Abstract
The purpose of the study was to investigate the adaptation process of hindlimb cortical bone subjected to free-fall landing training. Female Wistar rats (7 weeks old) were randomly assigned to four landing (L) groups and four age-matched control (C) groups (n = 12 per group): L1, L2, L4 L8, C1, C2, C4 and C8. Animals in the L1, L2, L4 and L8 groups were respectively subjected to 1, 2, 4 and 8 weeks of free-fall-landing training (40 cm height, 30 times/day and 5 days/week) while the C1, C2, C4 and C8 groups served as age-matched control groups. The tibiae of the L8 group were higher in cortical bone mineral content (BMC) than those in the C8 group (p < 0.05). Except for the higher bone mineralization over bone surface ratio (MS/BS, %) shown in the tibiae of the L1 group (p < 0.05), dynamic histomorphometry in the tibial and femoral cortical bone showed no difference between landing groups and their age-matched control groups. In the femora, the L1 group was lower than the C1 group in cortical bone area (Ct.Ar) and cortical thickness (Ct.Th) (p < 0.05); however, the L4 group was higher than the C4 group in Ct.Ar and Ct.Th (p <0 .05). In the tibiae, the moment of inertia about the antero-posterior axis (Iap), Ct.Ar and Ct.Th was significantly higher in the L8 group than in the C8 group (p < 0.05). In biomechanical testing, fracture load (FL) of femora was lower in the L1 group than in the C1 group (p < 0.05). Conversely, yield load (YL), FL and yield load energy (YE) of femora, as well as FL of tibiae were all significantly higher in the L8 group than in the C8 group (p < 0.05). Free-fall landing training may initially compromise bone material. However, over time, the current free-fall landing training induced improvements in biomechanical properties and/or the structure of growing bones.
Key points.
An 8-week landing training from a height of 40 cm improved cortical bone structure and strength in the hindlimbs of growing Wistar female rats.
Tibiae and femora showed different patterns of adaptive responses to the 8-week free-fall landing training.
Free-fall landing training may cause a slight and temporary compromise in bone material properties, but eventually induces an improvement in biomechanical properties and structures of growing bones.
Key words: Mechanical loading, exercise, bone strength, animal model
Introduction
Mechanical loading has a profound influence on the remodeling of cortical bone. Loss of loading (e.g. bed rest or spaceflight) results in an acceleration of bone turnover, in which bone resorption is uncoupled from bone formation and causes a rapid loss of bone mass (Bikle and Halloran, 1999). Conversely, mechanical loading caused by physical activity can generate micro-damages to bone tissue, stimulating bone turnover (Burr et al., 1985). If damage is accumulated faster than tissue can be repaired, more and larger micro-damages may develop and propagate to form fractures (Burr et al., 1985).
Along this unloading-to-overloading spectrum, it is known that high-impact exercise-induced mechanical loading generates positive changes in bone remodeling, structure and strength (Turner, 2006). However, the adaptive process undergone by cortical bone in response to this mechanical loading is unknown. According to Frost’s minimum effective strain (MES) proposal, a specific threshold of mechanotransduction is needed to evoke subsequent remodeling and adaptation (Frost, 2003). This bone remodeling cycle is initiated by bone resorption, which is a prerequisite for the following bone formation (Hattner et al., 1965). Hence, a period of compromise in bone material can be existent and detectable if the extra-mechanical loading is significant enough.
In animal studies, a variety of physical activity mimetic rodent models have been designed to explore the relationship between mechanical loading and cortical bone development, including endurance running (Chen et al., 1994; Huang et al., 2003), climbing-linked resistance training (Notomi et al., 2001; 2002) and jumping related movements (e.g. takeoff or landing) (Lin et al., 2011; 2013; Umemura et al., 1997; Umemura et al., 2008; Welch et al., 2004). Among those research models, high-impact jumping produces a relatively larger anabolic response due to the correspondingly higher mechanical impact. Furthermore, during a complete jumping movement, the landing phase is known to induce a larger ground reaction force (GRF) on limbs than the take-off phase (Kato et al., 2006), and previous study has indicated that bone tissue would be subjected to a higher strain rate with a larger GRF (Edwards et al., 2009). Therefore, the mechanical loading caused by landing would play a determinant role in bone’s adaptive process.
Prior animal studies investigating the effects of mechanical loading on cortical bone using free-fall landing models with rats have included only a single time point (e.g. 8 weeks or 1 week) (Lin et al., 2011; Lin et al., 2013; Welch et al., 2008; Welch et al., 2004). As a result, information regarding the process by which bone adapts to this type of intervention is not available. Thus, it would be valuable to design a time-course study to scrutinize the effects of free-fall landings on cortical bone development over the course of the intervention. Previous study has indicated that an 8-week free-fall landing program enhanced cortical bone strength and changed geometry in ulnae and radii, but not femora or tibiae (Welch et al., 2004; 2008). Moreover, a 1-week landing training program enhanced mineral apposition rate (MAR) and bone strength in ulnae (Lin et al., 2011), but compromised extrinsic mechanical properties and size-related measurements in femora (Lin et al., 2013); this suggests a site-specific adaptation rate in bone material and a latency period in rat femora in response to a landing-generated mechanical stimulus (Lin et al., 2013).
Because rodents’ hindlimbs have been found to receive greater impact during landing and to show material compromise after a 5-day free-fall landing training (Lin et al., 2013), the effects of free-fall landing on the cortical bone development of the hindlimbs are worthy of investigation. We hypothesized that landing training would initially cause a temporary compromise in cortical bone mechanical and geometric properties, such as bone strength and size. Further, we expected this initial change to be followed by a recovery and then an improvement in cortical bone mechanical and geometrical properties. The purpose of the current study is to investigate the adaptive processes of hindlimb cortical bone through a multiple time-point study. An 8-week free-fall landing protocol was designed for growing female Wistar rats and methods of biomechanical measurements, geometry, dynamic histomorphometry and densitometry were included in order to expose the details of this adaptive process.
Methods
Animals
Ninety-six female Wistar rats (4-week-old) were purchased and housed in National Cheng Kung University Animal Center under controlled conditions, including a room temperature of 22 ± 1°C with a 12:12 h light-dark cycle, and fed with standard Purina Rodent Chow 5001 (Labdiet®, Richmond, IN, USA) and tap water ad libitum. All procedures of the animal experiment followed the APS’s ‘‘Guiding Principles in the Care and Use of Animals’’ and were approved by the Committee of Animal Study at National Cheng Kung University (Document Serial No. 101166).
