Abstract
Boron neutron capture therapy (BNCT) can potentially deliver high linear energy transfer particles to tumor cells without causing severe damage to surrounding normal tissue, and may thus be beneficial for cases with characteristics of infiltrative growth, which need a wider irradiation field, such as glioblastoma multiforme. Hypoxia is an important factor contributing to resistance to anticancer therapies such as radiotherapy and chemotherapy. In this study, we investigated the impact of oxygen status on 10B uptake in glioblastoma cells in vitro in order to evaluate the potential impact of local hypoxia on BNCT. T98G and A172 glioblastoma cells were used in the present study, and we examined the influence of oxygen concentration on cell viability, mRNA expression of L-amino acid transporter 1 (LAT1), and the uptake amount of 10B-BPA. T98G and A172 glioblastoma cells became quiescent after 72 h under 1% hypoxia but remained viable. Uptake of 10B-BPA, which is one of the agents for BNCT in clinical use, decreased linearly as oxygen levels were reduced from 20% through to 10%, 3% and 1%. Hypoxia with <10% O2 significantly decreased mRNA expression of LAT1 in both cell lines, indicating that reduced uptake of 10B-BPA in glioblastoma in hypoxic conditions may be due to reduced expression of this important transporter protein. Hypoxia inhibits 10B-BPA uptake in glioblastoma cells in a linear fashion, meaning that approaches to overcoming local tumor hypoxia may be an effective method of improving the success of BNCT treatment.
Keywords: boron neutron capture therapy, boronophenylalanine, hypoxia, glioblastoma
INTRODUCTION
Boron neutron capture therapy (BNCT) can potentially deliver high linear energy transfer (LET) particles to tumor cells without causing severe damage to surrounding normal tissue, and may thus be beneficial for inoperable cancer cases and patients who have no other treatment options. The basic idea of BNCT was first outlined by Locher in 1936 [1]. In BNCT treatment, an intravenously injected boron (10B) carrier molecule accumulates preferentially in cancer cells compared with in normal cells. The patient is then irradiated with epithermal or thermal neutrons, which have sufficiently low energy not to harm normal tissues but which stimulate the 10B carrier molecules to release a substantial amount of localized energy in the form of alpha rays and lithium ions. Alpha rays have very high relative biological effect (RBE) and LET compared with X-rays, and a very short range of 9 μm which is generally equal to or less than the diameter of cells. These characteristics theoretically enable the highly selective killing of cancer cells that are tagged with 10B, without damage to non-tagged normal cells. However, this revolutionary method depends on the high accumulation in and selective delivery of 10B into the tumor tissue.
To date, only two types of boron carrier are available for use in this context—sodium mercaptoundecahydrododecaborate-10B (BSH) and boronophenylalanine (BPA). BSH is a water-soluble drug that is taken into cancer tissues passively according to the local concentration gradient. On the other hand, BPA which is an amino-acid derivative, is actively taken up by cancer cells via the L-type amino acid transporter 1 (LAT1), with a small amount also being transported by LAT2. Cancer cells are known to overexpress LAT1 compared with normal cells, but LAT2 is expressed by both cancer and non-cancer cells. However, since the amount of 10B-BPA taken up via LAT2 is less than that via LAT1, there is a preferential accumulation of BPA in tumor tissues [2]. Several studies have indicated the possibility of a new drug delivery system using liposomes to enhance the density of 10B in tumor cells [3–8]; however, adverse events associated with this approach have so far proved restrictive.
In the treatment of glioblastoma, surgery followed by radiation therapy and chemotherapy with agents such as temozolomide is standard; however, the prognosis for this disease remains extremely poor. One reason for this is that the typically invasive growth pattern makes complete surgical resection difficult. Because of the infiltrative growth pattern of glioblastoma, the irradiation field of radiation therapy with X-rays needs to be expanded. The large irradiation field also include normal tissues near the tumor bed and leads to complications; however, super-selective irradiation with BNCT enables us to damage malignant cells without damaging normal cells, even if the irradiation field is large. More recently, therefore, the targeted approach offered by BNCT has received significant attention; however, the results of BNCT studies are not yet convincing [9–16]. The rapid growth of glioblastoma and abnormal tumor vascularization produces a hypovascular and hypoxic tumor microenvironment, and this increases the fraction of cells resistant to chemotherapy and radiation therapy [17]. Actually, several studies have reported that there is a rich hypoxic area in glioblastoma tumor observed using positron emitting tomography (PET) with hypoxia tracer; 18F-Fluoromisonidazole (FMISO) [18–20] and 1-(2-[18F] fluoro-1-[hydryxymethyl]ehoxy)methyl-2-nitromidazole (FRP-170)] [21, 22], and it is known that increasing the size of the hypoxic area promotes the poor prognosis for conventional therapy [18]: operative resection followed chemoradiotherapy. Successful treatment with BNCT depends on the selective accumulation of 10B in cancer cells, but it is not yet clear how a hypoxic environment influences this uptake. We investigated the impact of oxygen pressure until the last minute prior to exposure to 10B-BPA on 10B uptake in glioblastoma.
