Abstract
Aims
Evidence is lacking regarding acute anticoagulation management in patients after intracerebral haemorrhage (ICH) with implanted mechanical heart valves (MHVs). Our objective was to investigate anticoagulation reversal and resumption strategies by evaluating incidences of haemorrhagic and thromboembolic complications, thereby defining an optimal time-window when to restart therapeutic anticoagulation (TA) in patients with MHV and ICH.
Methods and results
We pooled individual patient-data (n = 2504) from a nationwide multicentre cohort-study (RETRACE, conducted at 22 German centres) and eventually identified MHV-patients (n = 137) with anticoagulation-associated ICH for outcome analyses. The primary outcome consisted of major haemorrhagic complications analysed during hospital stay according to treatment exposure (restarted TA vs. no-TA). Secondary outcomes comprised thromboembolic complications, the composite outcome (haemorrhagic and thromboembolic complications), timing of TA, and mortality. Adjusted analyses involved propensity-score matching and multivariable cox-regressions to identify optimal timing of TA. In 66/137 (48%) of patients TA was restarted, being associated with increased haemorrhagic (TA = 17/66 (26%) vs. no-TA = 4/71 (6%); P < 0.01) and a trend to decreased thromboembolic complications (TA = 1/66 (2%) vs. no-TA = 7/71 (10%); P = 0.06). Controlling treatment crossovers provided an incidence rate-ratio [hazard ratio (HR) 10.31, 95% confidence interval (CI) 3.67–35.70; P < 0.01] in disadvantage of TA for haemorrhagic complications. Analyses of TA-timing displayed significant harm until Day 13 after ICH (HR 7.06, 95% CI 2.33–21.37; P < 0.01). The hazard for the composite—balancing both complications, was increased for restarted TA until Day 6 (HR 2.51, 95% CI 1.10–5.70; P = 0.03).
Conclusion
Restarting TA within less than 2 weeks after ICH in patients with MHV was associated with increased haemorrhagic complications. Optimal weighing—between least risks for thromboembolic and haemorrhagic complications—provided an earliest starting point of TA at Day 6, reserved only for patients at high thromboembolic risk.
Keywords: Intracerebral haemorrhage, Mechanical heart valve, Therapeutic anticoagulation
Introduction
Anticoagulation management in patients with acute intracerebral haemorrhage (ICH) and mechanical heart valves (MHVs) who require long-term oral anticoagulation (OAC) represents a growing therapeutic dilemma.1–4 During the hyper-acute phase of ICH, altered coagulation needs to be normalized as soon as possible to stabilize the haematoma,5,6 specifically by administering prothrombin complex concentrates to reduce international normalized ratio (INR) levels at least to less than 1.3.5,6 As a consequence of normalizing coagulation, the risk for thromboembolism is increased, which is why anticoagulation management in MHV-patients is intensely debated.7
International guidelines state that ‘early resumption of anticoagulation may be necessary’, without providing specific recommendations on safety and timing.8–11 This reflects an essential lack of randomized trials or sufficiently-sized observational studies exploring the optimal time-point for OAC re-initiation.9,11,12 Yet, a recent consensus statement by the European Society of Cardiology Working Group on Thrombosis suggested that anticoagulation with heparin may be safely restarted 3 days after ICH and that vitamin-K antagonists (VKAs) may be initiated at Day 7 without any major concerns for bleeding complications.13 However, this conclusion was based on a meta-analysis of small observational studies, the largest including on 52 MHV-patients of which 22 died.13,14 Hence, available analyses and data quality seems inadequate to draw firm conclusions, notably as none of the included studies was designed to investigate and compare strategies of restarting anticoagulation.15
The present nation-wide multicentre study pooled individual data of patients with OAC-associated ICH and MHV to investigate both anticoagulation reversal and anticoagulation-resumption by providing incidences of haemorrhagic vs. thromboembolic complications among patients with and without restarted therapeutic anticoagulation (TA). We aimed to establish an optimal time-window for restarting TA in MHV-patients with acute ICH using time-dependent safety and risk-benefit analyses.
Methods
Study design and participants
This observational cohort study represents a combined analysis of both parts of the registered (‘geRman-widE mulTicenter Analysis of oRal Anticoagulation associated intraCerebral hEmorrhage’) RETRACE-program; Part-1 conducted from 1 January 2006 until 31 December 2010 (NCT01829581)5 and Part-2 from 1 January 2011 until 31 December 2015 (NCT03093233). We integrated pooled individual patient data of 2504 consecutive OAC-ICH patients treated at 22 tertiary-care centres (Departments of Neurology) throughout Germany. We defined MHV-patients by having a MHV (i.e. bi-leaflet, single-leaflet, tilting disc, or caged-ball) in situ during occurrence of ICH and recorded valve positions (aortic, mitral, or both).7 Patients with exclusively bioprosthetic valves were not classified as MHV patients. In all patients with VKA-related ICH an effective intake was determined as INR-value equal or greater to 1.5 on hospital admission. We excluded patients with secondary ICH aetiologies, as previously described.5 For analyses of reversal management, we did not consider patients with early care limitation (withhold or withdrawal of therapy orders within 24 h).5,16 For analyses of anticoagulation management, we implemented a 72 h ‘quarantine period’, as previously suggested, to limit false positive attribution of treatment exposures with outcomes.17 The study was approved by the local ethics committees and institutional review boards based on the central vote from Friedrich-Alexander-University Erlangen-Nuremberg, Germany (Re.No-4409 & 30_16B). Individual consent was obtained from patients or legal representatives if not waived by local ethics committees.
Data acquisition
We assessed data on demographics, prior medical history, in-hospital and imaging parameters, laboratory data and reversal management (timing, doses, and agents used for reversal treatment) as previously described.5 Pre-existing comorbidities comprised arterial hypertension, diabetes mellitus, abnormal kidney or liver function, prior myocardial infarction, congestive heart failure, history of stroke, and antiplatelet medication, which were used to compute values for the CHADS2 and HAS-BLED scores. Clinical status on admission was obtained using the Glasgow Coma Scale (GCS) as noted in prospective data bases, medical charts, and emergency protocols. Severity of ICH was assessed using the most commonly used prognostic Scale, i.e. ICH Score (higher scores indicating poorer outcome).16,18 Laboratory parameters on admission and serial coagulation parameters [activated partial thromboplastin time (aPTT), INR] were recorded using institutional laboratory databases.
Diagnosis of ICH and imaging characteristics were determined on first-available cranial imaging after onset of ICH. Intracerebral haemorrhage volume was calculated using the ABC/2 and ABC/3 methods,19 haematoma growth was defined as greater 33% volume increase,20 and all serial follow-up imaging during the complete hospital stay were evaluated for detection of outcome measures in each patient.5 For analyses of reversal management, we dichotomized patients into sufficiently (INR-level reversed to less than 1.3 within at least 4 h) or non-sufficiently reversed, as previously established.5
Timing and mode of anticoagulation management during hospital stay were categorized into (i) no-TA, i.e. either no antithrombotic medication received during hospital stay or administration of heparins [unfractionated heparin, low molecular weight heparins (LMWH)] in prophylactic dosing for prevention of venous thromboembolism (VTE)21 or into (ii) TA, i.e. either restarted OAC using VKA (scored on first day with INR levels ≥ 1.5), continuous intravenous or subcutaneous application of unfractionated heparin (targeting a therapeutic range of 1.5-fold to 2.5-fold aPTT prolongation) or full weight-adjusted dosing of LMWH (targeting 0.5 to 1.0 anti-Xa units/mL).1,8,22 Dichotomous treatment exposure was classified according to the most aggressive anticoagulation therapy received.