Experimental design and treatment
Animals were randomly assigned to four landing groups (L1, L2, L4 and L8 groups) and four time-matched control groups (C1, C2, C4 and C8 groups), which were subjected to landing training or a sedentary life for 1, 2, 4, 8 weeks, respectively. For all animals, free-fall landing training was started at the age of 7 weeks. Since rats reach puberty at an average age of 50 days after birth (Sengupta, 2013), the animals were in early adolescence at the beginning of the current study. For landing training groups, female rats were subjected to free-fall landing 30 times per day, 5 days per week. In our previous studies (Lin et al., 2011; 2013), protocols of 10 and 30 landings per day have been used. In the current study, the 30-landing protocol was chosen to mimic daily amount of work for high-impact exercise or sport (e.g. basketball or volleyball). Briefly, the rats were gently held and released from a height of 40 cm and landed with four-feet simultaneously on a dry and flat surface. A 10-15-second interval was arranged between each landing, according to a previous study (Welch et al., 2004). The CON rats were held and similarly raised thirty times per day without free-fall landing. Rats were sacrificed after the 1, 2, 4 or 8-week experimental periods. According to our previous protocol for investigating dynamic bone turnover, intraperitoneal (i.p.) injections of calcein (15mg/kg b.w.) and alizarin (30 mg/kg b.w.) were given to all rats one day before and one day after their final 5-day landing training periods (Lin et al., 2013). Thus, the two fluorescent label injections were 6 days apart. Three days after the last fluorescent injection, animals were humanely euthanized by decapitation under deep anesthesia with an i.p. injection of Zoletil 50 (Virbac Taiwan Co., Ltd, Taiwan) at a dose of 50mg/kg b.w.. Before decapitation, animals were laid in a prone position and body length was measured from the tip of nose to the sacrum.
GRF measurement
Time serial GRF data were collected from animals of the L8 group (n=12) on the 1st and 5th days of each free-fall training week. A force plate (30cm×30cm), used in the current and our previous studies (Lin et al., 2011; Lin et al., 2013), was designed by our laboratory. It includes four load cells (10 lbs., MDB-10, Transducer Techniques®, Temecula, USA), an amplifier (InstruNet-100, GW Instruments, Inc., Somerville, USA) and a data acquisition card (InstruNet-230, GW Instruments, Inc., Somerville, USA) connected to a personal computer with a sampling rate at 100 Hz. Original landing data (unit: N) of the thirty free-fall landings for each rat were obtained from four load cells under the force platform. Average free-fall landing GRF data of bilateral hindlimbs were presented as folds of standing force (unit: N*N-1) (Figure 1A) and calculated based on the concept of force equilibrium for a force plate, which has been described in our previous study (Lin et al., 2013) and in Welch’s force platform design for concurrently measuring forefeet and hindfeet GRF in young female rats (Welch et al., 2009).
Figure 1.
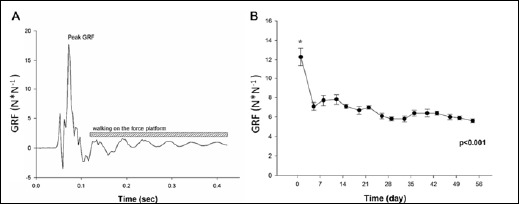
Free-fall landing and related measurements. An example of single ground reaction force (GRF) data acquired from one load cell A); average GRF on bilateral hindfeet during 8 weeks of free-fall landing training B). Values are means ± SEM. *, significantly higher than the other time points, p < 0.05. GRF, ground reaction force, which was presented as folds of standing force (unit: N*N-1).
Bone sample preparation
Immediately after animals were euthanized, femora and tibiae of each rat were dissected and freed of soft tissue, and the length of each femur and tibia was measured with a precision caliper (0.05 mm) as described by (Weinreb et al., 1991). Briefly, femur length was measured from the most superior point on the head of the femur to the most inferior point on the distal condyle. The distance from the lateral condyles to the tip of the medial malleolus was measured as tibia length. The long bones of left limbs were fixed in 70% ethanol for further micro-computed tomography (CT) scanning and analysis. Right femora and tibiae were wrapped in normal saline-soaked gauze and aluminum foil and stored at −2℃ for future biomechanical and geometric measurements.
Microcomputed tomography (μCT)
Distal femora and proximal tibiae were subjected to 3D scanning using a DCT scanner (Skyscan 1176, SKYSCAN, Kontich, Belgium) under x-ray conditions of 65 kV and 350μA with 1 mm aluminum filter per picture with 1100ms exposure time and 8.88 μm/pixel resolution. Reconstruction of sections was carried out with GPU-based scanner software (NRecon, Version, 1.6.5.0, SKYSCAN, Kontich, Belgium). Cortical bone measurements, including volumetric bone mineral density (vBMD) and bone mineral content (BMC) were conducted on diaphysis. Specifically, quantification of vBMD and BMC was performed in a defined cortical bone area located 6~7 mm below the growth plate of the proximal end of tibiae and distal end of femora using a CT-Analyzer (version 1.11.4.2, SKYSCAN, Kontich, Belgium).
Dynamic histomorphometry
After ACT scanning, bone samples from the left limbs were subjected to methylmethacrylate (MMA) embedding. A cross-sectional cut was made at the mid-shaft of each femoral and tibial sample using a diamond blade low-speed cutter (S150, PlusOver Co.Ltd., Kaohsiung, Taiwan). All sections were ground to a thickness of 100 ± 5 (μm) and photographed with a digital camera (COOLPIX 4500, Nikon, Japan) under a fluorescent light microscope (×25). Three dynamic histomorphometric parameters, bone mineralization over bone surface (MS/BS, %), mineral apposition rate (MAR, μm/day), and bone formation rate per bone surface (BFR/BS, /m3//m2/day), were measured in periosteal surfaces and endocortical surfaces according to Parfitt’s methods (Parfitt et al., 1987). All measurements were done by a histological expert who was blind to the original sample number.
