Abstract
Following an initial ankle sprain it is not unlikely that chronic ankle instability (CAI) will develop. CAI is associated with impaired perceived functional and mechanical properties of the ligaments. Nutritional supplementation with collagen peptides has been shown to improve the functional and mechanical properties of the connective tissue. The purpose of this study was to investigate the effectiveness of specific collagen peptide supplementation (SCP) to improve ankle stability in athletes with CAI. 50 male and female athletes with CAI completed a randomized, double-blinded and placebo-controlled study with a daily oral administration of either 5 g SCP or 5 g placebo (Maltodextrin) over a period of six months. Both, the Cumberland Ankle Instability Tool (CAIT) and the German version of the Foot and Ankle Ability Measure (FAAM-G) were used to measure the subjective perceived function of the ankle. Additionally, the mechanical stability was determined by measuring the ankle stiffness by an ankle arthrometer. Finally, a three-month follow-up was performed. ANOVA analysis indicated that the subjective ankle stability was improved in both the CAIT (p < 0.001) and the FAAM-G (p < 0.001) following SCP supplementation compared with placebo. No significant changes between the groups were detected in the results of the ankle arthrometer. After six month the subjective report of the ankle stability function significantly improved and the three month follow-up revealed a significant decline in the number of ankle joint injuries (p < 0.05). These data support the concept that specific collagen peptide supplementation in athletes with chronic ankle instability results in significant improvements in subjective perceived ankle stability. The reduction in the re-injury rate of ankle sprains in the follow-up period suggests that these findings have clinical relevance.
Key points.
Collagen peptides significantly improved subjective perceived function of the ankle in activities of daily living and sports exercise.
Decreases in ankle sprains and the risk of ankle injuries were shown in a three-month follow-up period.
These first results need to be confirmed by further investigations.
Key words: Ankle sprain, collagen, nutrition, ligaments
Introduction
One of the most widely spread sport injuries among athletes is ankle sprains. It is assumed that 31-40% of athletes with an ankle sprain suffer from permanent impairments. The progression of recurrent ankle sprains and residual symptoms is designated as chronic ankle instability (CAI). Athletes with CAI complain about chronic symptoms of giving way, pain, weakness and general instability, which can affect the activities of daily living, functional activities and physical exercise (Hubbard, 2008; Hubbard and Hertel, 2006).
Therapeutic approaches for CAI should primarily address the prophylaxis and occurrence of injuries. In addition to medical treatment, physiotherapy and sports therapeutic interventions focusing on the improvement of neuromuscular, proprioceptive and strength deficits (Holmes and Delahunt, 2009), nutritional supplementation should also be considered. The affected target tissue of the ankle joint is comprised of approximately 70% collagen. It is responsible for the elasticity and firmness of tendons, ligaments and connective tissues and therefore constitutes one of the most predominant components of the extracellular matrix (Kjaer et al., 2009).
Specific collagen peptides are produced for nutritional administration by a special hydrolysis of collagen. On the basis of their organoleptic and hydrological characteristics, collagen peptides can easily be digested in drinks or dietary supplements (Mohammad et al., 2014; Shoulders and Raines, 2009). In general, the high biological availability of the peptides is based on its low molecular weight and its lack of cross links. Preclinical (Oesser and Seifert, 2003; Walrand et al., 2008) and human studies (Iwai et al., 2005; McAlindon et al., 2011) indicate that collagen peptides are almost completely resorbed after oral intake and that a considerable proportion accumulates in tissue structures (Oesser et al., 1999). Approximately 10% of CP is directly transferred in intact form with a size of 1-10 kDa from the gastrointestinal tract into the blood and may stimulate extracellular matrix expression and biosynthesis within the connective tissue (Ohara et al., 2007; Watanabe-Kamiyama et al., 2010).
Regarding the health of ligaments and tendons, it has been demonstrated that SCP improve the functional and mechanical properties of the connective tissue. An in vitro experiment revealed a significantly increased RNA expression and biosynthesis of collagen type I, collagen type III, proteoglycans and elastin in both ligament fibroblasts and the Achilles tendon, which were isolated by enzymatic digestion and seeded in monolayer cultures in a humidified incubator (Schunck and Oesser, 2013). Moreover, an in vivo study demonstrated that collagen hydrolysate intake increased circulating blood levels of glycine, proline, hydroxyproline and hydroxylysine (Shaw et al., 2017). In this investigation, the functional effects of collagen supplementation were examined by means of a mechanical approach. The study demonstrated that the tensile load of the investigated ligaments, the material properties in terms of stiffness, and the ultimate tensile strength increased following collagen peptide supplementation.