We addressed crossover between treatment groups by calculating incidence rates per patient days for each day on specific treatment until the occurrence of complications or discharge. Complications consisted of intra- or extracranial haemorrhagic and thromboembolic events (details see below), which were serially evaluated and corroborated by pertinent medical charts, physicians’ letters, and imaging or echocardiography reports review during the entire hospital stay.23 Complications were adjudicated to treatment exposure (TA vs. no-TA), which was present at the day of clinical diagnosis of haemorrhagic and thromboembolic complications.
Investigated outcomes
Primary outcome
We defined major haemorrhagic complications as primary safety outcome measure consisting of (i) any intracranial haemorrhage, i.e. new ICH distant from the initial haematoma, any delayed haematoma enlargement >33% occurring beyond the 72 h quarantine period, and new subarachnoid or sub-/epidural haemorrhage, as well as (ii) any major extracranial haemorrhage, i.e. acute (<24 h) reduction of serum haemoglobin ≥3 g/dL, transfusion ≥2 units packed red blood cells, bleeding in critical site (intraspinal, intraocular, pericardial, articular, retroperitoneal), or fatal bleeding according to Bleeding Academic Research Consortium Type 3a or greater.23 Outcome measures in all patients were recorded during the entire acute hospitalization period.
Secondary outcomes
Secondary outcomes included (i) thromboembolic complications, (ii) the composite of haemorrhagic and thromboembolic complications, (iii) timing of TA, and (iv) mortality and functional outcome at discharge and Day 90. We defined thromboembolic complications as intracranial, i.e. ischaemic stroke (scored upon serial follow-up imaging), or extracranial thromboembolic complications,23 i.e. systemic embolism, myocardial infarction [ST-elevated myocardial infarction (STEMI) and non-STEMI with troponin elevation >99th percentile upper reference limit],24 valve thrombosis (evaluated through routine echocardiography or computed tomography), or symptomatic pulmonary embolism.25 Coincidence of intra- and extracranial thromboembolic complications were scored as intracranial, e.g. valve thrombosis with ischaemic stroke. We evaluated timing of TA for both haemorrhagic and thromboembolic complications to establish associations with primary and secondary outcomes according to the starting point of therapy after occurrence of ICH. We analysed mortality and functional outcome at discharge and 90 days after ICH using the modified Rankin scale (mRS, higher scores indicating poorer outcome)—categorized into favourable (mRS = 0–3) and unfavourable (mRS = 4–6).26
Statistical analyses
We performed statistical analyses using the SPSS 21.0 software package (www.spss.com) and R 2.12.0 (www.r-project.org). We conducted complete case analysis as the maximum rate of missing values was less than 3% among all analysed parameters included into outcome analyses. Statistical tests were two-sided and the significance level was set at α = 0.05. Data distribution was evaluated using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean (±standard deviation), compared using the Student’s t-test or univariate analysis of variance (ANOVA), and non-normally distributed data as median (interquartile range), analysed using the Mann–Whitney U-test or the Kruskal–Wallis H-test. Frequency distribution of categorized variables was compared using the Pearson’s χ2 test or the Fisher’s exact test, respectively the Freeman–Halton extension of the Fisher exact test for trichotomous group comparisons. All univariate analyses and post hoc tests were corrected for multiple comparisons using the Holm’s sequential Bonferroni procedure to minimize accumulation of type 1 errors. Sensitivity analyses were performed to investigate bias and confounding of reversal- with anticoagulation-management as well as for the doses of prothrombin complex concentrates (PCCs) with thromboembolic complications by receiver operating characteristics (ROC) analyses.
To account for potential confounding by indication, we additionally performed propensity score matching using parallel, balanced, variable ratio (1: many) nearest-neighbour approach at a calliper of 0.1.27 The propensity score was calculated using parameters showing differences for inter-group comparisons, i.e. age, GCS, and haematoma volume.
We calculated crude incidence rates (CIRs) per 100 patient days on each specific treatment for haemorrhagic complications, thromboembolic complications, and the composite of both. We compared incidence rates according to treatment exposure (with and without TA) presented as conditional maximum likelihood (CML) estimate of rate ratios 95% confidence interval (CI) and compared CIR using the Mid-P exact test. Analyses were censored after occurrence of aforementioned complications or patient death. Comparisons of CIR according to treatment exposure were conducted for (i) the overall cohort, (ii) the propensity score matched cohort, (iii) patients dichotomized according to valve position (aortic vs. mitral or both, preceded by CIR analyses based on valve position omitting treatment exposure), and (iv) MVH-patients with present atrial fibrillation vs. sinus rhythm.
Timing of TA was analysed in relation to outcome complications using stepwise-forward multivariable adjusted Cox regression modelling. We calculated hazard ratio (HR) estimates for each day after ICH derived from patient clusters (HR-estimate at the median of a 3 day interval, i.e. HR-estimate at Day 4 calculated using the interval from Day 3 to 5) comparing patients that started TA vs. no-TA. HR estimates were weighted and smoothed by the method of moving averages to correct for overestimation. Analyses were adjusted for haemorrhagic and thromboembolic risk (CHADS2 and HAS-BLED Scores) as well as for statistical imbalances among baseline clinical characteristics (GCS, ICH volume). The primary outcome—associations of TA with safety—was calculated using haemorrhagic complications as the dependent variable. The secondary outcome constituted both complications as dependent variable. The significance thresholds (intercept of the 5% CI with the HR of 1) allowed identification of a time interval at which patients were at significantly increased risk to experience the dependent outcome in relation to treatment exposure. Dichotomous functional outcome and mortality analyses at hospital discharge are presented for all patients receiving TA separately shown for the identified time-intervals and for the overall cohort comparing patients with TA vs. no-TA.
Results
Study analyses
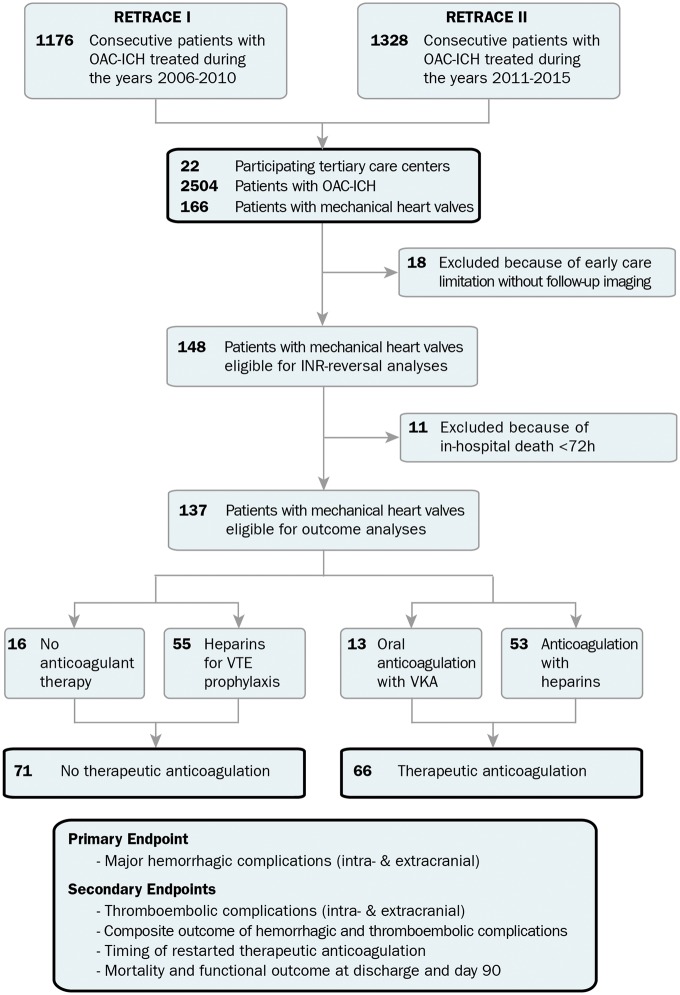
Of 2504 OAC-ICH patients (see Supplementary material online, Table S1) recruited among 22 tertiary care centres of the German nationwide RETRACE I and II study programs, a total of 166 patients with MHVs were identified (Figure 1). At first, reversal management was investigated in 148 patients after exclusion of 18 patients that received early care limitations (<24 h) without follow-up imaging. Secondly, analyses of anticoagulation management were conducted in all patients who survived the first 72 h leading to exclusion of another 11 patients (see Supplementary material online, Table S2 for details on excluded patients). The remaining 137 patients were grouped according to treatment exposure, i.e. 71 patients received no-TA (16 patients received no antithrombotic treatment and 55 patients’ heparins for VTE-prophylaxis) and 66 patients received TA (13 patients with VKA and 53 patients with unfractionated heparin or LMWH). All following analyses of anticoagulation management were calculated dichotomously for TA vs. no-TA as post hoc analyses (trichotomous inter-group comparison) of all possible modes of anticoagulation management and detailed comparison of patients anticoagulated with VKA and heparin suggested limited confounding (see Supplementary material online, Tables S3 A and B and S4).