Biomechanical three-point bending testing and geometry measurements
Three-point bending tests were conducted in the antero-posterior direction (posterior surface in tension) for femora and lateral-medial direction (medial surface in tension) for tibiae using a material testing system (MTS-858, MTS System, Minneapolis, MN, USA). Each right tibia and femur was subjected to loading to failure with a 20mm span of two support points and a deformation speed of 1 mm per second. Loading-deformation data were collected at a frequency of 200Hz, transported to a personal computer and acquired by the software Team490 (Version 4.10, Nicolet Instrument Technologies Inc., Madison, WI, USA). Load-deformation data were used for further calculating indices of extrinsic biomechanical properties, including yield load (YL), fracture load (FL), yield energy (YE), post-yield energy (PYE), fracture energy (FE) and stiffness. The failure sites of all specimens were photographed under ×25 magnification by a digital camera (COOLPIX 4500, Nikon, Japan) according to the methods in our previous study (Huang et al., 2003). Cross-sectional moments of inertia about the mediolateral axis (Iml) for femora and the antero-posterior axis for tibiae (Iap) were measured from the photographs using the software Image-Pro Plus 6.1 for Windows (Media Cybernetics, Silver Spring, MD, USA). Iml and Iap of failure sites were measured according to a tutorial for irregular cross sections set up by Turner and Burr (Turner and Burr, 1993), which is based on the assumption that a bone cross section is made up of numerous rectangular elements (pixels), using the following equation:
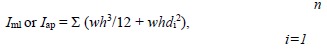 |
(1) |
where Iml is cross-sectional moments of inertia about the mediolateral axis for femora, Iap is cross-sectional moments of inertia about the antero-posterior axis for tibiae, n is the number of pixels, w and h are the height and width of each pixel, and di is the distance from the center of the element of the area to a given axis on the cross section. At the same region where Iml or Iap was measured, cortical bone area (Ct.Ar), total cross-sectional area inside the periosteal envelope (Tt.Ar), and average cortical thickness (Ct.Th) were also measured using the software Image-Pro Plus 6.1. Load-deformation data were then converted to stress-strain data using the following equations:
| (2) |
| (3) |
| (4) |
where σ is stress, I is Iml or Iap, ε is strain, c is the maximal distance from pixels located on the posterior surface of the femur and medial surface of the tibia to the line crossing the center of mass, F is the applied load (N), E is elastic modulus, d is the deformation (mm) and L is the span between the two support points of the bending fixture (mm). Because elastic beam theory is only valid for pre-yield investigation, a stress-strain curve was used to calculate yield stress (YS), yield toughness (YT) and elastic modulus (EM) as parameters of intrinsic biomechanical (tissue-level) properties. YL and YS were determined with the 0.2% offset method described previously (Turner and Burr, 1993).
Statistical analysis
Student’s t-test was performed to compare statistical differences between each landing group and its age-matched control group. One-way analysis of variance (ANOVA) was used to compare GRF values among 16 time points. Percentage differences between landing groups and their age-matched control groups were calculated for indices of geometry and biomechanical properties using the formula: [(Li − Cmean)/Cmean]× 100%, where Li is data from each landing rat, and Cmean is the average of the age-matched group. Also, one-way ANOVA was used to compare percentage differences among different time points, including a baseline (0 week) time point, which was assumed zero in percentage difference. P < 0.05 was considered significant, and Fisher’s LSD method was used for post hoc comparison. A statistical analysis software (SPSS 14.0 version, SPSS, Chicago, IL) was adopted to process statistical analyses.
Results
Body mass and linear growth
No difference in body mass (BM) was found between landing groups and age-matched control groups during the four training periods. Similarly, no differences were found between landing and control groups in body lengths or bone lengths as indices of linear growth (Table 1).
Table 1.
Body mass (BM), body length and bone lengths. Values are means (± SEM, n=12 for all groups).
| 1-Week | 2-Week | 4-Week | 8-Week | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | C1 | L2 | C2 | L4 | C4 | L8 | C8 | |
| Pre-training BM (g) | 202.6 (2.6) | 202.8 (3.3) | 207.6 (4.3) | 205.3 (4.0) | 208.0 (2.4) | 207.9 (2.7) | 210.3 (2.8) | 207.8 (2.1) |
| Post-training BM (g) | 223.0 (2.9) | 222.6 (4.5) | 243.8 (4.5) | 244.7 (5.3) | 257.7 (3.6) | 260.0 (4.0) | 290.3 (4.2) | 285.8 (7.2) |
| Body length (cm) | 20.3 (.1) | 20.2 (.1) | 20.2 (.1) | 20.2 (.1) | 21.1 (.1) | 21.1 (.1) | 22.0 (.1) | 21.8 (.1) |
| Femur length (mm) | 32.6 (.1) | 32.5 (.1) | 33.3 (.2) | 33.2 (.2) | 34.6 (.1) | 34.4 (.1) | 35.6 (.1) | 35.4 (.1) |
| Tibia length (mm) | 36.6 (.2) | 36.5 (.1) | 37.2 (.3) | 37.2 (.3) | 38.3 (.1) | 38.5 (.1) | 39.4 (.1) | 39.2 (.1) |
GRF
On the first day of free-fall landing training, average peak GRF generated on bilateral hindlimbs was 12.5-fold standing forces, which was significantly higher than those measured at subsequent time points (6~8-fold standing forces) (p < 0.05) (Figure 1B).
μCT
In cortical vBMD of tibiae and femora, no difference was found between landing groups and their time-matched control groups. However, the L8 group had higher cortical BMC in tibiae compared to the C8 group (p < 0.05) (Table 2).
Table 2.
Bone mineral measurements from μCT in femora and tibiae. Values are means (± SEM).
| 1-Week | 2-Week | 4-Week | 8-Week | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | C1 | L2 | C2 | L4 | C4 | L8 | C8 | |
| Femur | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=11 | n=12 |
| Ct. vBMD (g/cm3) | 1.173 (.003) | 1.178 (.004) | 1.200 (.006) | 1.213 (.010) | 1.264 (.004) | 1.262 (.006) | 1.298 (.004) | 1.298 (.005) |
| Ct. BMC (mg) | 5.2 (.1) | 4.9 (.3) | 5.8 (.1) | 5.9 (.1) | 3.5. (.3) | 4.1 (.6) | 7.5 (.1) | 7.4 (.1) |
| Tibia | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 |
| Ct. vBMD (g/cm3) | 1.137 (.005) | 1.147 (.005) | 1.174 (.007) | 1.178 (.005) | 1.220 (.004) | 1.210 (.003) | 1.265 (.004) | 1.268 (.006) |
| Ct. BMC (mg) | 3.5 (.1) | 3.7 (.1) | 4.0 (.1) | 4.0 (.1) | 4.7 (.2) | 4.5 (.1) | 5.3 (.1) | 5.0 (.1) * |
*, significant difference between landing group and age-matched control group, p < 0.05. Ct., cortical; vBMD (g/cm3), volumetric bone mineral density; BMC, bone mineral content (mg).
Geometry measurement
Cortical bone area and thickness of femoral cross-sections were significantly lower in the L1 group and higher in the L4 groups, respectively, compared to their age-matched control groups (p < 0.05) (Table 3). Furthermore, Iap, Ct.Ar and Ct.Th of tibiae were significantly higher in the L8 group than in the C8 group (p < 0.05) (Table 3).
Table 3.