With respect to joint-related discomforts, a randomized, double-blinded and placebo-controlled investigation showed a reduction in activity-related joint pain in athletes with functional knee discomfort following the oral administration of 5 g SCP over 12 weeks (Zdzieblik et al., 2017). An additional study demonstrated that the supplementation of 10 g collagen peptides per day over a period of 6 months improved the firmness of tissue and its resistance to mechanical stress. This effect was particularly observable in persons with a high joint laxity at the beginning of the study (Weh and Petau, 2001). However, evidence regarding the impact of collagen peptides on CAI is limited. To the best of our knowledge, there are currently no studies evaluating the influence of SCP supplementation on subjects with CAI.
Considering the clinical relevance of these results, the effects of SCP could also have a therapeutic benefit on subjects with CAI. The purpose of the present study was to investigate the impact of SCP vs. placebo ingestion in subjects with chronic ankle instability by measuring the subjective function and mechanical stability of the ankle. The hypothesis being that SCP may improve ankle stability.
Methods
Participants
Sixty male and female athletes aged 26.9 (SD 9.1); weight 69.4 (SD 11.5) kg; height 1.74 (SD 0.09) m; with a CAIT score of 18.14 (SD 5.4) were recruited via existing databases and an article in a local newspaper (Figure 1). In this study competitive athletes were included (e.g. soccer, track and field athletes, basketball). All athletes were on an amateur competition level with 2-3 training sessions per week, but were not in an active competitive season. The subjects engaged inside and outside training sessions, respectively. In conformity with the International Ankle Consortium (IAC), only participants with CAI were included in the investigation. In this context, a medical examination was conducted by the principal investigator to verify, whether the participants have a history of at least 1 significant ankle sprain (Gribble et al., 2014). Second, we used the Cumberland Ankle Instability Tool (CAIT), a specifically, self-reported ankle instability specific evidence-based questionnaire. The primary inclusion criterion was a CAIT score of ≤ 24, which is classified as noticeable or pathological (Donahue et al., 2011). In addition, subjects were only eligible if they were free of acute and chronic degenerative diseases and had neither administered collagen peptides nor any other nutritional supplements in the previous 6 months. All the participants underwent a comprehensive medical examination. Written informed consent was given by all subjects. The experiments were approved by the ethics committee of the University of Freiburg (394/15) and conducted in accordance with the Declaration of Helsinki.
Figure 1.
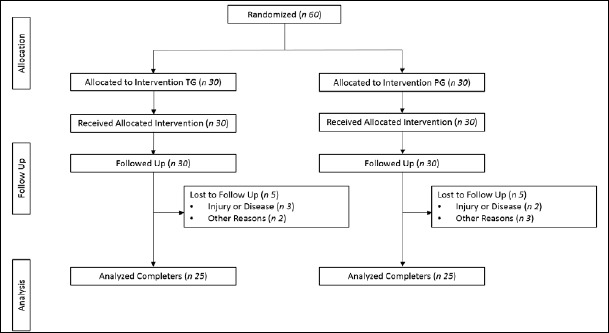
Flowchart of subject recruitment and dropouts before and during the study. TG, treatment group; PG, placebo group.
Design
The study was a randomized, double-blind and placebo-controlled design in which the participants supplemented either 5 g SCP (Gelita AG, Germany) or 5 g Maltodextrin (MDX). Both water-soluble preparations were comparable in terms of taste and appearance for daily oral ingestion over a period of 6 months. It is assumed that the acute and chronic loading of connective tissue is necessary to ensure the increased synthesis and turnover of extracellular matrix proteins, especially for collagen formation and degradation (Kjaer et al., 2009). Therefore, a mechanical loading protocol was applied in both groups that consisted of 3 home-based exercise sessions per week, which were performed within 10 minutes. They were scheduled with a day of rest between each session (e.g. Monday, Wednesday and Friday) and performed in the following order: rope skipping, squats, and one-legged heel raises. Rope skipping took 5 minutes. The squats and one-legged heel raises were performed in 1 set with 15 repetitions. The intensity was not increased throughout the entire intervention period. The participants consumed the supplements within 1 hour after completing each mechanical loading session.