Figure 1.
Study profile and numbers of participants. Individual level data of 2504 patients with anticoagulation-associated intracerebral haemorrhage were pooled and screened for the study. We identified 166 patients with mechanical heart valves in situ. For analysis of anticoagulation reversal, we excluded 18 patients because of early treatment restriction without follow-up imaging. For analysis of anticoagulation resumption, because of the predefined quarantine period of 72 h, we excluded additional 11 patients with early (<72 h) in-hospital death. For outcome analyses, 137 mechanical heart valve patients were eligible and were dichotomized into patients restarted on therapeutic anticoagulation vs. those without therapeutic anticoagulation. Therapeutic anticoagulation consisted either of restarting vitamin-K antagonists (n = 13) or systemic heparinization (n = 53). We defined major haemorrhagic complications as primary safety outcome measure consisting of (i) any intracranial haemorrhage, i.e. new intracerebral haemorrhage distant from the initial haematoma, any delayed haematoma enlargement >33% occurring beyond the 72 h quarantine period, and new subarachnoid or sub-/epidural haemorrhage, as well as (ii) any major extracranial hemorrhage [i.e. acute decrease (<24 h) in haemoglobin ≥3 g/dL, transfusion ≥2 units packed red blood cells, bleeding in critical site: intraspinal, intraocular, pericardial, articular, retroperitoneal, or fatal bleeding). We defined thromboembolic complications as intracranial, i.e. ischaemic stroke (unrelated to intracranial interventions scored upon serial follow-up imaging), or extracranial thromboembolic complications, i.e. systemic embolism, myocardial infarction (ST-elevated myocardial infarction and non-ST-elevated myocardial infarction with troponin elevation >99th percentile upper reference limit), valve thrombosis (evaluated through routine echocardiography or computed tomography), or symptomatic pulmonary embolism. Coincidence of intra- and extracranial thromboembolic complications were scored as intracranial, e.g. valve thrombosis with ischemic stroke. ICH, intracerebral haemorrhage; OAC, oral anticoagulation; VTE, venous thromboembolism.
Analyses of reversal management
Sufficient INR-reversal (Table 1) was achieved in 37/148 (25.0%) of patients and was associated with a significantly decreased rate of haematoma enlargement [6/30 (20.0%) vs. 49/100 (49.0%); P < 0.01]. Reversal was mainly carried out using PCC 136/148 (91.9%). Characteristics of patients sufficiently reversed revealed a poorer admission status [GCS: 9 (3–14) vs. 14 (10–15); P < 0.01], more frequent intraventricular haemorrhage [23/37 (62.2%) vs. 40/111 (36.0%); P < 0.01], and increased initial ICH volumes [28.1 mL (14.8–63.0) vs. 19.4 mL (7.8–43.0); P = 0.05]. Overall, sensitivity analyses provided neither signals that INR-reversal was associated with acute (<72 h) or delayed (≥72 h) occurrence of thromboembolic complications, nor that the total cumulative dose of PCC given correlated with an increased thromboembolic risk [area under the curve (AUC): 0.67, 95% (0.49–0.87); P = 0.09]. These data suggested that clinically less severely affected patients received less aggressive reversal management and thus carry an increased risk to experience haematoma enlargement (Table 1). Of note, the proportion of patients who were restarted on TA was not statistically different among patients with vs. without sufficient reversal [16/37 (43.2%) vs. 53/111 (47.7%); P = 0.63, Table 1].
Table 1.
Clinical characteristics of patients with oral anticoagulation-ICH and mechanical heart valve who received sufficient oral anticoagulation reversal or insufficient oral anticoagulation reversal (n = 148)
| Sufficient INR reversal (n = 37) | Insufficient INR reversal (n = 111) | P-value | |
|---|---|---|---|
| Age (years), mean (±SD) | 69 (59–75) | 70 (62–76) | 0.44 |
| Female sex, n (%) | 11 (29.7) | 42 (37.8) | 0.37 |
| Pre-mRS, mean (±SD)a | 0 (0–1) | 0 (0–2) | 0.38 |
| Symptom onset–onset admission, (min), median (IQR) | 125 (70–202) | 129 (60–348) | 0.73 |
| Glasgow coma scale, median (IQR)b | 9 (3–14) | 14 (10–15) | <0.01 |
| Mechanical heart valve positions, n (%) | |||
| Aortic valve | 27 (73.0) | 69 (62.2) | 0.23 |
| Mitral valve | 10 (27.0) | 32 (28.8) | 0.84 |
| Both locations | 0 (0.0) | 10 (9.0) | 0.12 |
| Imaging | |||
| Deep ICH, n (%) | 15 (40.5) | 45 (40.5) | 1.00 |
| Lobar ICH, n (%) | 18 (48.6) | 51 (45.9) | 0.78 |
| Other ICH locations, n (%) | 4 (5.4) | 15 (9.0) | 0.78 |
| ICH volume (mL), median (IQR) | 28.1 (14.8–63.0) | 19.4 (7.8–43.0) | 0.05c |
| Intraventricular haemorrhage, n (%) | 23 (62.2) | 40 (36.0) | <0.01 |
| Reversal treatment | |||
| Admission reversal (min), median (IQR) | 80 (54–120) | 101 (60–235) | 0.15 |
| Any reversal treatment, n (%) | 37 (100.0) | 109 (98.2) | 1.00 |
| PCC, n (%) | 37 (100.0) | 99 (89.2) | 0.07 |
| Dose (IU), median (IQR) | 2500 (1800–3200) | 2000 (1500–3000) | 0.15 |
| FFP, n (%) | 7 (18.9) | 16 (14.4) | 0.51 |
| Konakion, n (%) | 31 (83.8) | 84 (75.7) | 0.31 |
| Blood pressure control (mmHg), median (IQR) | |||
| Initial systolic BP | 162 (133–187) | 156 (140–186) | 0.85 |
| Initial diastolic BP | 89 (72–98) | 80 (70–99) | 0.89 |
| Initial mean arterial BP | 108 (89–127) | 107 (96–127) | 0.77 |
| Systolic BP (4 h) | 141 (120–159) | 141 (122–152) | 0.99 |
| Diastolic BP (4 h) | 65 (60–74) | 70 (60–80) | 0.31 |
| Mean arterial BP (4 h) | 93 (79–101) | 91 (83–103) | 0.65 |
| Systolic BP (8 h) | 133 (125–141) | 132 (117–142) | 0.41 |
| Diastolic BP (8 h) | 68 (60–73) | 65 (58–74) | 0.63 |
| Mean arterial BP (8 h) | 89 (83–98) | 87 (80–96) | 0.36 |
| Systolic BP (12 h) | 127 (110–149) | 135 (123–145) | 0.22 |
| Diastolic BP (12 h) | 60 (56–73) | 66 (57–75) | 0.43 |
| Mean arterial BP (12 h) | 83 (75–100) | 89 (80–96) | 0.33 |
| Coagulation parameters | |||
| INR on admission, median (IQR) | 2.83 (2.4.6–3.67) | 2.70 (2.40–3.44) | 0.27 |
| 1st INR after reversal, median (IQR) | 1.17 (1.11–1.22) | 1.39 (1.27–1.60) | <0.01 |
| INR <1.3 on 1st INR after reversal, n (%) | 37 (100.0) | 34 (30.6) | <0.01 |
| INR <1.3 within 4 h, n (%) | 37 (100.0) | 0 (0.0) | <0.01 |
| INR after 24 h, median (IQR) | 1.26 (1.20–1.37) | 1.27 (1.20–1.39) | 0.64 |
| INR after 48 h, median (IQR) | 1.24 (1.13–1.34) | 1.26 (1.16–1.41) | 0.41 |
| INR after 72 h, median (IQR) | 1.23 (1.11–1.37) | 1.25 (1.14–1.46) | 0.33 |
| Complications ≤72 h after ICH, n (%) | |||
| Haemorrhagic complications | |||
| Hematoma enlargement | 6/30 (20) | 49/100 (49) | <0.01 |
| Other intracranial haemorrhage | 1 (2.7) | 0 (0.0) | 0.25 |
| Extracranial haemorrhage | 0 (0.0) | 0 (0.0) | 1.00 |
| Thromboembolic complication | 0 (0.0) | 0 (0.0) | 1.00 |
| Complications ≥72 h after ICH, n (%) | |||
| Haemorrhagic complications | 5 (13.5) | 16 (14.4) | 0.89 |
| Thromboembolic complication | 2 (5.4) | 6 (5.4) | 1.00 |
| Anticoagulation management | |||
| Therapeutic Anticoagulation, n (%) | 16 (43.2) | 53 (47.7) | 0.63 |
| Day after ICH, median (IQR) | 7 (1–17) | 4 (1–11) | 0.89 |
| <72 h after ICH, n (%) | 7 (18.9) | 20 (18.0) | 0.88 |
Significant parameters are presented in bold. Sufficient OAC reversal was defined as achieving INR values <1.3 within 4 h after admission.