Geometry measurement in cross-section of cortical bone. Values are means (±SEM).
| 1-Week | 2-Week | 4-Week | 8-Week | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | C1 | L2 | C2 | L4 | C4 | L8 | C8 | |
| Femur | n=12 | n=12 | n=12 | n=12 | n=12 | n=11 | n=12 | n=12 |
| Iml (mm4) | 6.67 (.30) | 6.96 (.31) | 6.46 (.36) | 6.70 (.37) | 8.40 (.45) | 7.41 (.37) | 8.79 (.51) | 8.18 (.35) |
| Ct.Ar (mm2) | 6.24 (.15) | 6.78 (.12) | 6.43 (.26) | 6.45 (.13) | 7.25 (.09) | 6.81 (.10)** | 7.80 (.10) | 7.59 (.14) |
| Tt.Ar (mm2) | 10.77 (.21) | 10.85 (.31) | 10.67 (.28) | 10.76 (.27) | 11.67 (.23) | 11.00 (.27) | 11.92 (.26) | 11.74 (.18) |
| Ct.Th (mm) | .63 (.01) | .69 (.01)*** | .66 (.02) | .66 (.01) | .72 (.01) | .69 (.01)* | .78 (.01) | .75 (.01) |
| Tibia | n=10 | n=12 | n=10 | n=9 | n=12 | n=12 | n=12 | n=12 |
| Ct. vBMD (g/cm3) | 3.50 (.22) | 3.50 (.29) | 2.91 (.19) | 3.17 (.24) | 3.56 (.20) | 3.40 (.16) | 3.87 ± 0.10 | 3.41 (.16)* |
| Ct. BMC (mg) | 4.50 (.13) | 4.32 (.15) | 4.23 (.21) | 4.59 (.19) | 5.13 (.11) | 5.07 (.11) | 5.43 ± 0.07 | 5.09 (.10)** |
| Ct. vBMD (g/cm3) | 7.51 (.22) | 7.40 (.35) | 6.86 (.31) | 6.87 (.33) | 7.44 (.20) | 7.31 (.16) | 7.58 ± 0.12 | 7.33 (.17) |
| Ct. BMC (mg) | .55 (.02) | .52 (.02) | .54 (.03) | .59 (.01) | .65 (.01) | .65 (.01) | 0.70 ± 0.02 | .65 (.01)** |
*,** and *** denote p ≤ 0.05, 0.01 and 0.001, respectively, between landing group to the time-matched sedentary control group. Iml, moment of inertia about the mediolateral axis for femora; Iap, moment of inertia about the antero-posterior axis for tibiae; Ct.Ar, cortical bone area; Tt.Ar, total cross-sectional area inside the periosteal envelope; Ct.Th, average cortical thickness.
Dynamic histomorphometry
No significant difference was found in dynamic histomorphometric analyses in regions of periosteal surfaces and endocortical surfaces of femora and tibiae, except for in the L1group, which showed a significantly higher MS/BS in the tibial periosteum than the C1 group (Table 4).
Table 4.
Dynamic histomorphometry of cortical bone in growing rats. Values are means (±SEM).
| 1-Week | 2-Week | 4-Week | 8-Week | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | C1 | L2 | C2 | L4 | C4 | L8 | C8 | |
| Femur | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 |
| Periostium | ||||||||
| MS/BS | 36.6 (3.6) | 32.6 (2.6) | 41.2 (4.7) | 39.9 (4.5) | 43.2 (4.9) | 34.0 (4.8) | 33.0 (4.7) | 36.2 (2.8) |
| MAR | 1.8 (.79) | 3.0 (.73) | 2.9 (.94) | 2.3 (.6) | 1.7 (.40) | 2.3 (.44) | 1.5 (.43) | 1.8 (.47) |
| BFR/BS | .83 (.38) | 1.07 (.25) | 1.42 (.46) | 1.07 (.34) | .87 (.22) | .90 (.19) | .57 (.18) | .75 (.21) |
| Endosteum | ||||||||
| MS/BS | 37.3 (3.9) | 41.5 (5.4) | 39.3 (3.0) | 35.2 (3.5) | 38.4 (5.9) | 36.9 (4.8) | 27.5 (6.6) | 21.7 (4.2) |
| MAR | 1.3 (.57) | 3.4 (.93) | 1.3 .64) | 1.6 (.54) | 1.4 (.32) | 1.6 (.32) | 1.7 (.33) | 1.9 (.68) |
| BFR/BS | .64 (.31) | 1.70 (.45) | .62 (.35) | .74 (.28) | .70 (.17) | .72 (.16) | .55 (.15) | .50 (.15) |
| Tibia | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 |
| Periostium | ||||||||
| MS/BS | 40.3 (2.0) | 30.2 (3.3)* | 40.1 (3.8) | 37.9 (3.6) | 35.4 (2.6) | 28.3 (3.2) | 21.5 (3.9) | 29.0 (2.7) |
| MAR | 1.0 (.41) | .6 (.32) | 1.2 (.53) | 1.0 (.38) | .8 (.34) | .5 (.29) | .7 (.40) | .7 (.35) |
| BFR/BS | .42 (.18) | .23 (.13) | .61 (.27) | .48 (.19) | .35 (.16) | .19 (.11) | .27 (.15) | .19 (.11) |
| Endosteum | ||||||||
| MS/BS | 37.8 (5.4) | 28.1 (3.4) | 35.0 (3.8) | 39.2 (3.5) | 37.6 (4.2) | 38.4 (3.9) | 27.5 (4.7) | 32.5 (3.3) |
| MAR | 1.7 (.62) | 1.1 (.45) | 1.5 (.70) | 1.3 (.39) | 1.7 .(43) | 1.7 (.52) | 1.0 (.45) | 1.2 (.36) |
| BFR/BS | .84 (.32) | .43 (.20) | .69 (.33) | .57 (.19) | .73 (.20) | .72 (.22) | .38 (.24) | .45 (.14) |
*, significant difference was shown between landing group to the time-matched sedentary control group, p < 0.05. BFR/BS, bone formation rate per bone surface, μm3/μm2/day; MAR, mineral apposition rate, μm/day. MS/BS, bone mineralization over bone surface, %.
Biomechanical properties
In femora, whole bone strength was trending toward significance and significantly lower in YL (p = 0.092) and FL (p = 0.049), respectively, in the L1 group than in the time-matched control group. Conversely, the L8 group had significantly higher YL, FL and YE, and they are trending toward significance in YS (p = 0.067) and YT (p = 0.091) when compared to the C8 group (p < 0.05) (Table 5). Additionally, FL of tibia was higher in the L8 group than in the C8 group (p < 0.05) (Table 5).
Table 5.