On days without mechanical loading protocol, subjects were instructed to intake the supplement at the same day time. Both, the mechanical loading sessions and the supplementation of SCP or placebo were documented using a compliance calendar. Participants were excluded from the study if they failed to fulfil > 20% of these conditions.
Furthermore, dietary intake and physical activity were evaluated before and after the investigation. On the basis of a 3-day nutritional protocol, dietary intake was analyzed using NutriGuide® (Nutri-Science, Version 4.6). Daily nutritional and physical habits should not be changed over the entire study period. Physical activity was quantified by the Freiburg Questionnaire of physical activity. Subjects were asked to fill out the 12 standardized questions, related to basic, leisure, and sports-type activities.
In conformity with the International Ankle Consortium chronical ankle instability was measured by the by the Cumberland Ankle Instability Tool (CAIT). This self-reported questionnaire consists of 9 items with a maximum 30-point scale for measuring the severity of subjective perceived function of ankle (Hiller et al., 2006). Furthermore, CAI was examined with the German version of the Foot and Ankle Ability Measure (FAAM-G) to distinguish activities of daily living (FAAM-G ADL) and sports exercise (FAAM-G sports). Subjects were assigned a score in the range of 0-100%, where the FAAM-G score of 100% represents a pain free and unlimited level of physiological function (Lohrer et al., 2015).
Moreover we tested mechanical stability of the ankle. Ankle stiffness was examined by an ankle arthrometer (SGAM 2013; Elmako Medizintechnik), which determined anterior talar drawer. Basically, the non-radiographic device pulls the heel anteriorly in relation to the fixed lower leg and reflects the fibro elastic properties of the ankle joint complex. Ankle stiffness values from the load displacement curves were quantified between 40 and 60 N (Nauck et al., 2010).
A three-month follow-up phase was conducted after the investigation. This allowed a thorough evaluation using a shortened questionnaire based on the CAIT and FAAM-G. The questions focused on the participants’ experience over the past three months with regard to: (I) ankle sprains are reduced, (II) during both activities of daily living (ADL) and sports, the ankle is more frequently stable, (III) ankle injuries are reduced. These issues and the criteria selected were formulated as questions to be answered with “Yes” or “No”. Only after the subjects had filled out the questionnaire the blinding of the investigation was repealed.
Statistical analysis
The data were analyzed using SPSS (IBM, Version 24®). All the parameters are presented as mean values, standard deviation, and standard error in bar charts.
In accordance with the Kolmogorov-Smirnov test, the parameters did not differ significantly from normal distribution at base line. Furthermore, the Levene test for homogeneity of variances indicated no significant differences between the corresponding parameters. Baseline differences were examined by the unpaired samples t test. Testing alterations between examinations at baseline and following the 6-month intervention within groups were quantified by the Wilcoxon test for paired samples.
A two-way repeated measures analysis of variance (ANOVA) was conducted to identify significant effects attributable to treatment, time, or both. Treatment × time interaction was considered statistical significant with p-value of < 0.05. If a significant treatment × time interaction for an ANOVA was observed, a Bonferroni correction as a post hoc test was conducted.
In addition, data were tested for correlations between the subject’s perceived function of ankle stability and mechanical ankle stability. For this purpose, both the Pearson’s correlation coefficients and the p-values between the CAIT and stiffness were analyzed. An r-value of 0.50 was regarded as a strong effect, and a p-value of < 0.05 was statistically significant.
Finally, the strength of the connection between the treatment group (TG) and placebo group (PG) in the follow-up was assessed using the chi-square test based on Pearson. In total 30 subjects were included for each group, estimating a dropout rate of 20%.
Results
A total of 50 subjects completed the investigation and were included in the analysis. Neither the TG (age 27.8 (SD 10.3); weight 69.4 (SD 11.3) kg; height 1.72 (SD 0.09) m) nor the PG (age 25.9 (SD 7.9); weight 69.4 (SD 11.9) kg; 174.7 (SD 9.3) differed significantly in age, height or weight. All 10 dropouts failed to comply with the study design. The excluded participants predominantly took less than 80% of their supplements or could not perform their mechanical loading protocol sufficiently due to illness, injury or other reasons (Figure 1). No dropouts occurred through side effects of the supplemented SCP or placebo. Moreover, no pathological findings were observed in the routine blood test.