ICH, intracerebral haemorrhage; INR, international normalized ratio; IQR, interquartile range; IU, international unit; mRS, modified Rankin Scale; SD, standard deviation.
Modified Rankin Scale range 0–6, from no disability to death.
Glasgow coma scale range 3–15, from deep coma to alert.
Not significant after Holm’s sequential Bonferroni correction.
Analyses of anticoagulation management
Baseline characteristics (Table 2) of eligible patients (n = 137) showed that patients who received TA were less severely affected by ICH [i.e. smaller ICH volumes: 14.7 mL (6.0–38.1) vs. 23.9 mL (10.7-65.4) P = 0.02; more favourable neurological status: GCS 14 (13–15) vs. 12 (5–15), P < 0.01; and decreased ICH severity: ICH-Score 1 (0–2) vs. 2 (1–3), P < 0.01]. The median length of hospital stay between treatment groups was comparable [TA: 15 (9–25) days vs. no-TA: 13 (6–20) days; P = 0.07] and serial INR levels after acute reversal management did not differ between TA and no-TA patients neither before nor at the beginning of outcome assessment (Table 2).
Table 2.
Baseline characteristics of patients with oral anticoagulation-ICH and mechanical heart valve
| No therapeutic anticoagulation (n = 71) | Therapeutic anticoagulation (n = 66) | P-value | |
|---|---|---|---|
| Age (years), mean (±SD) | 71 (62–77) | 69 (60–73) | 0.14 |
| Female sex, n (%) | 26 (36.6) | 21 (31.8) | 0.55 |
| Pre-mRS, mean (±SD)a | 0 (0–1) | 0 (0–1) | 0.67 |
| Glasgow coma scale, median (IQR)d | 12 (5–15) | 14 (13–15) | <0.01 |
| ICH score, median (IQR)e | 2 (1–3) | 1 (0–2) | <0.01 |
| Prior medical history, n (%) | |||
| Hypertension | 57 (80.3) | 56 (84.8) | 0.48 |
| Diabetes mellitus | 22 (31.0) | 17 (25.8) | 0.50 |
| Ischaemic stroke | 14 (19.7) | 12 (18.2) | 0.82 |
| Congestive heart failure | 14 (19.7) | 14 (21.2) | 0.83 |
| Abnormal kidney function | 20 (28.2) | 26 (39.4) | 0.16 |
| Abnormal liver function | 4 (5.6) | 5 (7.6) | 0.74 |
| Additional antiplatelet medication | 9 (12.7) | 7 (10.6) | 0.71 |
| Mechanical heart valve positions, n (%) | |||
| Aortic valve | 51 (71.8) | 39 (59.1) | 0.12 |
| Mitral valve | 15 (21.1) | 22 (33.3) | 0.11 |
| Both locations | 5 (7.0) | 5 (7.6) | 1.00 |
| OAC scores | |||
| CHADS2b | |||
| Mean (±SD) | 2.0 (±1.4) | 2.1 (±1.2) | |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 0.99 |
| High-risk (≥2), n (%) | 44 (62.0) | 39 (59.1) | 0.73 |
| HAS-BLEDc | |||
| Mean (±SD) | 2.5 (±1.3) | 2.6 (±1.1) | |
| Median (IQR) | 2 (1–4) | 3 (2–3) | 0.32 |
| High-risk (≥3), n (%) | 29 (40.8) | 37 (56.1) | 0.08 |
| Imaging | |||
| Deep ICH, n (%) | 28 (39.4) | 29 (43.9) | 0.59 |
| Lobar ICH, n (%) | 34 (47.9) | 27 (40.9) | 0.41 |
| Cerebellar ICH, n (%) | 6 (8.5) | 6 (9.1) | 0.90 |
| Brainstem ICH, n (%) | 1 (1.4) | 2 (3.0) | 0.61 |
| Primary IVH, n (%) | 2 (2.8) | 2 (3.0) | 1.00 |
| ICH volume (mL), median (IQR) | 23.9 (10.7–65.4) | 14.7 (6.0–38.1) | 0.02f |
| Intraventricular haemorrhage, n (%) | 32 (45.1) | 26 (39.4) | 0.50 |
| Coagulation parameters, median (IQR) | |||
| INR on admission | 2.68 (2.15–3.39) | 2.76 (2.43–3.51) | 0.29 |
| 1st INR after reversal | 1.28 (1.16–1.47) | 1.33 (1.17–1.56) | 0.34 |
| INR after 24 h | 1.27 (1.18–1.39) | 1.30 (1.21–1.38) | 0.31 |
| INR after 48 h | 1.23 (1.14–1.38) | 1.27 (1.17–1.38) | 0.31 |
| INR after 72 h | 1.24 (1.12–1.46) | 1.25 (1.18–1.40) | 0.72 |
Significant parameters are presented in bold. ICH, intracerebral haemorrhage; INR, international normalized ratio; IQR, interquartile range; mRS, modified Rankin Scale; SD, standard deviation.
Modified Rankin Scale range 0–6, from no disability to death.
CHADS2 score range 0–6, from low to high risk of thromboembolism.
HAS-BLED score range 0–9, from low to high risk of bleeding under oral anticoagulation.
Glasgow coma scale range 3–15, from deep coma to alert.
ICH score range 0–6, from low to high risk of short-term mortality. Significant P-values are presented in bold.
Not significant after Holm’s sequential Bonferroni correction.
Analyses of primary and secondary outcomes
We recorded a total of 21/137 (15.3%) haemorrhagic complications, at a significantly increased rate in patients restarted on TA (17/66, 25.8%) compared to patients without TA (4/71, 5.6%; P = 0.001; Figure 2, Table 3). We recorded a total of 8/137 (5.8%) thromboembolic complications, revealing a trend towards reduced rates in TA patients [1/66(1.5%) vs. 7/71 (9.9%) no-TA; P = 0.06].