MATERIALS AND METHODS
Cell lines
The human glioblastoma multiforme tumor cell lines T98G and A172 were provided by the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). Cells were cultured in serum-free Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/F12 1:1; Gibco Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, Saint Louis, MO) and 1% penicillin/streptomycin (Gibco Life Technologies), and maintained at 37°C in a 5% CO2 atmosphere.
Hypoxic conditions
Hypoxic conditions were achieved by culturing cells in modular incubator chambers (Billups-Rothenberg Inc., Del Mar, CA, USA). The chambers, in which cell culture dishes and distilled water were housed, were flushed with mixed gas (95% nitrogen/5% carbon dioxide) to achieve the target oxygen pressure monitored using a JKO-02 Ver. III monitor (JIKCO, Tokyo, Japan), then sealed and incubated at 37°C.
Trypan blue dye viability assay
Cells were incubated in 60-mm dishes with 4 ml of culture medium, at 1 × 106 cells/dish under either normoxic conditions, or hypoxic conditions (1% O2) for 0, 24, 48 and 72 h. For the trypan blue dye exclusion test, cells were stained using phosphate-buffered saline (PBS, Sigma-Aldrich, Saint Louis, MO) containing 0.5% trypan blue (Nacalai Tesque, Inc., Kyoto, Japan). Cell viability was assessed by counting the number of both stained and unstained cells using cell-count slides.
Cell cycle analysis
The cell cycle phase distribution was analyzed with propidium iodide (PI) solution (PI/RNase, Cosmo Bio Co., Ltd, Tokyo, Japan) staining according to the manufacturer’s instructions. Briefly, cells were harvested following 0, 24, 48 or 72 h incubation in a 60-mm culture dish (Iwaki) under normoxia/hypoxia, washed with the binding buffer, then suspended in the buffer containing PI. Stained cells were analyzed with a Cytomics FC 500 flow cytometer (Beckman Coulter, Tokyo, Japan).
Boron accumulation study
10B-enriched L-BPA, D-sorbitol, standard boron solution, and yttrium ICP standard solution were kindly supplied by Stella Pharma Corporation (Osaka, Japan). L-BPA was used as a D-sorbitol complex as a stock solution containing 30 mg 10B/ml. The two human glioblastoma cell lines T98G and A172 were cultured in 10 cm culture dishes with either 9 × 106 cells (T98G) or 3 × 106 cells (A172) for 72 h under normoxic or hypoxic (10%, 3% or 1% O2) conditions. After trypsinization, cell suspensions were collected in 15 ml centrifuge tubes at 1 × 106 cells per tube, exposed to BPA at either 10, 20 or 30 μg 10B/ml in the medium for 2 h under normoxic condition, washed with PBS, centrifuged, and the supernatant discarded. Cell pellets were digested with 0.67 ml of perchloric acid (HClO4) and 1.33 ml of hydrogen peroxide (H2O2) for 6 h at 70°C, before being mixed with standard yttrium solution (1 ml) and diluted with distilled water to a total volume of 10 ml. The 10B concentration in each sample was analyzed with inductively coupled plasma atomic emission spectroscopy (ICP-AES) using an ICPE-9000 (Shimadzu, Kyoto, Japan) at a wavelength of 249.772 nm. The calibration curve obtained from dilutions of the standard boron solution was linear in the range of 0.0025–0.25 μg10B/ml.
Total RNA extraction
T98G and A172 were cultured in 10 cm culture dishes with either 9 × 106 (T98G) or 3 × 106 cells (A172) for 72 h under normoxic or hypoxic (10% or 1% oxygen) conditions at 37°C. After 72 h incubation, total RNA was extracted using the Agencourt RNAdvance Cell v2® system (Beckman Coulter, Danvers, MA) using Agencourt’s patented SPRI paramagnetic bead technology, according to the manufacturer’s instructions. Briefly, cultured cells were lysed with lysis buffer and proteinase K, and transferred into new 96-multi-well plates (Beckman Coulter). After total RNA was mixed with paramagnetic beads, the beads were washed with wash buffer and 70% ethanol, and separated from contaminants using Agencourt SPRIPlate 96R. Subsequently, DNase I solution (Thermo Fisher Scientific, Waltham, MA, USA) was added into each well to digest the genomic DNA. Total RNA was re-bound to the beads and contaminants removed by washing. Finally, total RNA was eluted from the magnetic particles with nuclease-free water (Thermo Fisher Scientific).