Mechanical properties in femora and tibiae. Values are means (±SEM).
| 1-Week | 2-Week | 4-Week | 8-Week | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | C1 | L2 | C2 | L4 | C4 | L8 | C8 | |
| Femur | n=12 | n=12 | n=12 | n=11 | n=12 | n=12 | n=12 | n=12 |
| YL (N) | 82.4 (1.2) | 86.2 (1.8) | 95.2 (4.2) | 87.1 (5.6) | 101.5 (2.7) | 101.4 (6.9) | 117.8 (4.4) | 99.1 (3.5)** |
| FL (N) | 67.1 (3.4) | 76.8 (3.2)* | 86.6 (1.9) | 86.8 (4.4) | 115.4 (3.0) | 119.3 (3.5) | 126.7 (4.1) | 111.9 (3.2)** |
| YE (mJ) | 39.8 (1.6) | 41.0 (4.6) | 40.7 (2.8) | 35.9 (2.8) | 25.5 (2.5) | 28.4 (3.2) | 36.4 (2.4) | 27.7 (2.7)* |
| FE (mJ) | 79.6 (4.4) | 75.3 (4.1) | 75.2 (7.7) | 66.7 (6.7) | 50.4 (3.3) | 54.8 (3.1) | 48.5 (3.0) | 42.1 (2.6) |
| PYE (mJ) | 39.8 (4.6) | 34.3 (4.2) | 34.5 (6.2) | 30.8 (6.3) | 24.8 (3.7) | 25.8 (3.3) | 12.1 (2.0) | 14.4 (2.9) |
| Stiffness (N/mm) | 107.1 (4.2) | 104.2 (5.2) | 154.9 (12.1) | 141.3 (15.7) | 315.2 (18.7) | 265.0 (22.6) | 267.0 (18.7) | 271.5 (21.5) |
| YS (MPa) | 118.9 (4.5) | 117.3 (5.4) | 138.2 (7.7) | 126.0 (9.3) | 120.3 (5.3) | 130.1 (9.0) | 131.2 (5.7) | 116.0 (5.5) |
| YT (mJ/mm3) | 3.27 (.22) | 3.38 (.32) | 3.26 (.25) | 2.90 (.24) | 1.74 (.15) | 2.03 (.22) | 2.32 (.15) | 1.87 (.21) |
| EM (Gpa) | 2.73 (.16) | 2.51 (.10) | 4.10 (.37) | 3.74 (.47) | 6.41 (.50) | 6.34 (.49) | 5.31 (.58) | 5.58 (.46) |
| Tibia | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 | n=12 |
| YL (N) | 56.7 (2.2) | 60.5 (3.0) | 68.6 (3.4) | 70.3 (2.4) | 81.2 (2.5) | 79.4 (2.5) | 90.0 (2.53) | 85.1 (3.7) |
| FL (N) | 67.6 (3.4) | 66.6 (2.6) | 70.4 (4.8) | 71.8 (3.4) | 86.4 (3.3) | 82.2 (3.6) | 98.6 (2.49) | 88.9 (2.4)** |
| YE (mJ) | 18.3 (1.1) | 21.4 (1.8) | 27.0 (2.3) | 27.2 (1.8) | 26.1 (2.2) | 25.6 (1.5) | 28.4 (1.98) | 30.2 (3.4) |
| FE (mJ) | 53.9 (5.7) | 58.2 (4.1) | 60.6 (5.9) | 76.3 (9.9) | 75.2 (12.1) | 68.3 (5.5) | 65.5 (4.39) | 61.1 (7.1) |
| PYE (mJ) | 36.1 (6.8) | 36.8 (3.2) | 35.8 (6.9) | 49.9 (11.5) | 49.1 (11.1) | 42.8 (4.6) | 37.1 (3.83) | 31.0 (4.8) |
| Stiffness (N/mm) | 109.0 (5.0) | 107.7 (5.7) | 115.6 (19.2) | 106.1 (9.3) | 171.2 (11.7) | 156.5 (9.3) | 183.2 (9.3) | 162.9 (12.1) |
| YS (MPa) | 139.1 (5.5) | 155.0 (11.1) | 179.6 (7.9) | 173.1 (9.5) | 175.5 (7.2) | 173.4 (4.1) | 183.1 (5.1) | 187.4 (3.7) |
| YT (mJ/mm3) | 2.28 (.15) | 2.74 (.21) | 3.18 (.29) | 3.06 (.25) | 2.53 (.22) | 2.46 (.15) | 2.72 (.20) | 2.94 (.28) |
| EM (Gpa) | 5.35 (.31) | 5.50 (.52) | 6.55 (.68) | 6.07 (.50) | 8.46 (.90) | 7.77 (.47) | 8.00 (.52) | 8.17 (.63) |
* and ** denote p ≤ 0.05 and 0.01, respectively, between landing group to the time-matched sedentary control group. EM, elastic modulus; FE, fracture load energy; FL, fracture load; PYE, post-yield energy; YE, yield energy; YL, yield load; YS, yield stress; YT, yield stress.
Percentage differences between landing and control groups in bone geometry and biomechanical properties
In order to further demonstrate the impact of free-fall landing, percentage differences between the landing groups and their age-matched control groups were calculated in geometric and biomechanical indices and summarized in Figure 2. In femora, percentage difference in geometric and/or biomechanical properties were comprehensively lower in the 1st week, but higher in the 4th or 8th weeks than at the baseline (p < 0.05) (Figure 2A, B, D, E). In tibiae, percentage differences in five geometric and biomechanical indices were all significantly higher in the 8th week than at the baseline (p < 0.05) (Figure 2F, G, H, I, J).
Figure 2.
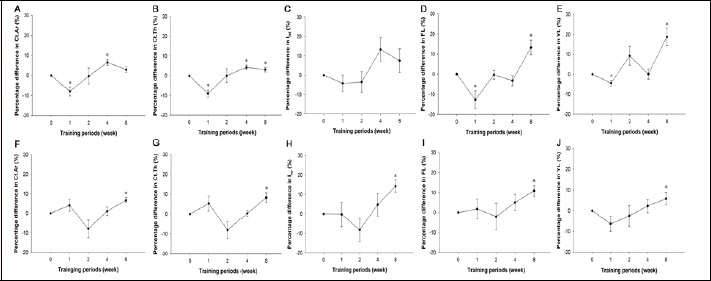
Percentage differences in various geometric and biomechanical properties. Percentage differences between landing groups and their age-matched control groups were calculated for indices of geometry and biomechanical properties in femora (A-E) and tibiae (F-J). Values are means ± SEM. *, significantly different from 0 week. Ct.Ar, cortical bone area; Ct.Th, cortical bone thickness; Iml, moment of inertia about the mediolateral axis for femora; Iap, moment of inertia about the antero-posterior axis for tibiae; FL, fracture load; YL, yield load.
Discussion
Long-term strengthening and temporary compromise
The present study verified that an 8-week free-fall landing training from a height of 40 cm would improve cortical bone strength and bone size for the hindlimbs of growing female rats. Previous animal and human studies have had similar findings. Welch and colleagues demonstrated that an 8-week free-fall landing training from a height of 30 cm and 60 cm increased cortical bone strength (e.g. ultimate force, stiffness, work to failure) in the forelimbs of growing Fischer 344 female rats (Welch et al., 2004). Additionally, an 8-week 45 cm jumping training increased strength in the tibial cortical bone of growing Wistar female rats (Umemura et al., 2008) and femora of growing Sprague Dawley male rats (Notomi et al., 2000). In human studies, a 12-week drop-jump training protocol (25 repetitions/day, 5 days/week) from a height of 45 cm increased leg BMC of girls (3-18 year-old) (Johannsen et al., 2003), while a 7-month landing training program (61cm, 100 repetitions/day, 3 days/week) increased areal BMD and/or BMC in hip and lumbar of children (5.9-9.8 year-old) (Fuchs et al., 2001). In addition, a jumping exercise program (10-50 cm, 50-100 repetitions, 3 times per week for 7 months) improved areal BMD, BMC and bone structural indices for early-pubertal girls (Petit et al., 2002). In general, the findings agree that high-impact loading on bones generates positive effects, though the details of these benefits were inconsistent, possibly due to differences in age, species, gender, and anatomy etc.