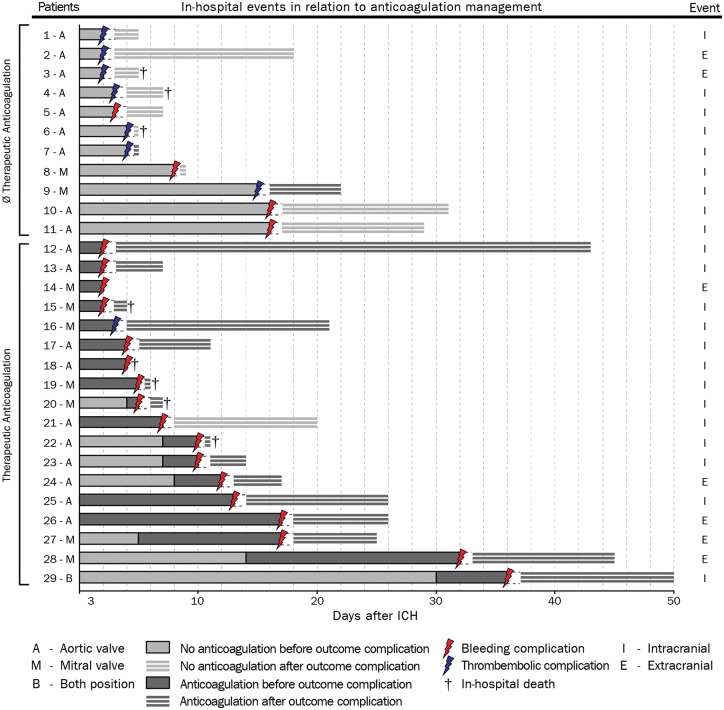
Figure 2.
Details on haemorrhagic and thromboembolic complications during hospital stay. Haemorrhagic or thromboembolic complications occurred in 29 patients with mechanical heart valves, dichotomized according to treatment exposure (therapeutic anticoagulation shown dark grey bars, no therapeutic anticoagulation shown as light grey bars). Complications were adjudicated according to anticoagulation treatment present immediately prior to clinical occurrence or diagnosis of haemorrhagic and thromboembolic complications. The mechanical valve position is indicated by ‘A’, ‘M’, ‘B’, either ‘aortic’, ‘mitral’, or ‘both’. The time-point of occurrence and the type of complication is depicted by coloured arrows (haemorrhagic shown as red, thromboembolic as blue). The classification of complications as intra- or extracranial is shown at the right hand side, ‘I’, intracranial and ‘E’, extracranial. The cross indicates the time-point of in-hospital mortality. The length of hospital stay after the censoring complication is depicted as light grey bars with white horizontal stripes. For definitions of haemorrhagic and thromboembolic complications please see methods. ICH, intracerebral haemorrhage.
Table 3.
Analysis of primary and secondary outcomes according to treatment exposure
| No. of patients | No. of outcomes | P-valuea | No. of patient days | Incidence rate per 100 patient days (95% CI) | CML estimate of rate ratio (95% CI) | P-valueb | |
|---|---|---|---|---|---|---|---|
| Haemorrhagic complication | |||||||
| TA | 66 | 17 | <0.01 | 482 | 3.53 (2.05–5.65) | 10.31 (3.67–35.70) | <0.01 |
| No TA | 71 | 4 | 1169 | 0.34 (0.09–0.88) | |||
| Thromboembolic complication | |||||||
| TA | 66 | 1 | 0.06 | 482 | 0.21 (0.01–1.15) | 0.35 (0.02–2.24) | 0.34 |
| No TA | 71 | 7 | 1169 | 0.60 (0.24–1.23) | |||
| Composite endpoint | |||||||
| TA | 66 | 18 | 0.09 | 482 | 3.73 (2.21–5.90) | 3.97 (1.88–8.69) | <0.01 |
| No TA | 71 | 11 | 1169 | 0.94 (0.47–1.68) | |||
Significant P-values are presented in bold. For definitions of haemorrhagic and thromboembolic complications please see Methods.
CI, confidence interval; CML, conditional maximum likelihood; No, number; No TA, i.e. either no antithrombotic medication received during hospital stay or administration of heparins [unfractionated heparin, low molecular weight heparins (LMWH)] in prophylactic dosing for prevention of venous thromboembolism (VTE); TA, i.e. either restarted therapeutic anticoagulation using VKA (scored on first day with INR levels ≥ 1.5), continuous or subcutaneous heparinization (targeting a therapeutic range of aPTT extended by 1.5–2.5) and or full weight adjusted dosing of LMWH (targeting 0.5–.0 anti-Xa units/mL).
Compared using the Pearson's χ2 or the Fisher’s exact test as appropriate.
Compared using the Mid-P exact test.
Figure 2 provides a detailed overview of all complication characteristics in relation to mode of treatment, valve position and mortality. Of all 29 complications the majorities were intracranial (haemorrhagic complications: 16 intracranial, 5 extracranial; thromboembolic complications: 6 intracranial, 2 extracranial). There were no significant differences in baseline characteristics (see Supplementary material online, Table S4) and no significant difference in the rate of haemorrhagic complications during hospital stay among patients who resumed TA using VKA vs. heparins [VKA: 3/13 (23.1%) vs. heparin: 14/53 (26.4%); P = 0.81].
Comprehensive analyses of the primary outcome (correcting for treatment crossover; i.e. switching from prophylactic to therapeutic dosing or vice versa) were conducted according to time spent on each specific treatment and are presented as CIR per 100 patient days (Table 3). In patients with TA, the CIR for haemorrhagic complications was significantly increased (TA: CIR 3.53, 95% CI 2.05–5.65 vs. no-TA: CIR 0.34, 95% CI 0.09–0.88) resulting in a significantly increased CML incidence rate ratio (10.31, 95% CI 3.68–35.70; P < 0.01). Sub-analyses of potential baseline confounders in patients resumed on TA experiencing intracranial haemorrhagic complications did not provide significant differences (see Supplementary material online, Table S5). Comparing the CIRs between VKA (CIR 5.36, 95% CI 1.08–15.6) and heparin-treated patients (CIR 3.29, 95% CI 1.79–5.51) did not provide significant associations with increased haemorrhagic complications with one or the other treatment (CML incidence rate ratio 1.63, 95% CI 0.38–5.26; P = 0.12).
The CIR per 100 patient days for the occurrence of thromboembolic complications (Table 3) were not significantly different between both (TA vs. no-TA) treatment groups (CML incidence rate ratio 0.35, 95% CI 0.02–2.24; P = 0.34). For the composite outcome of haemorrhagic and thromboembolic complications, the CIR per 100 patient days showed a significantly increased rate in patients receiving TA (TA: CIR 3.73, 95% CI 2.21–5.90 vs. no-TA: CIR 0.94, 95% CI 0.47–1.68; CML incidence rate ratio 3.97, 95% CI 1.88–8.69; P < 0.01).
To establish that these findings were not confounded by differences in baseline characteristics (Table 2), we performed a propensity score matching to balance inter-group differences (see Supplementary material online, Table S6). Analyses of primary and secondary outcomes in the propensity score matched cohort verified that the CIR per 100 patient days for haemorrhagic complications (CML incidence rate ratio 8.02, 95% CI 2.73–28.54; P < 0.01), and for the composite of haemorrhagic and thromboembolic complications (CML incidence rate ratio 3.14, 95% CI 1.41–7.12; P < 0.01), were significantly increased in disadvantage of patients receiving TA (see Supplementary material online, Table S7).
Timing of therapeutic anticoagulation after intracerebral haemorrhage
Patients with TA that experienced a haemorrhagic complication restarted TA significantly earlier than patients without a haemorrhagic complication [median of 3 (2–10) days after ICH vs. median of 8 (5–19) days after ICH; P < 0.01]. Timing of TA was not significantly different (P = 0.10) among patients restarting VKA [7 (4–30) days] vs. heparin [3 (2–10) days]. In all patients who received continuous intravenous unfractionated heparin, aPTT levels were within the targeted therapeutic range within 72 h after heparin initiation (see Supplementary material online, Figure S1).