The subjective perceived function of ankle was significantly improved with SCP vs. placebo (Table 1), as a clinical relevant benefit in the CAIT (treatment × time interaction: p < 0.001) was identified after 6 months’ intervention. CAIT clearly increased by 5.28 (SEM 1.16) score (p < 0.001) in the TG. In contrast, no pronounced change 0.65 (SEM 0.88) score (p > 0.05) was analyzed in the PG (Figure 2). The observed group difference after 6 months was statistically significant (p < 0.01). Nevertheless, the significant differences at baseline between treatment group and placebo group (Table 1) have to be considered for CAIT (p = 0.001).
Table 1.
Measures of chronical ankle instability in the subjects before and after supplementation with SCP or placebo.
| Parameter | Group | t1 Mean | t1 SD | t2 Mean | t2 SD | ANOVA |
|---|---|---|---|---|---|---|
| CAIT (Score) | TG | 15.48† | 4.86 | 20.76*** | 5.42 | < 0.05 |
| PG | 20.80 | 4.77 | 21.45 | 4.18 | ||
| FAAM-G ADL (%) | TG | 81.56† | 12.79 | 91.32*** | 9.91 | < 0.05 |
| PG | 90.28 | 9.29 | 91.44 | 8.13 | ||
| FAAM-G Sport (%) | TG | 60.48† | 18.43 | 80.36*** | 16.90 | < 0.05 |
| PG | 76.24 | 18.15 | 76.52 | 18.10 | ||
| Stiffness (N/mm) | TG | 9.12 | 4.65 | 9.19 | 4.45 | NS |
| PG | 9.62 | 2.97 | 8.85 | 2.89 |
NS, not significant.
* p < 0.05 within the group from baseline to final examination
** p < 0.005 within the group from baseline to final examination
*** p <0.001 within the group from baseline to final examination.
† significant difference at baseline between treatment group and placebo group (p < 0.05).
Figure 2.
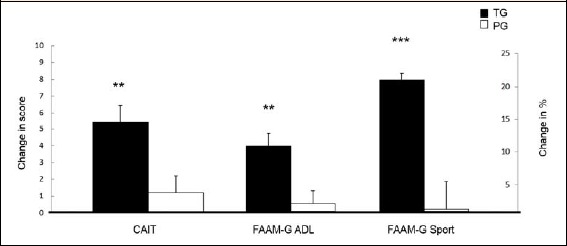
Change in CAIT, FAAM-G ADL and FAAM-G Sports after 6 months intervention in subjects with CAI following collagen peptide supplementation (TG, n 25) or placebo (PG, n 25). Values are means, with their standard errors represented by vertical bars. Significance was tested by an ANOVA with a time × treatment interaction. ** Mean value was significantly different from that of the placebo group (P < 0.01). *** Mean value was highly significantly different from that of the placebo group (P < 0.001).
Additionally, FAAM-G ADL (treatment × time interaction: p < 0.001) and FAAM-G Sport (treatment × time interaction: p < 0.001) significantly improved in the TG compared to PG (Table 1). The higher CAIT after CP supplementation corresponds to a mean change of 9.76 (SEM 1.90) % (p < 0.001) in FAAM-G ADL and 19.88 (SEM 2.89) % (p < 0.001) in FAAM-G Sports of the TG, respectively. In the PG neither FAAM-G ADL nor FAAM-G Sports improved within the intervention period (p > 0.05). Both the FAAM-G ADL (p < 0.01) and the FAAM-G Sports (p < 0.001) indicated a statistically significant group difference and demonstrates the positive effects of a specific collagen peptide intake. Again, the significant differences at baseline between both groups (Table 1) should be taken into account for FAAM-G ADL (p = 0.007) and FAAM-G Sport (p = 0.011).
Regarding to mechanical stability of the ankle, no statistically significant increase in ankle stiffness (treatment × time interaction: p > 0.05) was detected in the TG vs. PG at the end of the investigation. While the 6-month administration of SCP was accompanied by an increase of stiffness to 0.08 (SEM 0.96) N/mm, a decrease of -0.77 (SEM 0.59) N/mm was revealed in the PG (Table 1).