In addition to verifying the positive effects of an 8-week landing training protocol, the current time-course study demonstrated a compromise in bone structure (e.g. Ct.Th, Ct.Ar) and strength (e.g. FL) in femora after 1-week of landing training, which was similar to, but not exactly the same as, the results of our previous short-term landing study, which showed a reduction in Tt.Ar, Ct.Th, Ct.Ar, PYE and FE after 1 week (Lin et al., 2013). In addition, similar phenomena have been found in other experimental models. For example, mechanical environmental changes caused by extracting right maxillary molars induced a wave of remodeling activity initiated by osteoclastogenesis on the periosteal surface of the alveolar bone (Tran Van et al., 1982). Furthermore, using apparatus-generated axial compressive loading (Hsieh and Silva, 2002) reduced whole bone strength indices (e.g. ultimate force and stiffness) of ulnae immediately and 6 days after a single bout of cyclic fatigue loading. Ultimate force and stiffness returned to normal levels and eventually showed a 20% increase on the 12th and 18th days, respectively, after loading (Hsieh and Silva, 2002). These data are in agreement with the existence of a latency period in the adaptation of bone mechanical properties to changes caused by compressive loading or free-fall landing.
In summary, temporary compromises in bone material were found in structural and mechanical indices after one week of landing training, which were then followed by a recovery and an improvement after a longer period of similar training.
Possible mechanisms of the temporary compromise in bone material
Subsequent to accumulated damages caused by mechanical loading, bone material is capable of self-repair through bone resorption activity initiated with a simultaneous compromise in structural and biomechanical properties, followed by bone formation activity (Clarke, 2008; Robling et al., 2006; Tran Van et al., 1982). Though molecular and cellular analyses were unavailable in the current study, changes in the mechanical environment will affect molecular signals produced by osteocytes in cortical bone. Evidence from previous studies verified that osteocytes are capable of sensing and responding to mechanical strain via changes in the release of nitric oxide, prostaglandin, and sclerostin, as well as nuclear factor kappa-B ligand (RANKL) (Forwood, 1996; Klein-Nulend et al., 2014; Robling et al., 2008; Xiong and O’Brien, 2012), which would subsequently induce osteoclastogenesis as well as bone remodeling near the loading site (Verborgt et al., 2000). The development of high-resolution computed tomography (e.g. CT or nano-CT) has led to research capable of measuring bone remodeling in-vivo at multiple time points (Schulte et al., 2013). Future study using high-resolution CT technology to investigate the distribution and shifting of the bone remodeling area throughout a free-fall landing regimen will be valuable.
Differences in adaptive responses
In the current study, the biomechanical properties of tibiae and femora adapted differently to free-fall landing training. In tibiae, no change was shown in geometric or mechanical properties in the initial phase, but an upgrade in bone mineral accumulation (Table 2), geometry (Table 3 and Figure 2) and mechanical strength (Table 5 and Figure 2) was found after 8 weeks of landing training. This implies site-specific differences in the ways in which bones adapt to mechanical loading. Moreover, forelimb bone showed an even faster adaptive response. After 5 days of free-fall landing, the ulnae of young female rats had no losses in extrinsic strength and even had increases in post-yield bending energy (Lin et al., 2011). One plausible explanation for the adaptive timing difference between forelimbs and hindlimbs could be differences in gravitational loading (e.g. GRF). The GRF received by forelimbs (4-5-fold standing force) (Lin et al., 2011), was relatively higher than the GRF received by hindlimbs (~12-fold standing force) during the initial phase. This relatively higher GRF on hindlimbs was the opposite of the results in previous studies using growing female F-344 rats, which had higher GRF on the forefeet (12.04~16.74-fold standing force) than on the hindfeet (4.46~7.7-fold standing force) (Welch et al., 2009). It could be that a relatively higher hindlimb GRF temporarily compromises bone mechanical properties. In addition, though the psychological stress and landing skill were not evaluated at the beginning of the training protocol in the current study, mental fear and inappropriate skill (e.g. incoordinate muscle contractions) in the 40-cm free-fall landing may lead to a greater impact force and short-term compromises in bone material. Although there have been arguments about whether bone’s response to mechanical signals can be dominated by gravitational loading (Judex and Carlson, 2009) or muscle forces (Robling, 2009), both are factors in adaptation. From a more macroscopic view, terrestrial mammal skeletons adapt to increased external loading by increasing the effective mechanical advantage of limbs to decrease mass-specific forces (Biewener, 1991). This allows them to maintain a similar mechanical stress distribution and safety for a specific region of bone (Biewener, 1991). Bone size (e.g., cross-sectional area and moment of inertia) (Biewener and Bertram, 1994; Rubin and Lanyon, 1985) has been identified as one of the most important mechanical advantages. Therefore, the femur, as the largest limb bone, is assumed to receive a higher integrated mechanical loading during a given free-fall landing movement, which could then lead to a temporary, but detectable, compromise in bone dimensions and strength measurements.
In addition to the initial differences in adaptive responses, femora also had a distinctive adaptation process in bone material properties throughout the training course. Despite a significant improvement in whole bone strength indices after 8 weeks of training, femora of landing groups did not maintain the relative improvement in bone geometry shown after 4 weeks of training. There are several explanations for the difference in adaptation between femora and tibiae. First, the loading intensity of the 40-cm landing could become relatively lower for femora in the later training phase. In a study by Welch and colleagues, growing Fisher 344 female rats showed no adaptive response to an 8-week free-fall landing course (Welch et al., 2004). A progressive increase in landing height would probably be helpful for maintaining adaptive gains shown at 4 weeks. Second, bone matrix organization (e.g. collagen orientation, levels of cross-link or mineral to matrix ratio) can play a role in bone mechanical properties (Garnero et al., 2006; Takata et al., 2011). Though related measurements were not available in the current study, femoral tissue could eventually adapt to this long-term 40-cm free-fall landing through an optimized integration between collagen and cross-link rather than through size-related development.
Though there was a short period of compromise in bone material, neither linear growth (e.g. bone lengths) nor bone mineralization status (e.g. dynamic histomorphometry and vBMD) were negatively impacted by the free-fall landing protocol. Similar findings in previous short-term (Lin et al., 2013) and long-term (Welch et al., 2004) studies suggest that the current landing regimen may lead growing bone to go through a short-term geometric development latency but eventually generate an improvement in bone material.