Quantitative real-time reverse transcription-polymerase chain reaction
First-strand cDNA was synthesized with an iScript RT Supermix for RT-qPCR® (Bio-Rad, Hercules, CA) from extracted total RNA according to the manufacturer’s instructions. Gene expression was assessed using real-time reverse transcription-polymerase chain reaction (qRT-PCR) (SsoAdvanced Universal SYBR Green Supermix®; Bio-Rad), with typical amplification parameters being 95°C for 30 s, followed by 40 cycles at 98°C for 10 s and 60°C for 30 s. Relative differences were determined by the crossing point method with a standard curve. The mRNA expression of each hypoxic condition after 72 h was compared with the expression under normoxia after normalization with the housekeeping gene GAPDH. The oligonucleotide primer sets used for real-time PCR purchased from TAKARA Bio Inc. (Otsu, Shiga, Japan) were as follows: GAPDH, forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3’; SLC7A5, forward 5′-GCATCGGCTTCACCATCATC-3′ and reverse 5′-ACCACCTGCATGAGCTTCTGAC-3′; SLC7A8, forward 5′-TGTATGTCTTTGCCAATGTCGCTTA-3′ and reverse 5′-ATGATCCAGGCCATGACTCCTA-3′.
Statistical analysis
Significance differences were determined using Student’s two-tailed t-test or Welch’s t-test, depending on the data distribution, with P < 0.05 considered to indicate statistical significance. Excel 2010 software (Microsoft Corporation, Redmond, WA, USA) with the add-in software Statcel 3 was used for statistical analysis.
RESULTS
Influence of hypoxic condition on cell growth and viability
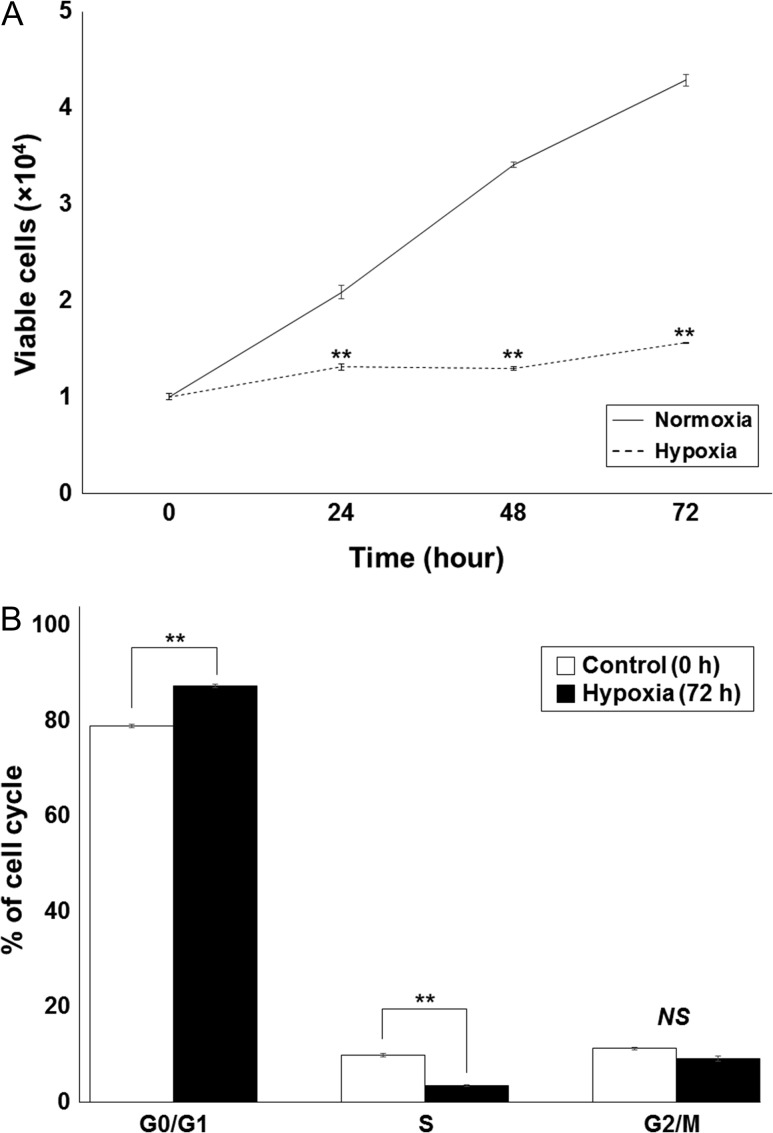
We first investigated the baseline cytotoxicity of hypoxic conditions on T98G and A172. Cells incubated for 0 h, 24 h, 48 h and 72 h under either normoxic or hypoxic (1% O2) conditions, were assessed by trypan blue viability assay and cell cycle analysis. Hypoxic conditions significantly inhibited T98G cell growth; however, viable cells remained (comparable with the number of cells seen at 0 h under normoxic conditions), even after 72 h in 1% O2 conditions (Fig. 1A). The same trend was found with the A172 cell line (data not shown). With longer incubation periods under hypoxia, more cells were found to have stopped in G0/G1 phase, and there were fewer in S phase. There was no correlation observed, however, between the number of cells in G2/M phase and the hypoxia incubation time (Fig. 1B).