The increase of stiffness in the TG correlates with the increased CAIT score (r = 0.42; p < 0.05), whereas the reduced stiffness in the PG demonstrates no correlation with the CAIT score (r = 0.30; p > 0.05).
The group comparison (p < 0.001) in the three-month follow-up showed that ankle sprains were significantly more reduced in subjects that had received the specific collagen peptides. Furthermore, ankle stability improved more in the treatment group during both activities of daily living (ADL) and sports compared with the placebo (p < 0.001). Table 2 shows that ankle injuries decreased significantly more in subjects after SCP supplementation (p < 0.05) than in the control group.
Table 2.
Difference in three-month follow-up in subjects with CAI following collagen peptide supplementation (TG, n 25) or placebo (PG, n 25). Values are means.
| Group | Yes (n) | No (n) | Chi-square test | |
|---|---|---|---|---|
|
Reduced ankle sprains |
TG | 24 | 1 | < 0.001 |
| PG | 9 | 16 | ||
|
Improved ankle stability |
TG | 21 | 4 | < 0.001 |
| PG | 5 | 20 | ||
|
Reduced ankle injuries |
TG | 23 | 2 | < 0.05 |
| PG | 15 | 10 |
Significance was analyzed by Chi-square test based on person. p < 0.05 Difference in the three-month follow-up between TG and PG. p < 0.001 Difference in the three-month follow-up between TG and PG.
The analysis of dietary behavior was performed for macronutrient intake. Baseline data showed no significant difference between the groups for carbohydrates, fats and proteins (p > 0.05). No significant differences for the dietary intake were reported of the calculated variables during pre and post-tests in both groups (p > 0.05). During the six-month intervention physical exercise status was evaluated for energy consumption and demonstrated no significant differences for baseline data (p > 0.05) and pre and post-tests in both groups (p > 0.05).
Discussion
The main finding of the present study is that the daily dosage of 5 g of specific collagen peptides positively influenced ankle stability in subjects with chronic ankle instability over a study period of 6 months. SCP clearly improved subjective perceived function after the intervention compared to the control group. Both the Cumberland Ankle Instability Tool and the German version of the Foot and Ankle Ability Measure increased equally in the treatment group, while the placebo group was virtually unchanged across both measurements. More specifically, SCP treated athletes suffered less from the feeling of the joint ‘giving way’, pain and swelling during sports activities. In regard to subjective assessment the ingestion of 5 g SCP supplementation improved the function in the ankle joint complex during activities of daily living and sports activities in contrast to the placebo group. SCP ingestion also resulted in a decreased relapsing-remitting ankle trauma, which demonstrated a beneficial role in injury prevention and tissue repair.
The findings of this study are in accordance with previous investigations of collagen peptide supplementation. Zdzieblik et al. (2017) reported a significant decline in functional knee discomfort following the ingestion of 5 g of bioactive collagen peptides per day over a period of 12 weeks. Athletes in particular suffered less from pain during activity after the intervention compared to placebo. An assessment of the outcomes led to the presumption that SCP improved the connective tissue function and structure in collagen within the knee. The results of a current investigation underline this theory by observing a clear increase in collagen type II synthesis with a simultaneous minimized proteoglycan degradation compared to the placebo group (McAlindon et al., 2017). Furthermore, an in-vitro study by Schunck and Oesser (2013) on isolated primary fibroblasts derived from human ligaments and tendons shows that the supplementation of SCP leads to a stimulated matrix molecule biosynthesis in the treated tendon and ligament cells.