Study conditions, clinical applications and possible limitations
Compared to previous apparatus-generated mechanical loading (e.g. axial compression on ulnae) (Hsieh and Silva, 2002) or surgery-induced mechanical environment changes (Tran Van et al., 1982), the current free-fall landing-induced impact on bone is a relatively practical and easy-to-handle experimental model. The hindlimbs of rodents perform a knee-bending movement, similar to that of humans, to cushion the impact of landing. Furthermore, during the landing training period, animals are free from excessively invasive procedures, such as anesthesia, surgery or loading by apparatus. Finally, landing-caused mechanical loading can be measured by an economical and easily-assembled force plate.
In terms of clinical applicability, the current study and previous jumping-related studies adopted mechanical loading protocols simulating general physical activity interventions rather than damage- or fracture-causing experimental models. Although most previous findings corroborate the positive effects of various jumping interventions on bone (Boudreaux et al., 2014; Honda et al., 2001; Ju et al., 2008; Lin et al., 2011; Umemura et al., 1997; Welch et al., 2004), the current study duplicated a short period of femoral compromise found in our previous study (Lin et al., 2013). This compromise has not been seen in other long bones (e.g.tibiae or ulnae), suggesting that femora undergo a relatively vigorous adaptation process at the beginning of being subjected to a free-fall landing regimen. Further studies would be valuable in clinic practice to investigate whether increasing the height or repetitions in the initial phase would elevate the material compromise found in week one of this study to the level of stress fractures.
Conclusion
The current study demonstrated changes in the structure and biomechanical properties of cortical bone in rats subjected to a period of free-fall landing training. Results of biomechanical assays suggest that, in the initial phase of landing training, a temporary compromise in dimension and bending strength of long bone diaphysis is existent. However, after a longer period of training, the free-fall landing protocol eventually generated positive effects for growing long bones, as shown by improvements in bone mechanical properties and/or increased bone size-related measurements.
Acknowledgements
This study was supported by a grant from the National Science Council (NSC99-2410-H-006-114-MY2, Taiwan). We thank Miss Jae Cody for her editorial assistance in English. The authors have no conflicts of interest to declare. All experiments comply with the current laws of the country.
Biographies

Hsin-Shih LIN
Employment
National Cheng Kung University (Taiwan), Adjunct Assistant Professor
Degree
PhD
Research interests
Exercise and bone metabolism
E-mail: 897300180@ntnu.edu.tw

Ho-Seng WANG
Employment
National Taiwan Normal University (Taiwan), Associate Professor
Degree
PhD
Research interests
Exercise physiology
E-mail: t08019@ntnu.edu.tw

Hung-Ta CHIU
Employment
National Cheng Kung University (Taiwan), Associate Professor
Degree
PhD
Research interests
Sports Biomechanics
E-mail: htchiu@mail.ncku.edu.tw

Kuang-You B. CHENG
Employment
National Cheng Kung University (Taiwan), Professor
Degree
PhD
Research interests
Sports Biomechanics
E-mail: kybcheng@mail.ncku.edu.tw

Ar-Tyan HSU
Employment
National Cheng Kung University (Taiwan) Professor
Degree
PhD
Research interests
Orthopedic physical therapy, Biomechanics, Finite element analysis of bone
E-mail: arthsu@mail.ncku.edu.tw

Tsang-Hai HUANG
Employment
National Cheng Kung University (Taiwan), Professor
Degree
PhD
Research interests
Exercise and bone metabolism
E-mail: tsanghai@mail.ncku.edu.tw
References
- Biewener A.A. (1991) Musculoskeletal design in relation to body size. Journal of Biomechanics 24 Suppl 1, 19-29. [DOI] [PubMed] [Google Scholar]
- Biewener A.A., Bertram J.E. (1994) Structural response of growing bone to exercise and disuse. Journal of Applied Physiology 76, 946-955. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Halloran B.P. (1999) The response of bone to unloading. Journal of Bone and Mineral Metabolism 17, 233-244. [DOI] [PubMed] [Google Scholar]
- Boudreaux R.D., Swift J.M., Gasier H.G., Wiggs M.P., Hogan H.A., Fluckey J.D., Bloomfield S.A. (2014) Increased resistance during jump exercise does not enhance cortical bone formation. Medicine and Science in Sports and Exercise 46, 982-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D.B., Martin R.B., Schaffler M.B., Radin E.L. (1985) Bone remodeling in response to in vivo fatigue microdamage. Journal of Biomechanics 18, 189-200. [DOI] [PubMed] [Google Scholar]
- Chen M.M., Yeh J.K., Aloia J.F., Tierney J.M., Sprintz S. (1994) Effect of treadmill exercise on tibial cortical bone in aged female rats: a histomorphometry and dual energy x-ray absorptiometry study. Bone 15, 313-319. [DOI] [PubMed] [Google Scholar]
- Clarke B. (2008) Normal bone anatomy and physiology. Clinical Journal of the American Society of Nephrology 3 Suppl 3, S131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards W.B., Ward E.D., Meardon S.A., Derrick T.R. (2009) The use of external transducers for estimating bone strain at the distal tibia during impact activity. Journal of Biomechanical Engineering 131, 051009. [DOI] [PubMed] [Google Scholar]
- Forwood M.R. (1996) Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. Journal of Bone and Mineral Research 11, 1688-1693. [DOI] [PubMed] [Google Scholar]
- Frost H.M. (2003) Bone’s mechanostat: a 2003 update. The Anatomcial Record. Part A Discoveries in Molecular Cellular and Evolutionary Biology 275, 1081-101. [DOI] [PubMed] [Google Scholar]
- Fuchs R.K., Bauer J.J., Snow C.M. (2001) Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. Journal of Bone and Mineral Research 16, 148-156. [DOI] [PubMed] [Google Scholar]
- Garnero P., Borel O., Gineyts E., Duboeuf F., Solberg H., Bouxsein M.L., Christiansen C., Delmas P.D. (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38, 300-309. [DOI] [PubMed] [Google Scholar]
- Hattner R., Epker B.N., Frost H.M. (1965) Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 206, 489-490. [DOI] [PubMed] [Google Scholar]
- Honda A., Umemura Y., Nagasawa S. (2001) Effect of high-impact and low-repetition training on bones in ovariectomized rats. Journal of Bone and Mineral Research 16, 1688-1693. [DOI] [PubMed] [Google Scholar]
- Hsieh Y.F., Silva M.J. (2002) In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. Journal of Orthopedic Research 20, 764-771. [DOI] [PubMed] [Google Scholar]