Fig. 1.
The influence of 1% hypoxia on survival and cell growth in the T98G glioblastoma cell line. (A) A significant difference was observed between normoxia and hypoxia cell growth; however, viable cells could be identified even after incubation under hypoxia for 72 h. (B) Cells were incubated under hypoxia for 0 h or 72 h, and the cell cycle was analyzed by flow cytometry. Hypoxic conditions increased the percentage of T98G cells in G0/G1 phase and decreased the percentage of cells in S phase. No significant difference was found for G2/M phases. Values are expressed as the mean ± standard error; two asterisks indicate P < 0.01 compared with control cells at 0 h; NS = no significant difference.
10B-BPA uptake in human malignant glioblastoma cells under hypoxia
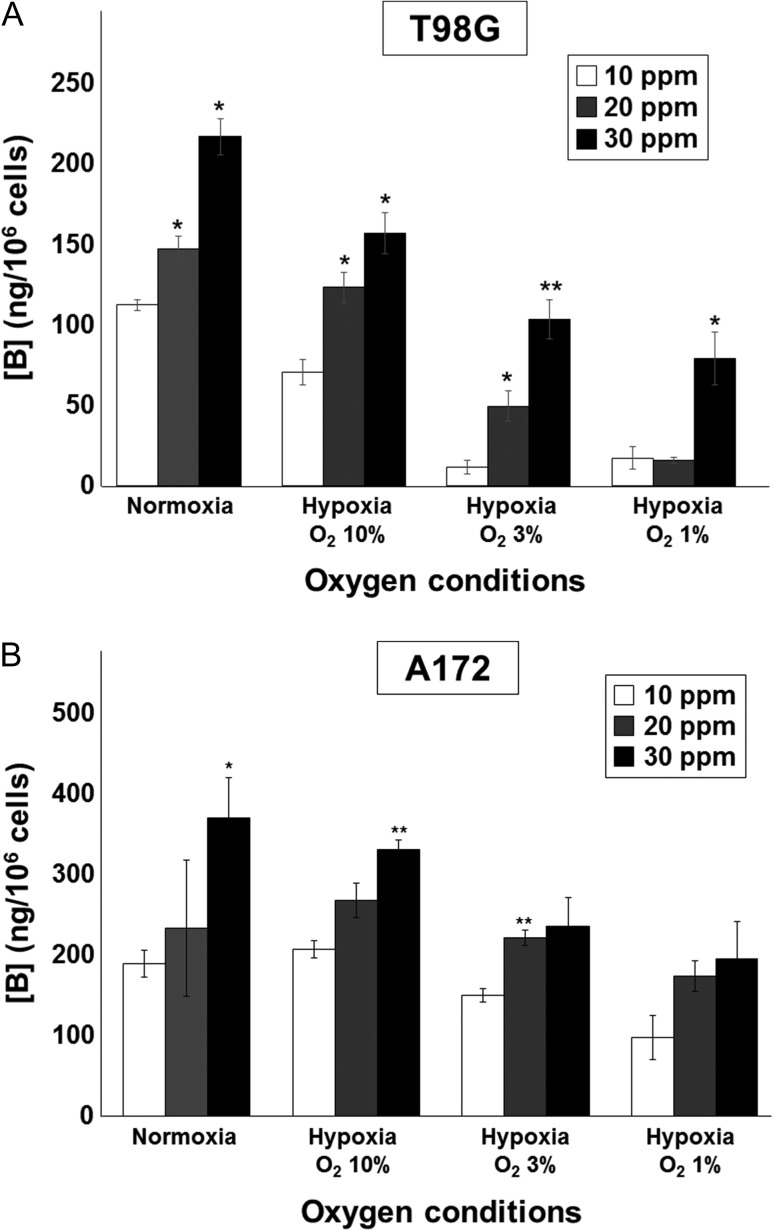
Next, we investigated the influence of oxygen levels on the 10B uptake of human glioblastoma cells. Previous work has indicated that 10B-BPA uptake in human head and neck squamous carcinoma cell lines is decreased under hypoxia with 0.28% O2, compared with normoxic conditions [23]; however, it is unknown whether this effect has a critical threshold or whether 10B-BPA uptake gradually decreases according to the oxygen concentration. In the present study, we incubated T98G and A172 under several oxygen conditions (20.8%, 10%, 3% and 1% O2) for 72 h, exposed them to 10B-BPA at concentrations of 10, 20 and 30 ppm for 2 h, then analyzed 10B-BPA uptake using ICP-AES. We had previously investigated that adding 10B-BPA into culture medium did not cause a cytotoxic effect by preliminary experiment (date not shown). As shown in Fig. 2, the cellular accumulation of 10B increased according to the BPA concentration in the culture medium under all oxygen conditions, while the 10B concentration tended to decrease gradually according to the decrease in oxygen concentration. In the T98G cell line, significant differences in uptake at 30 ppm 10B-BPA could be seen between normoxia and hypoxia at 10% O2 (P = 0.03), 3% O2 (P = 0.02), and 1% O2 (P = 0.02). In the A172 cell line, significant differences could be seen at 10 ppm 10B-BPA between 10% and 3% O2 (P = 0.01), and between 10% and 1% O2 (P = 0.03), and at 20 ppm 10B-BPA between 10% and 1% O2 (P = 0.03). These results demonstrate that 10B-BPA uptake in glioblastoma is affected in a continuous fashion by changes in oxygen concentration.