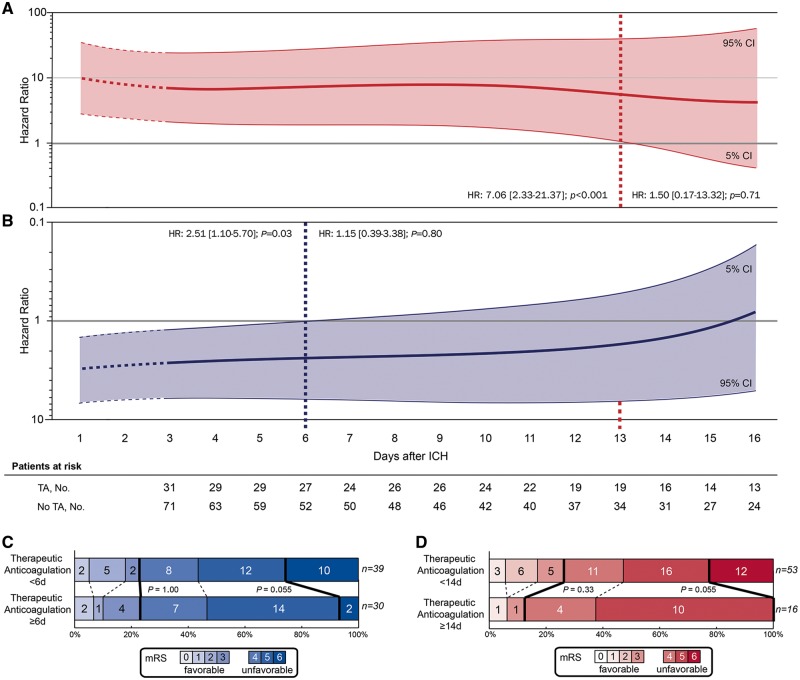
We calculated adjusted Cox regression analyses to explore the associations of re-initiating TA—detailed assessment of timing for each day after ICH during the period of hospital stay—with haemorrhagic and thromboembolic complications (Figure 3). Regarding the primary outcome, re-initiation of TA was associated with a significantly increased hazard ratio for haemorrhagic complications until 13 days after ICH (HR 7.06, 95% CI 2.33–21.37; P < 0.01), whereas TA initiated ≥14 days after ICH until hospital discharge was no longer significantly related to haemorrhagic complications (HR 1.50, 95% CI 0.17–13.32; P = 0.71) (Figure 3A). We investigated the clinical relevance of this association by comparing patients with TA initiated ≥14 days after ICH to patients with earlier TA (Figure 3D). This analysis demonstrated a statistical trend towards a reduced discharge mortality rate in patients who received TA ≥14 days [mortality: 0/16 (0%) vs. 12/53 (22.6%); P = 0.055], yet without influence on functional outcome at discharge [mRS = 0–3: 2/16 (12.5%) vs. 14/53 (26.4%); P = 0.33].
Figure 3.
Timing of therapeutic anticoagulation and clinical outcome. Adjusted cox proportional hazard models were used to visualize the association between the day of restarting therapeutic anticoagulation after intracerebral haemorrhage and (A) haemorrhagic complications and (B) the composite of haemorrhagic and thromboembolic complications during hospital stay. Patients were dichotomized according to treatment exposure (therapeutic anticoagulation vs. no therapeutic anticoagulation) and sequentially included into analyses at the day therapeutic anticoagulation was restarted. Specifically, we calculated hazard ratio estimates (y-axis) for both outcomes at each day after intracerebral haemorrhage using time-patient-clusters (3 day intervals) of patients that restarted therapeutic anticoagulation at a median of the presented day on the x-axis, and compared these with patients without therapeutic anticoagulation with available datapoints within these time-patient-clusters. Hazard ratio estimates were weighted and smoothed by the method of moving averages to correct for overestimation. ’Patients at risk‘ represents the number of individuals with attributed treatment exposure (either therapeutic anticoagulation or no therapeutic anticoagulation) at that specific day post-intracerebral haemorrhage. Stepwise-forward Cox proportional hazard modelling was adjusted for haemorrhagic and thromboembolic risk (CHADS2 and HAS-BLED scores) as well as for statistical imbalances among baseline characteristics (Glasgow Coma Scale, intracerebral haemorrhage volume). The thick lines (red for haemorrhagic complications, blue for the composite of haemorrhagic and thromboembolic complications) represent the hazard ratio estimates generated for every single day after intracerebral haemorrhage; the thin lines with shaded area indicate the 95% confidence intervals. The distribution of mortality and functional outcome (C and D) at discharge is displayed using the modified Rankin Scale. Analyses of in-hospital mortality and functional outcome at discharge in patients who received therapeutic anticoagulation (including three patients who died before 72 h but were restarted on therapeutic anticoagulation before) is displayed as dichotomized comparison at the identified time-threshold for (D) haemorrhagic complications and for the (C) composite of haemorrhagic and thromboembolic complications. Each score on the modified Rankin Scale is separated by dashed lines. Thick lines separate the proportion of patients with favourable (modified Rankin Scale 0–3) and unfavourable (modified Rankin Scale 4–6) outcome as well as patients with and without in-hospital mortality. HR, hazard ratio; ICH, intracerebral haemorrhage.
Balancing haemorrhagic and thromboembolic complications according to timing of re-initiated TA, there was a significant association with an increased hazard ratio for the composite outcome until 5 days after incident ICH (HR 2.51, 95% CI 1.10–5.70; P = 0.03), whereas TA started ≥6 days after ICH until hospital discharge was no longer significantly related to the composite of haemorrhagic and thromboembolic complications (HR 1.15, 95% CI 0.39–3.38; P = 0.80; Figure 3B). To investigate the clinical significance of this association we compared patients started on TA ≥6 days after ICH to patients with earlier TA (Figure 3C). This analysis demonstrated a trend towards a reduced discharge mortality [mortality: 2/30 (6.7%) vs. 10/39 (25.6%); P = 0.055]. No difference was observed regarding functional outcome [mRS = 0–3: 7/30 (23.3%) vs. 9/39 (23.0%); P = 1].
Influence of therapeutic anticoagulation on mortality and functional outcome
Functional outcome and mortality at discharge and 3 months is provided in Supplementary material online, Figure S2. There was no significant difference, neither in mortality nor in the proportion of patients achieving a favourable functional outcome, among patients who received vs. who did not receive TA at the time of hospital discharge and 90 days after ICH (mortality at discharge: P = 0.25, at 3 months: P = 1; favourable functional outcome at discharge: P = 0.92, at 3 months: P = 0.81, Supplementary material online, Figure S2).
Sub-analyses according to valve position
The comparison of patients with aortic valve vs. patients with mitral valve prostheses, or with both aortic and mitral valve prostheses, revealed no significant differences in the proportion of patients with haemorrhagic complications [aortic valve: 13/90 (14.4%) vs. mitral valve or both: 8/47 (17.0%); P = 0.69], thromboembolic complications [aortic valve: 6/90 (6.7%) vs. mitral valve or both: 2/47 (4.2%); P = 0.71], or the composite endpoint (see Supplementary material online, Table S8). Analyses of patients with different valve position according to treatment exposure (see Supplementary material online, Table S9) did also not provide significant differences; i.e. thromboembolic complications without TA compared between valve positions [aortic valve: 6/51 (11.7%) vs. mitral valve or both: 1/20 (5.0%); P = 0.66; CML incidence rate ratio 2.65, 95% CI 0.39–61.34; P = 0.39].