However, the mechanism leading to the improved collagen synthesis in the extracellular matrix after SCP administration still requires further investigation. In the study of Schunck and Oesser (2013), SCP stimulated the biosynthesis of collagen type I, collagen type III, proteoglycans and elastin on the bases of a higher availability of SCP. This data suggests that the increased expression of extracellular matrix molecules in tendon and ligament cells is accompanied by an enhanced firmness of the tissue. These findings were associated with a decreased laxity. It is currently unclear whether the enhanced firmness of the tissue results from an increased collagen density or thickness. It could be speculated that the administration of SCP leads to a reduced enzymatic or activity-induced stimulation of inflammatory messengers, or both. In addition to the direct stimulatory effect of collagen peptide intake, the higher bioavailability of collagen peptides could result in the development of an amino acid pool within the body, which supports the reformation of connective tissue through fibrocytes (Weh and Petau, 2001). The recent clinical trial by Shaw et al. (2017) confirmed that collagen peptide administration to an intermittent mechanical loading protocol elevates the biosynthesis of collagen and might be crucial in injury prevention and tissue repair. In this in vivo study, subjects had an increased concentration in circulating glycine, proline, hydroxyproline and hydroxylysine after one hour of collagen supplementation in contrast to placebo. Furthermore, functional effects of the engineered ligaments were mechanically tested to failure. Thereby, the tensile load of the ligaments, material properties of the stiffness and ultimate tensile strength definitely increased for all treatment groups as a result of 6 days collagen peptides administration and an intermittent mechanical loading protocol. Besides collagen peptides supplementation, both the acute and chronic loading of connective tissue seem to be necessary to ensure an increased synthesis and turnover of extracellular matrix proteins, especially for collagen formation and degradation (Kjaer et al., 2009). Although subjective perceived function of ankle improved in the current investigation, we did not examine a similar response in mechanical ankle stability by measuring the ankle stiffness following SCP intake. Ankle stiffness was analyzed through an anterior talar drawer. This ankle arthrometer has previously been used to quantify the diagnostic validity of different subgroups in subjects or patients with CAI (Lohrer et al., 2015). Specifically, at low levels of force application deformation or stiffness measures are known to best distinguish between the tibiotalar translation and the rigidity of the ankle and its encompassing soft tissues with the talus already anteriorly translated to its end position (Kovaleski et al., 2014; Nauck et al., 2010; Tohyama et al., 2003).
While a slight improvement in ankle stiffness was detected in the TG in the current study, a decrease in the PG was observed. However, the slight rise in ankle stiffness correlated with a significantly higher CAIT score after 5 g supplementation of SCP per day over a period of 6 months, which indicates a clinical relevance.
This data demonstrated that the mechanical improvement in the ankle-joint complex was supported by collagen peptide intervention, which is also reflected in the three-month follow-up. After 3 months, subjects with SCP administration reported on tangibly beneficial effects with respect to a less common feeling of the joint ‘giving way’, a more stable ankle during activities of daily living and sports, as well as a decrease in ankle injuries, compared to the placebo group. On the basis of the compliance and feasibility of the investigation, no long term follow-up was conducted to analyze the absence of ankle sprain and giving away. Further studies should evaluate a six-month follow-up, to be consistent with the recommendation of the IAC. Thereby, participants should report at least 2 episodes of giving way in the 6 months prior to study enrollment. In this context, the present trial has several limitations. Baseline data from CAIT and both FAAM-G clearly differ between TG and PG. No counterbalancing measures were used when randomizing participants into their respective groups, because in general 24 men and 26 women completed the investigation with homogeneous distribution in both groups. It could be speculated that the lower CAIT score in the TG at the beginning of the study led to an enhanced improvement compared to PG. Further studies may consider minimizing this difference in the baseline across groups by using lower inclusion criteria with a CAIT ≤ 16. Furthermore, according to the IAC a scoring < 24 on the CAIT presents a standard inclusion criterion for CAI. Although the treatment group improved in terms of subjective function, in mean (20.76) athletes were still classified as CAI based on the CAIT. As the level of disability of the cohort was not within the focus on the research question, the FAAM-G was no inclusion criterion. However, for both of the FAAM scores, improvements were analyzed and pursuant to IAC in which athletes were above the inclusion criteria (ADL scale > 90%; Sport scale > 80%) for CAI (Gribble et al., 2014). These subjective perceived improvements in the TG supported the clinical relevance and may constitute a new desideratum of research.
Additionally, no pronounced group difference was identified in mechanical ankle stability by following a daily oral dosage of 5 g SCP in the arthrometer examination. It remains to be determined if a higher dosage would have induced significant effects.