Fig. 2.
Reduced oxygen concentration results in lower 10B-BPA uptake in glioblastoma cell lines. Cells were incubated under several oxygen concentrations—20.8% (normoxia), 10%, 3% or 1% oxygen (hypoxia)—for 72 h, exposed to 10B-BPA with a 10B concentration of 10, 20 or 30 ppm for 2 h under normoxic conditions, and analyzed by ICP-AES. (A) In T98G cells, 10B-BPA uptake gradually decreased in parallel with the decrease in oxygen. There was a significant correlation between 10B-BPA uptake and the 10B-BPA concentration of the culture medium. (B) In A172 cells the same trend was observed (i.e. a gradual decrease in 10B-BPA uptake with decrease in oxygen concentration), although this did not reach statistical significance. Values are expressed as the mean ± standard error; one asterisk indicates P < 0.05 and two asterisks indicate P < 0.01, compared with 10 ppm of 10B, at each oxygen concentration.
Effect of oxygen levels on LAT1/2 expression
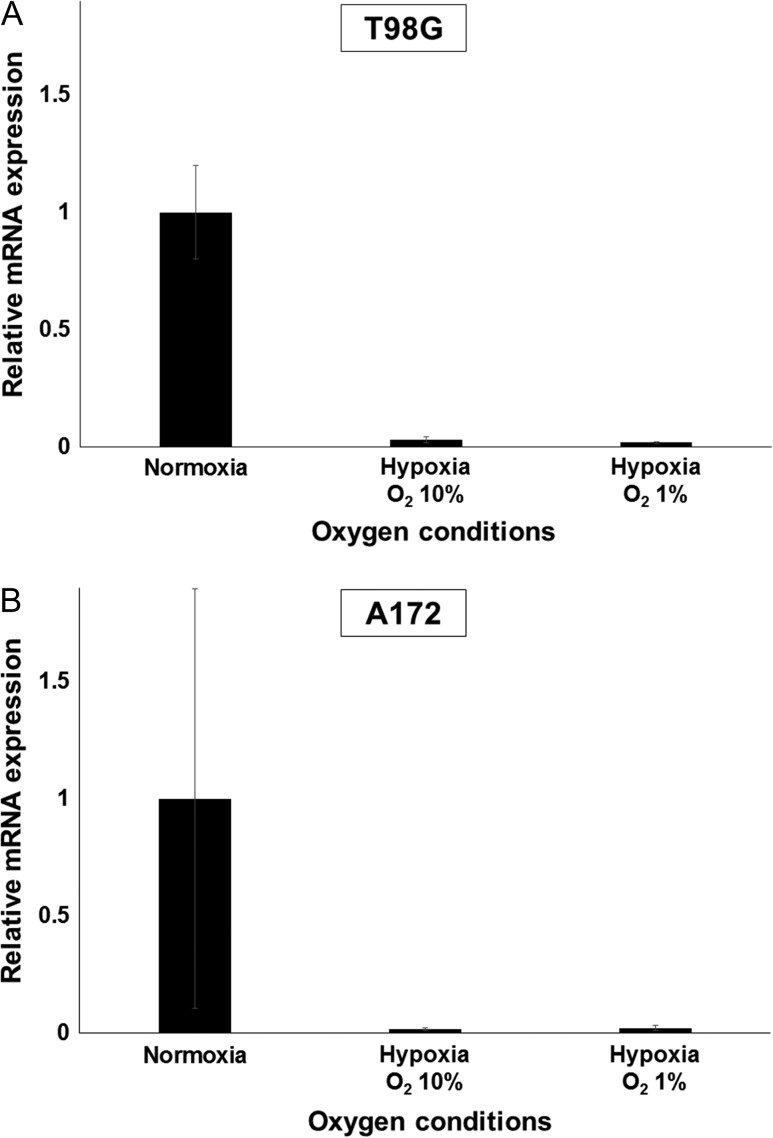
The relationship between oxygen concentration and 10B uptake in the glioblastoma cell lines that we observed above could be due to changes in the mRNA expression level of LAT1/2 or to inactivation of the LAT1/2 transporter. We thus investigated the influence of hypoxia on LAT1 and LAT2 expression in the glioblastoma cell lines. As shown in Fig. 3, relative mRNA expression levels of LAT1 were decreased significantly by both hypoxic conditions tested (10% and 1%). However, LAT2 expression levels in both cell lines were very low, even when incubated under normoxia. The fluorescence from cells under any oxygen conditions could not be detected until after more than 35 cycles of denaturation and annealing/extension processes (data not shown). These results support the concept that hypoxia can reduce 10B-BPA uptake via a reduction in the mRNA expression of LAT1, but do not rule out the possibility that functional inactivation of LAT1/2 at the protein level may also be involved in this response.