Sub-analyses of mechanical heart valve patients with atrial fibrillation vs. sinus rhythm
Analyses of MHV-patients in sinus rhythm vs. atrial fibrillation (see Supplementary material online, Table S10) showed that MHV patients with concomitant atrial fibrillation were at increased risk for both haemorrhagic (CML incidence rate ratio 0.38, 95% CI 0.16–0.96; P = 0.04) and thromboembolic complications (CML incidence rate ratio 0.29, 95% CI 0.07–1.29; P = 0.09). Thus, the composite outcome was significantly increased in MHV-patients with atrial fibrillation [sinus rhythm: 16/99 (16.1%) vs. atrial fibrillation: 13/38 (34.2%); P = 0.02]. Evaluating the associations of heart rhythm and treatment exposure (see Supplementary material online, Table S11); comparing patients with sinus rhythm vs. atrial fibrillation restarted on TA showed no difference regarding haemorrhagic complications (CML incidence rate ratio 0.54, 95% CI 0.20–1.58; P = 0.24). Patients with concomitant atrial fibrillation not restarted on TA during hospital stay showed an increased incidence for thromboembolic complications (1.53, 95% CI 0.41–3.91 vs. 0.33, 95% CI 0.07–0.97) resulting in an CML incidence rate ratio (0.22, 95% CI 0.04–1.05; P = 0.06). Therefore, the composite outcome in MHV-patients with atrial fibrillation not restarted on TA was significantly increased [sinus rhythm: 4/50 (8.0%) vs. atrial fibrillation: 7/21 (33.3%); P = 0.01].
Discussion
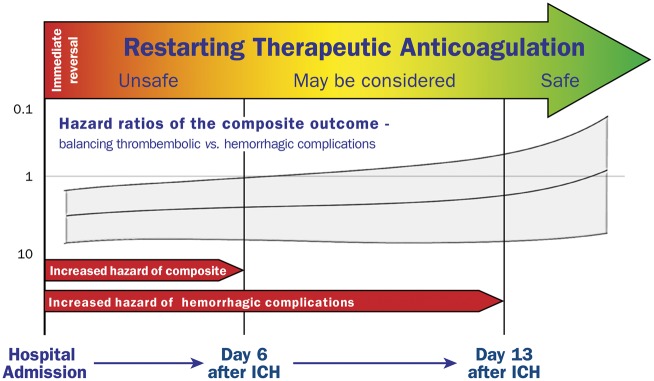
To our knowledge this study represents the largest analysis of patients with acute parenchymal ICH related to the intake of vitamin-K-antagonists in the presence of MHV. Regarding reversal management, our results showed that sufficient INR-reversal with PCC was associated with decreased haematoma enlargement without increasing thromboembolic events. Analyses of anticoagulation strategies demonstrated that early re-initiation of TA was associated with increased rates of haemorrhagic complications until 13 days after initial ICH and with respect to safety should not be routinely restarted before 14 days. Thromboembolic complications occurred notably in patients without TA, however at a significantly lower incidence rate than haemorrhagic complications under TA. Therefore, concerning the composite of haemorrhagic and thromboembolic complications, our findings suggest that TA should not be reinitiated earlier than 6 days after initial ICH and may be considered thereafter only in high-risk patients to optimally balance between least haemorrhagic and thromboembolic complications (see take home figure). Several aspects need discussion.
Take home figure.
In-hospital management of therapeutic anticoagulation in patients with ICH and MHV – from reversal until restarting therapy.
First, regarding reversal management, in contrast to primary ICH haematoma enlargement is known to occur more frequently and protracted in OAC-ICH.28,29 This may be explained by the altered coagulation not completely or not sustainably normalized after initial reversal treatment.28,29 As recently demonstrated, elevated INR levels need to be rapidly and fully reversed using PCC to stabilize the haematoma.5,6 The present analysis verified that patients not completely reversed were at higher risks to experience haematoma enlargement. This aspect is of specific relevance as clinically less severely affected patients underwent less aggressive reversal management which—contrary to the intended effect—resulted in poorer outcome. In addition, we here demonstrated that completely reversed patients were not at risk for increased ischaemic complications, which argues in favour of an immediate and complete anticoagulation reversal in all OAC-ICH patients—including those with MHV.5,6
Second, regarding anticoagulation resumption, what seems to be causative for the observed high incidence rates (>3.5% per day) of haemorrhagic complications in patients with early TA and OAC-ICH? In OAC-ICH the stability of the haematoma may be less definitive, which is why early anticoagulation may counterbalance the intracranial tamponade effect leading to an increased rate of delayed intracerebral bleeding complications.30 In addition, meta-analyses and large trials in ischaemic stroke patients have previously established that heparin at dosages greater than 5000 IU twice daily cause an overall increase of haemorrhagic complications within the first 14 days.31,32 Moreover, in the updated Cochrane review which integrates data of over 20 000 ischaemic stroke patients early anticoagulation (<14 days) compared with aspirin, showed a greater risk for both extracranial and intracranial haemorrhages.31 These aspects highlight the negative safety profile of early anticoagulation even in ischaemic stroke patients.
The risk of thromboembolic complications in MHV-patients is feared and intuitively considered higher than what is actually known from previous investigations why treating physicians tend to restart anticoagulation early.7,15,33–35 The acute risk for valve thrombosis or thromboembolism even in timely anticoagulated patients amounts up to 1% per day for valve thrombosis and 0.2% per day for systemic thromboembolisms including ischaemic stroke and is greatest immediately after surgical implantation.36,37 In line with these data, we here noticed a thromboembolism rate of 0.2% per day in anticoagulated patients, and in patients without anticoagulation there was a rather modestly increased rate of 0.6% per day. Now, comparing incidence rates of haemorrhagic complications we documented a 10 times higher hazard with early anticoagulation. Hypothetically translated, this would result in a number needed to harm of 31 patients per day to experience a haemorrhagic complication if anticoagulated vs. a number need to harm of 256 patients per day to experience a thromboembolic complications if not anticoagulated. Hence, these findings argue against aggressive early anticoagulation and it seems acceptable to withhold that treatment in the acute phase of ICH in order to avoid severe bleeding complications.
Current investigations regarding the optimal time-point of OAC-resumption focused on ICH patients with atrial fibrillation, and although there are various observational studies and meta-analyses showing a benefit for OAC-re-initiation on the long-term, it is still uncertain when to restart OAC.5,38–40 A recent large-sized registry study suggested an optimal time-point for resumption of 7–8 weeks in patients with atrial fibrillation.40 This timeframe however appears not to meet the specific demands of MHV-patients. In this special subset of ICH patients no larger study and no treatment recommendation, neither in cardiologic nor neurological international guidelines, exists.8,10,11,41 A recent short report suggested an optimal time frame for anticoagulation resumption between 7 and 10 days after ICH, but needs to be interpreted with caution as this retrospective clinical database query (1996–2011) did not address ICH characteristics, reversal management (era before PCC availability in USA), and specific modes of anticoagulation strategies further lacking statistical adjustments for confounding and bias.42 Contrary to OAC resumption in ICH-patients with atrial fibrillation (NCT02830152, NCT02565693), it seems very unlikely that randomized trials for MHV-patients in the short or long-term future will be conducted. This multicentre nation-wide study provides the most robust basis to date facilitating routine management and further research in this field. We suggest to reinitiate TA not before Day 6 after ICH, however to restrict this treatment strategy to high-risk MHV-patients (e.g. MHV-patients with concomitant atrial fibrillation, mitral valve prosthesis, or cage-ball prosthesis, etc.). In general, given that the hazard ratio for both complications (composite outcome), similar to the significance level for haemorrhagic complications only, remained in disfavour of early anticoagulation until Day 15, we suggest restarting anticoagulation not before 2 weeks to balance between thromboembolic and haemorrhagic complications in the gross of MHV-patients.