Conclusions
In conclusion, to the best of our knowledge, this is the first study showing that the administration of SCP significantly improved subjective perceived function of ankle in subjects with chronic ankle instability. Both the Cumberland Ankle Instability Tool and the German version of the Foot and Ankle Ability Measure statistically significantly improved following the ingestion of specific collagen peptides compared with placebo. These results were supported by the data from the three-month follow-up period in which subjects reported on improved ankle stability, reduced ankle sprains and the risk of ankle injuries. No significant changes between the groups were detected in mechanical stability by measuring the ankle stiffness. Finally, the present data indicate that SCP supplementation could play a beneficial role in improving the function of the ankle in subjects with chronic ankle instability in a clinical context.
Acknowledgements
All experiments comply with the current laws of Germany. Parts of the costs were paid by Gelita AG, Uferstraße 7, Eberbach, Germany. We thank ELMAKO GmbH & Co. KG, Industriestraße 8, Iffezheim, Germany, for providing the SGAM 2013 ankle arthrometer. There are no conflicts of interest.
Biographies

Patrick DRESSLER
Employment
Department of Sport Science, University of Freiburg, Germany
Degree
MA
Research interests
Influence of collagen peptides supplementation in combination with physical activity on muscles, tendons and ligaments
E-mail:
patrick.dressler@sport.uni-freiburg.de

Dominic GEHRING
Employment
Lecturer of the Department of Sport Science, University of Freiburg, Germany
Degree
PhD
Research interests
Clinical biomechanics with a specific focus on ACL injuries, ankle sprains, chronic ankle instability
E-mail:
dominic.gehring@sport.uni-freiburg.de

Denise ZDZIEBLIK
Employment
Department of Sport Science, University of Freiburg, Germany
Degree
MSc
Research interests
Effects of macro- and micronutrients on the functionality of the musculoskeletal system
E-mail:
denise.zdzieblik@sport.uni-freiburg.de

Steffen OESSER
Employment
Director of the Collagen Research Institute (CRI) in Kiel, Germany
Degree
PhD
Research interests
Prevention and treatment of degenerative diseases. Physical activities and nutritional aspects in sports medicine
E-mail: steffen.oesser@CRI-mail.org

Albert GOLLHOFER
Employment
Director of the Department of Sport Science, University of Freiburg, Germany
Degree
Prof. Dr.
Research interests
Neuromechanics and functional adaptation to training
E-mail:
albert.gollhofer@sport.uni-freiburg.de

Daniel KÖNIG
Employment
Head of the Division of Nutrition at the Department of Sport Science, University of Freiburg, Germany
Degree
Prof. Dr. med
Research interests
Nutritional, preventive and sports medicine, lifestyle intervention, Nutrients and chronic disease
E-mail:
daniel.koenig@sport.uni-freiburg.de
References
- Donahue M., Simon J., Docherty C.L. (2011) Critical review of self-reported functional ankle instability measures. Foot & Ankle International 32(12), 1140-1146. [DOI] [PubMed] [Google Scholar]
- Gribble P.A., Delahunt E., Bleakley C.M., Caulfield B., Docherty C.L., Fong D.T., Fourchet F., Hertel J., Hiller C.E., Kaminski T.W., McKeon P.O., Refshauge K.M., van der Wees P., Vicenzino W., Wikstrom E.A. (2014) Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Journal of Athletic Training 49(1), 121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller C.E., Refshauge K.M., Bundy A.C., Herbert R.D., Kilbreath S.L. (2006) The Cumberland ankle instability tool: a report of validity and reliability testing. Archives of Physical Medicine and Rehabilitation 87(9), 1235-1241. [DOI] [PubMed] [Google Scholar]
- Holmes A., Delahunt E. (2009) Treatment of common deficits associated with chronic ankle instability. Sports Medicine 39(3), 207-224. [DOI] [PubMed] [Google Scholar]
- Hubbard T.J. (2008) Ligament laxity following inversion injury with and without chronic ankle instability. Foot & Ankle International 29(3), 305-311. [DOI] [PubMed] [Google Scholar]
- Hubbard T.J., Hertel J. (2006) Mechanical contributions to chronic lateral ankle instability. Sports Medicine 36(3), 263-277. [DOI] [PubMed] [Google Scholar]