Fig. 3.
Reduced oxygen conditions decrease mRNA expression levels of LAT1. Cells were incubated at oxygen concentrations of 20.8% (normoxia), 10% or 1% oxygen (hypoxia) for 72 h, followed by total RNA extraction and qRT-PCR. (A) In T98G, relative expression of LAT1 was significantly lower than in cells incubated under normoxia. (B) In A172, similar results were obtained as for the T98G cell line. Values are expressed as the mean ± standard error.
DISCUSSION
In the present study, we investigated the influence of hypoxia on the ability of human glioblastoma cells to take up 10B-BPA. The findings are consistent with those of other studies [23, 24]; however, ours is the first to assess the effect of several different levels of hypoxia. We showed that the 10B-BPA uptake of human glioblastoma cells decreases gradually, according to the decrease in oxygen concentration, i.e. without a critical threshold. These results suggest that maintaining normoxic oxygen conditions in the cancer cell microenvironment is likely to be very important for successful BNCT.
It has been previously reported that hypoxic conditions can trigger tumor cells to become quiescent (i.e. not actively dividing), and that these quiescent cells uptake less 10B-BPA or 10B-BSH compared with non-quiescent cells [25]. This negative effect was reported to be stronger with 10B-BPA than with 10B-BSH, an observation thought to be due to the fact that 10B-BSH largely accumulates in cells via diffusion, while 10B-BPA is actively taken up via LAT1/2 transporters [26, 27]. The cell cycle is known to be an important factor affecting 10B absorption, with cells in G2/M phase accumulating more 10B than cells in G0/G1 phase [28]. In the present study, we found impairment of cell growth and a greater proportion of cells in G0/G1 phase after 72 h in hypoxic conditions, consistent with the idea that suppression of the cell cycle and that of growth by hypoxia inhibits the uptake of 10B-BPA.
These findings suggest that BNCT treatment may be less effective under conditions of local hypoxia, since the therapeutic effect is largely dependent on the amount of 10B-BPA taken up by tumor cells. This may suggest a significant challenge, because the tumor microenvironment may be severely hypoxic as the result of rapid tumor growth and a dramatic increase in oxygen consumption with insufficient, or abnormal, vascularization [29]. Recently, several reports [30–32] have indicated that 4-borono-2-[18F] fluoro-phenylalanine PET (18FBPA-PET) could predict approximate accumulation of BPA in a tumor before BNCT; however, this prediction is limited by the spatial resolution of the PET scanner; therefore, the precise accumulation cannot be reflected [33]. Previous studies using a PET image with a hypoxic tracer reported there was a rich hypoxic area in the glioblastoma mass, and in consideration of the present results, the dose distribution in hypoxic areas may be overestimated compared with the actual distribution in BNCT. This may be largely related to poor results of the clinical trial [15]; operative resection followed BNCT for newly diagnosed glioblastoma. For the success of BNCT, techniques are therefore required in order to allow cancer cells under hypoxia to accumulate as much 10B-BPA as they would under normoxic conditions.
To date, several approaches have been tried for overcoming the hypoxic disadvantage of BNCT. These strategies can be roughly classified into two methods—first, developing improved 10B carriers, and second, increasing the oxygen concentration at the tumor site. Masunaga et al. tested new 10B carriers synthesized from a hypoxia-specific cytotoxic bioreductive agent (TX-2100) in vivo, and reported not only that the new chemistry exhibited a radiosensitizing effect in hypoxic tumor cells [34], but that this positive effect was enhanced when combined with mild hyperthermia [35]. Similarly, Luderer et al. evaluated a new 10B carrier (a boronated 2-nitroimidazole derivative) designed to have preferential retention in hypoxic glioma cells, and reported low cytotoxicity in normal cells and higher long-term tumor retention compared with 10B-BPA [36]. However, these new carriers have not yet been brought into the clinical setting.
