmr.2016.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai L, Zu K, Zhang X, et al. [Protective effects and possible mechanisms of hepatic fibrosis against APAP-induced lethal injury]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:161–65. doi: 10.3760/cma.j.issn.1007-3418.2015.03.001. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 10.Bai L, Liu X, Zheng Q, et al. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci Rep. 2017;7:10518. doi: 10.1038/s41598-017-11303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa Y, Hannun YA, Proia RL, et al. Roles of AKT and sphingosine kinase in the antiapoptotic effects of bile duct ligation in mouse liver. Hepatology. 2005;42:1320–28. doi: 10.1002/hep.20967. [DOI] [PubMed] [Google Scholar]
- 12.Osawa Y, Seki E, Adachi M, et al. Role of acid sphingomyelinase of Kupffer cells in cholestatic liver injury in mice. Hepatology. 2010;51:237–45. doi: 10.1002/hep.23262. [DOI] [PubMed] [Google Scholar]
- 13.Bourbonnais E, Raymond VA, Ethier C, et al. Liver fibrosis protects mice from acute hepatocellular injury. Gastroenterology. 2012;142:130–39e4. doi: 10.1053/j.gastro.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–62. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–76. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–27. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamidzadeh K, Christensen SM, Dalby E, et al. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol. 2017;79:567–92. doi: 10.1146/annurev-physiol-022516-034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng QF, Bai L, Duan ZP, et al. M2-like Kupffer cells in fibrotic liver may protect against acute insult. World J Gastroenterol. 2017;23:3655–63. doi: 10.3748/wjg.v23.i20.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tedesco S, Bolego C, Toniolo A, et al. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology. 2015;220:545–54. doi: 10.1016/j.imbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Vinken M, Maes M, Oliveira AG, et al. Primary hepatocytes and their cultures in liver apoptosis research. Arch Toxicol. 2014;88:199–212. doi: 10.1007/s00204-013-1123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Possamai LA, Thursz MR, Wendon JA, et al. Modulation of monocyte/macrophage function: A therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439–45. doi: 10.1016/j.jhep.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Sun YY, Li XF, Meng XM, et al. Macrophage phenotype in liver injury and repair. Scand J Immunol. 2017;85:166–74. doi: 10.1111/sji.12468. [DOI] [PubMed] [Google Scholar]
- 23.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–42. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 24.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 25.Heymann F, Tacke F. Immunology in the liver – from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 26.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–96. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Wan J, Benkdane M, Teixeira-Clerc F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and non-alcoholic fatty liver disease. Hepatology. 2014;59(1):130–42. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]