- Iwai K., Hasegawa T., Taguchi Y., Morimatsu F., Sato K., Nakamura Y., Higashi A., Kido Y., Nakabo Y., Ohtsuki K. (2005) Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. Journal of Agricultural and Food Chemistry 53(16), 6531-6536. [DOI] [PubMed] [Google Scholar]
- Kjaer M., Langberg H., Heinemeier K., Bayer M.L., Hansen M., Holm L., Doessing S., Kongsgaard M., Krogsgaard M.R., Magnusson S.P. (2009) From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine Science in Sports 19(4), 500-510. [DOI] [PubMed] [Google Scholar]
- Kovaleski J.E., Heitman R.J., Gurchiek L.R., Hollis J.M., Liu W., Pearsall A.W.t. (2014) Joint stability characteristics of the ankle complex after lateral ligamentous injury, part I: a laboratory comparison using arthrometric measurement. Journal of Athletic Training 49(2), 192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrer H., Nauck T., Gehring D., Wissler S., Braag B., Gollhofer A. (2015) Differences between mechanically stable and unstable chronic ankle instability subgroups when examined by arthrometer and FAAM-G. Journal of Orthopaedic Surgery and Research 10(1), 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon T., Bartnik E., Ried J.S., Teichert L., Herrmann M. (2017) Determination of serum biomarkers in osteoarthritis patients: a previous interventional imaging study revisited. The Journal of Biomedical Research 31(1), 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon T.E., Nuite M., Krishnan N., Ruthazer R., Price L.L., Burstein D., Griffith J., Flechsenhar K. (2011) Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis. Cartilage 19(4), 399-405. [DOI] [PubMed] [Google Scholar]
- Mohammad A.W., Suhimi N.M., Aziz A.G.K.A., Jahim J.M. (2014) Process for Production of Hydrolysed Collagen from Agriculture Resources: Potential for Further Development. Journal of Applied Sciences 14(12), 1319-1323. [Google Scholar]
- Nauck T., Lohrer H., Gollhofer A. (2010) Clinical evaluation of a new noninvasive ankle arthrometer. The Physician and Sportsmedicine 38(2), 55-61. [DOI] [PubMed] [Google Scholar]
- Oesser S., Adam M., Babel W., Seifert J. (1999) Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). The Journal of Nutrition 129(10), 1891-1895. [DOI] [PubMed] [Google Scholar]
- Oesser S., Seifert J. (2003) Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Research 311(3), 393-399. [DOI] [PubMed] [Google Scholar]
- Ohara H., Matsumoto H., Ito K., Iwai K., Sato K. (2007) Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. Journal of Agricultural and Food Chemistry 55(4), 1532-1535. [DOI] [PubMed] [Google Scholar]
- Schunck M., Oesser S. (2013) Specific collagen peptides benefit the biosynthesis of matrix molecules of tendons and ligaments. Journal of the International Society of Sports Nutrition 10(1), 23.23617897 [Google Scholar]
- Shaw G., Lee-Barthel A., Ross M.L., Wang B., Baar K. (2017) Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. American Journal of Clinical Nutrition 105(1), 136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders M.D., Raines R.T. (2009) Collagen structure and stability. Annuel Review of Biochemistry 78(1), 929-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama H., Yasuda K., Ohkoshi Y., Beynnon B.D., Renstrom P.A. (2003) Anterior drawer test for acute anterior talofibular ligament injuries of the ankle. How much load should be applied during the test? The American Journal of Sports Medicine 31(2), 226-232. [DOI] [PubMed] [Google Scholar]
- Walrand S., Chiotelli E., Noirt F., Mwewa S., Lassel T. (2008) Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. Journal of Agricultural and Food Chemistry 56(17), 7790-7795. [DOI] [PubMed] [Google Scholar]
- Watanabe-Kamiyama M., Shimizu M., Kamiyama S., Taguchi Y., Sone H., Morimatsu F., Shirakawa H., Furukawa Y., Komai M. (2010) Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. Journal of Agriculture in Food and Chemistry 58(2), 835-841. [DOI] [PubMed] [Google Scholar]
- Weh L., Petau C. (2001) Change in the Properties of Tissue Through the Administration of Gelatine: a Biomechanical In-Vivo Pilot Study. Extracta Orthopaedica 24(4), 12-16. [Google Scholar]
- Zdzieblik D., Oesser S., Gollhofer A., König D. (2017) Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Applied Physiology in Nutrition and Metabolism 42(6), 588-595. [DOI] [PubMed] [Google Scholar]