Alternatively, since the hypoxic effect is stronger when using 10B-BPA compared with 10B-BSH because of the difference in uptake mechanism [23, 24], the combined use with both 10B-BPA and 10B-BSH may improve treatment efficacy, but this approach has yet to be formally evaluated. In regard to improving local hypoxia, Masunaga et al. demonstrated the potential benefit of mild hyperthermia in combination with administration of nicotinamide (a vitamin B3 analogue that prevents the development of acute hypoxia) for effective BNCT in vivo [24]. Hyperbaric oxygen therapy prior to 10B carrier administration may be a further strategy; however, further study of the effects of re-oxygenation, including the timing and duration of this type of intervention is needed.
In conclusion, local hypoxia is likely to have a negative influence on the efficacy of BNCT through reducing the amount of 10B-BPA taken up by cancer cells, which in turn reduces the cytotoxic effect following neutron irradiation. The present study suggests that this negative influence correlates linearly with oxygen concentration, without a critical threshold, such that maintaining the local oxygen concentration in tumors may be a promising method for decreasing the rate of recurrence of glioblastoma after BNCT.
ACKNOWLEDGEMENTS
We are very grateful to Mr Masahiro Endo for his extensive technical help with ICP-AES. We have presented the results of the present study at the 136th Northern Japan Regional Meeting of the Japan Radiological Society.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by Japan Society for the Promotion of Science KAKENHI [Grant Number JP15K15442].
REFERENCES
- 1. Locher GL. Biological effects and therapeutic possibilities of neutrons. Am J Reontgenol Radium Ther 1936;36:1–13. [Google Scholar]
- 2. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res 2000;153:173–80. [DOI] [PubMed] [Google Scholar]
- 3. Maruyama K, Ishida O, Kasaoka S et al. . Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J Control Release 2004;98:195–207. [DOI] [PubMed] [Google Scholar]
- 4. Yanagie H, Tomita T, Kobayashi H et al. . Application of boronated anti-CEA immunoliposome to tumour cell growth inhibition in in vitro boron neutron capture therapy model. Br J Cancer 1991;63:522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shelly K, Feakes DA, Hawthorne MF et al. . Model studies directed toward the boron neutron-capture therapy of cancer: boron delivery to murine tumors with liposomes. Proc Natl Acad Sci U S A 1992;89:9039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan XQ, Wang H, Shukla S et al. . Boron-containing folate receptor-targeted liposomes as potential delivery agents for neutron capture therapy. Bioconjug Chem 2002;13:435–42. [DOI] [PubMed] [Google Scholar]
- 7. Masunaga S, Kasaoka S, Maruyama K et al. . The potential of transferrin-pendant-type polyethyleneglycol liposomes encapsulating decahydrodecaborate-10B (GB-10) as 10B-carriers for boron neutron capture therapy. Int J Radiat Oncol Biol Phys 2006;66:1515–22. [DOI] [PubMed] [Google Scholar]
- 8. Miyajima Y, Nakamura H, Kuwata Y et al. . Transferrin-loaded nido-carborane liposomes: tumor-targeting boron delivery system for neutron capture therapy. Bioconjug Chem 2006;17:1314–20. [DOI] [PubMed] [Google Scholar]
- 9. Andoh T, Fujimoto T, Sudo T et al. . Boron neutron capture therapy as new treatment for clear cell sarcoma: trial on different animal model. Appl Radiat Isot 2014;88:59–63. [DOI] [PubMed] [Google Scholar]
- 10. Bialek-Pietras M, Olejniczak AB, Tachikawa S et al. . Towards new boron carriers for boron neutron capture therapy: metallacarboranes bearing cobalt, iron and chromium and their cholesterol conjugates. Bioorg Med Chem 2013;21:1136–42. [DOI] [PubMed] [Google Scholar]
- 11. Kageji T, Nagahiro S, Mizobuchi Y et al. . Boron neutron capture therapy (BNCT) for newly diagnosed glioblastoma: comparison of clinical results obtained with BNCT and conventional treatment. J Med Invest 2014;61:254–63. [DOI] [PubMed] [Google Scholar]
- 12. Kanygin VV, Kichigin AI, Gubanova NV et al. . Possibilities of boron neutron capture therapy in the treatment of malignant brain tumors. Vestn Rentgenol Radiol 2015;6:36–42. [PubMed] [Google Scholar]
- 13. Andoh T, Fujimoto T, Suzuki M et al. . Boron neutron capture therapy (BNCT) as a new approach for clear cell sarcoma (CCS) treatment: trial using a lung metastasis model of CCS. Appl Radiat Isot 2015;106:195–201. [DOI] [PubMed] [Google Scholar]