- Huang T.H., Lin S.C., Chang F.L., Hsieh S.S., Liu S.H., Yang R.S. (2003) Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. Journal of Applied Physiology 95, 300-307. [DOI] [PubMed] [Google Scholar]
- Johannsen N., Binkley T., Englert V., Neiderauer G., Specker B. (2003) Bone reponse to jumping is site-specific in children: a randomized trial. Bone 33, 533-539. [DOI] [PubMed] [Google Scholar]
- Ju Y.I., Sone T., Okamoto T., Fukunaga M. (2008) Jump exercise during remobilization restores integrity of the trabecular architecture after tail suspension in young rats. Journal of Applied Physiology 104, 1594-1600. [DOI] [PubMed] [Google Scholar]
- Judex S., Carlson K.J. (2009) Is bone’s response to mechanical signals dominated by gravitational loading? Medicine and Science in Sports and Exercise 41, 2037-2043. [DOI] [PubMed] [Google Scholar]
- Kato T., Terashima T., Yamashita T., Hatanaka Y., Honda A., Umemura Y. (2006) Effect of low-repetition jump training on bone mineral density in young women. Journal of Applied Physiology 100, 839-843. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J., van Oers R.F., Bakker A.D., Bacabac R.G. (2014) Nitric oxide signaling in mechanical adaptation of bone. Osteoporosis International 25, 1427-1437. [DOI] [PubMed] [Google Scholar]
- Lin H.S., Huang T.H., Mao S.W., Tai Y.S., Chiu H.T., Cheng K.Y.B., Yang R.S. (2011) A Short-Term Free-Fall Landing Enhances Bone Formation and Bone Material Properties. Journal of Mechanics in Medicine and Biology 11, 1125-1139. [Google Scholar]
- Lin H.S., Huang T.H., Wang H.S., Mao S.W., Tai Y.S., Chiu H.T., Cheng K.Y., Yang R.S. (2013) Short-term free-fall landing causes reduced bone size and bending energy in femora of growing rats. Journal of Sports Science and Medicine 12, 1-9. [PMC free article] [PubMed] [Google Scholar]
- Notomi T., Lee S.J., Okimoto N., Okazaki Y., Takamoto T., Nakamura T., Suzuki M. (2000) Effects of resistance exercise training on mass, strength, and turnover of bone in growing rats. European of Journal Applied Physiology 82, 268-274. [DOI] [PubMed] [Google Scholar]
- Notomi T., Okazaki Y., Okimoto N., Tanaka Y., Nakamura T., Suzuki M. (2002) Effects of tower climbing exercise on bone mass, strength, and turnover in orchidectomized growing rats. Journal of Applied Physiology 93, 1152-1158. [DOI] [PubMed] [Google Scholar]
- Notomi T., Okimoto N., Okazaki Y., Tanaka Y., Nakamura T., Suzuki M. (2001) Effects of tower climbing exercise on bone mass, strength, and turnover in growing rats. Journal of Bone and Mineral Research 16, 166-174. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research 2, 595-610. [DOI] [PubMed] [Google Scholar]
- Petit M.A., McKay H.A., MacKelvie K.J., Heinonen A., Khan K.M., Beck T.J. (2002) A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. Journal of Bone and Mineral Research 17, 363-372. [DOI] [PubMed] [Google Scholar]
- Robling A.G. (2009) Is bone’s response to mechanical signals dominated by muscle forces? Medicine and Science in Sports Exercise 41, 2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling A.G., Castillo A.B., Turner C.H. (2006) Biomechanical and molecular regulation of bone remodeling. Annual Review of Biomedical Engineering 8, 455-498. [DOI] [PubMed] [Google Scholar]
- Robling A.G., Niziolek P.J., Baldridge L.A., Condon K.W., Allen M.R., Alam I., Mantila S.M., Gluhak-Heinrich J., Bellido T.M., Harris S.E., Turner C.H. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. Journal of Biologcial Chemistry 283, 5866-5875. [DOI] [PubMed] [Google Scholar]
- Rubin C.T., Lanyon L.E. (1985) Regulation of bone mass by mechanical strain magnitude. Calcified. Tissue International. 37, 411-417. [DOI] [PubMed] [Google Scholar]
- Schulte F.A., Zwahlen A., Lambers F.M., Kuhn G., Ruffoni D., Betts D., Webster D.J., Muller R. (2013) Strain-adaptive in silico modeling of bone adaptation--a computer simulation validated by in vivo micro-computed tomography data. Bone 52, 485-492. [DOI] [PubMed] [Google Scholar]
- Sengupta P. (2013) The Laboratory Rat: Relating Its Age With Human’s. International Journal of Preventive Medicine 4, 624-630. [PMC free article] [PubMed] [Google Scholar]
- Takata S., Yonezu H., Shibata A., Enishi T., Sato N., Takahashi M., Nakao S., Komatsu K., Yasui N. (2011) Mineral to matrix ratio determines biomaterial and biomechanical properties of rat femur--application of Fourier transform infrared spectroscopy. Journal of Medical Investigation 58, 197-202. [DOI] [PubMed] [Google Scholar]
- Van Tran P.T., Vignery A., Baron R. (1982) Cellular kinetics of the bone remodeling sequence in the rat. The Anatomical Record 202, 445-451. [DOI] [PubMed] [Google Scholar]
- Turner C.H. (2006) Bone strength: current concepts. Annals of the New York Academy Sciences 1068, 429-446. [DOI] [PubMed] [Google Scholar]
- Turner C.H., Burr D.B. (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14, 595-608. [DOI] [PubMed] [Google Scholar]
- Umemura Y., Ishiko T., Yamauchi T., Kurono M., Mashiko S. (1997) Five jumps per day increase bone mass and breaking force in rats. Journal of Bone and Mineral Research 12, 1480-1485. [DOI] [PubMed] [Google Scholar]
- Umemura Y., Nagasawa S., Honda A., Singh R. (2008) High-impact exercise frequency per week or day for osteogenic response in rats. Journal of Bone and Mineral Metabolism 26, 456-460. [DOI] [PubMed] [Google Scholar]
- Verborgt O., Gibson G.J., Schaffler M.B. (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. Journal of Bone and Mineral Research 15, 60-67. [DOI] [PubMed] [Google Scholar]
- Weinreb M., Rodan G.A., Thompson D.D. (1991) Depression of osteoblastic activity in immobilized limbs of suckling rats. Journal of Bone and Mineral Research 6, 725-731. [DOI] [PubMed] [Google Scholar]
- Welch J.M., Turner C.H., Devareddy L., Arjmandi B.H., Weaver C.M. (2008) High impact exercise is more beneficial than dietary calcium for building bone strength in the growing rat skeleton. Bone 42, 660-668. [DOI] [PubMed] [Google Scholar]
- Welch J.M., Wade J.A., Hillberry B.M., Weaver C.M. (2009) Force platform for rats measures fore and hind forces concurrently. Journal of Biomechanics 42, 2734-2738. [DOI] [PubMed] [Google Scholar]
- Welch J.M., Weaver C.M., Turner C.H. (2004) Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. Journal of Applied Physiology 97, 1859-65. [DOI] [PubMed] [Google Scholar]
- Xiong J., O’Brien C.A. (2012) Osteocyte RANKL: new insights into the control of bone remodeling. Journal of Bone and Mineral Research 27, 499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]