- 14. Miyatake S, Kawabata S, Hiramatsu R et al. . Boron neutron capture therapy for malignant brain tumors. Neurol Med Chir 2016;56:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawabata S, Miyatake S, Nonoguchi N et al. . Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot 2009;67:S15–8. [DOI] [PubMed] [Google Scholar]
- 16. Kawabata S, Miyatake S, Kuroiwa T et al. . Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 2009;50:51–60. [DOI] [PubMed] [Google Scholar]
- 17. Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol 2009;92:317–35. [DOI] [PubMed] [Google Scholar]
- 18. Gerstner ER, Zhang Z, Fink JR et al. . ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin Cancer Res 2016;22:5079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell C, Dowson N, Fay M et al. . Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Semin Nucl Med 2015;45:136–50. [DOI] [PubMed] [Google Scholar]
- 20. Bekaert L, Valable S, Lechapt-Zalcman E et al. . [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 2017;44:1383–92. [DOI] [PubMed] [Google Scholar]
- 21. Kaneta T, Takai Y, Iwata R et al. . Initial evaluation of dynamic human imaging using 18F-FRP170 as a new PET tracer for imaging hypoxia. Ann Nucl Med 2007;21:101–7. [DOI] [PubMed] [Google Scholar]
- 22. Beppu T, Sasaki T, Terasaki K et al. . High-uptake areas on positron emission tomography with the hypoxic radiotracer (18)F-FRP170 in glioblastomas include regions retaining proliferative activity under hypoxia. Ann Nucl Med 2015;29:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masunaga S, Tatebe H, Nishimura Y et al. . Effect of oxygen pressure during incubation with a 10B-carrier on 10B uptake capacity of cultured p53 wild-type and mutated tumor cells: dependency on p53 status of tumor cells and types of 10B-carriers. Int J Radiat Biol 2016;92:187–94. [DOI] [PubMed] [Google Scholar]
- 24. Masunaga S, Sakurai Y, Tanaka H et al. . Effects of employing a 10B-carrier and manipulating intratumour hypoxia on local tumour response and lung metastatic potential in boron neutron capture therapy. Br J Radiol 2012;85:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masunaga S, Sakurai Y, Tanaka H et al. . Radiosensitivity of pimonidazole-unlabelled intratumour quiescent cell population to gamma-rays, accelerated carbon ion beams and boron neutron capture reaction. Br J Radiol 2013;86:20120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soloway AH, Hatanaka H, Davis MA. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. J Med Chem 1967;10:714–7. [DOI] [PubMed] [Google Scholar]
- 27. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res 2000;153:173–80. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida F, Matsumura A, Shibata Y et al. . Cell cycle dependence of boron uptake from two boron compounds used for clinical neutron capture therapy. Cancer Lett 2002;187:135–41. [DOI] [PubMed] [Google Scholar]
- 29. Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198–206. [DOI] [PubMed] [Google Scholar]
- 30. Watabe T, Hanaoka K, Naka S et al. . Practical calculation method to estimate the absolute boron concentration in tissues using 18F-FBPA PET. Ann Nucl Med 2017;31:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nariai T, Ishiwata K, Kimura Y et al. . PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl Radiat Isot 2009;67:S348–50. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi Y, Imahori Y, Mineura K et al. . Prognostic and therapeutic indicator of fluoroboronophenylalanine positron emission tomography in patients with gliomas. Clin Cancer Res 2003;9:5888–95. [PubMed] [Google Scholar]
- 33. Shimosegawa E, Isohashi K, Naka S et al. . Assessment of 10B concentration in boron neutron capture therapy: potential of image-guided therapy using 18FBPA PET. Ann Nucl Med 2016;30:749–55. [DOI] [PubMed] [Google Scholar]
- 34. Masunaga S, Nagasawa H, Gotoh K et al. . Evaluation of hypoxia-specific cytotoxic bioreductive agent-sodium borocaptate-10B conjugates as 10B-carriers in boron neutron capture therapy. Radiat Med 2006;24:98–107. [DOI] [PubMed] [Google Scholar]
- 35. Masunaga S, Nagasawa H, Sakurai Y et al. . The usefulness of mild temperature hyperthermia combined with a newly developed hypoxia-oriented 10B conjugate compound, TX-2100, for boron neutron capture therapy. Int J Hyperthermia 2006;22:287–99. [DOI] [PubMed] [Google Scholar]
- 36. Luderer MJ, Muz B, de la Puente P et al. . A hypoxia-targeted boron neutron capture therapy agent for the treatment of glioma. Pharm Res 2016;33:2530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]