Several limitations need to be discussed. First, the observational and retrospective nature infers the potential for bias by indication and ICH-severity. Blinding or randomization was not feasible and may have led to a favoured assignment of TA in patients with a more favourable prognosis. Routine clinical management was executed at each individual centre, yet equally timed and standardized diagnostics (cranial or cardiac computed tomography, or cardiac sonography, etc.) were not conducted possibly underscoring evaluated outcomes. Yet, due to this apparent limitation in relation to study design, we focused specifically on safety as primary outcome and believe that false negative attribution of major haemorrhagic complications remains rather low. Although sophisticated statistical tools have been applied aimed at controlling confounding, centre effects cannot be fully excluded. Further, the number of MHV-patients and outcome events may have been too small to detect minor statistical differences in sub-analyses to fully refute type two errors.
Conclusion
In summary, restarting TA within 2 weeks after ICH in patients with MHV was associated with increased haemorrhagic complications. Optimal weighing—between least risks for thromboembolic and haemorrhagic complications—provided an earliest starting point of TA at Day 6, reserved only for patients at high thromboembolic risk.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We would like to thank Antje Milker, MD and Petra Burkhard, PhD, (University of Erlangen, Germany) for helping with logistics and data monitoring. We thank the following collaborators from sites (Departments of Neurology) across Germany for helping with data acquisition in the context of RETRACE 1: Joachim Hüwel (Dr Horst Schmidt Klinikum Wiesbaden), Christoph Terborg and Frank Trostdorf (Asklepios Klinik St. Georg, Hamburg), Tobias Neumann-Haefelin and Andras E. Racs (Hospital Fulda).
Funding
Johannes & Frieda-Marohn Foundation (FWN/Zo-Hutt/2011) and ELAN fonds (ELAN 12.01.04.1), University of Erlangen, Germany. The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The contributing equally first authors had full access to all the data of the study and the corresponding author had final responsibility for the decision to submit for publication.
Conflict of interest: Dr Kuramatsu reports grants from Covidien (Medtronic), personal fees from Bayer, personal fees from Pfizer, personal fees from Sanofi, outside the submitted work; Dr Endres reports grants and other from Bayer, other from Boehringer Ingelheim, other from BMS/Pfizer, other from Daiichi Sankyo, during the conduct of the study; grants from DFG, grants from BMBF, grants from EU, grants from Corona Foundation, grants from Fondation Leducq, other from Amgen, other from GSK, other from Novartis, other from Sanofi, other from Covidien, outside the submitted work; Dr Häusler reports grants and personal fees from Bayer, personal fees from Daiichi Sankyo, personal fees from BMS, personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Sanifi-Aventis, personal fees from Edwards Lifesciences, non-financial support from Getemed AG, personal fees from EIP Pharma, personal fees from Medtronic, outside the submitted work; Dr Sobesky reports personal fees from Bayer, personal fees from Pfizer/BMS, personal fees from daiichi, personal fees from boehringer, outside the submitted work; Dr Ringleb reports personal fees from Boehringer Ingelheim, personal fees from Bayer, personal fees from Daichii Sankyo, personal fees from Pfizer, outside the submitted work; Dr Purrucker reports personal fees from Boehringer Ingelheim, personal fees from Pfizer, outside the submitted work; Dr Rizos reports personal fees from BMS Pfizer, personal fees from Bayer Healthcare, personal fees from Daiichi Sankyo, personal fees from Boehringer Ingelheim, outside the submitted work; Dr Müllges reports personal fees from Boehringer Ingelheim, personal fees from Bayer Pharma, outside the submitted work; Dr Kraft reports personal fees from Bayer, personal fees from Boehringer-Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Pfizer/Bristol-Myers Squibb, outside the submitted work; Dr Erbguth reports grants and personal fees from Boehringer Ingelheim, personal fees from Bayer Pharma, personal fees from Pfizer Pharma, personal fees from Bristol-Myers Squibb, personal fees from Daiichi Sankyo, outside the submitted work; Dr Nueckel reports personal fees from Speaker's fee Pfizer/BMS, personal fees from Speaker's fee Boehringer Ingelheim, outside the submitted work; Dr Schellinger reports personal fees from Boehringer Ingelheim, personal fees from Bayer, personal fees from BMS/Pfizer, personal fees from Daiichi, personal fees from Medtronic, outside the submitted work; Dr Glahn reports personal fees from Pfizer, outside the submitted work; Dr Knappe reports other from Daiichi-Sankyo, other from Bayer, outside the submitted work; Dr Fink reports personal fees from Bayer, personal fees from Boehringer, outside the submitted work; Dr Minnerup reports personal fees from Boerhinger Ingelheim, personal fees from Bayer Healthcare, outside the submitted work; Dr Neugebauer reports personal fees from Boehringer Ingelheim, personal fees from Daiichi, outside the submitted work; Dr Roether reports personal fees from Bayer, personal fees from Boehringer, personal fees from Pfizer, personal fees from Bristol Myers Squibb, outside the submitted work; Dr Reimann reports personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from Bayer, grants from Daiichi, outside the submitted work; Dr Bäzner reports personal fees from Honoraria for lectures from Bayer Vital, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, outside the submitted work; Dr Schwert reports grants from Bayer Health Care, outside the submitted work; Dr Palm reports personal fees from Pfizer/BMS, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, outside the submitted work; Dr We reports personal fees from Boehringer Ingelheim Pharma GmbH&Co.KG, personal fees from Daiichi Sankyo Pharma GmbH, outside the submitted work; Dr Günther reports personal fees from Daiichi Sankyo, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Bristol-Myers Squibb/Pfizer, outside the submitted work; Dr Hamann reports participation in the Respect-ESUS trial. Dr Schwab reports personal fees from Boehringer Ingelheim, grants from Daiichi, outside the submitted work; Dr Huttner reports personal fees from Boehringer Ingelheim, grants from Medtronic, personal fees from Daiichi Sankyo, and Novartis, outside the submitted work.
References
- 1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 2. Head SJ, Celik M, Kappetein AP.. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183–2191. [DOI] [PubMed] [Google Scholar]
- 3. Iung B, Rodes-Cabau J.. The optimal management of anti-thrombotic therapy after valve replacement: certainties and uncertainties. Eur Heart J 2014;35:2942–2949. [DOI] [PubMed] [Google Scholar]
- 4. Alfieri O, Vahanian A.. The year in cardiology 2016: valvular heart disease. Eur Heart J 2017;38:628–633. [DOI] [PubMed] [Google Scholar]
- 5. Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Juttler E, Grau A, Palm F, Rother J, Michels P, Hamann GF, Huwel J, Hagemann G, Barber B, Terborg C, Trostdorf F, Bazner H, Roth A, Wohrle J, Keller M, Schwarz M, Reimann G, Volkmann J, Mullges W, Kraft P, Classen J, Hobohm C, Horn M, Milewski A, Reichmann H, Schneider H, Schimmel E, Fink GR, Dohmen C, Stetefeld H, Witte O, Gunther A, Neumann-Haefelin T, Racs AE, Nueckel M, Erbguth F, Kloska SP, Dorfler A, Kohrmann M, Schwab S, Huttner HB.. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824–836. [DOI] [PubMed] [Google Scholar]
- 6. Steiner T, Poli S, Griebe M, Husing J, Hajda J, Freiberger A, Bendszus M, Bosel J, Christensen H, Dohmen C, Hennerici M, Kollmer J, Stetefeld H, Wartenberg KE, Weimar C, Hacke W, Veltkamp R.. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016;15:566–573. [DOI] [PubMed] [Google Scholar]
- 7. Sun JC, Davidson MJ, Lamy A, Eikelboom JW.. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet 2009;374:565–576. [DOI] [PubMed] [Google Scholar]
- 8. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M.. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 9. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A.. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 10. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJM, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M.. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014;9:840–855. [DOI] [PubMed] [Google Scholar]
- 11. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 12. Passaglia LG, de Barros GM, de Sousa MR.. Early postoperative bridging anticoagulation after mechanical heart valve replacement: a systematic review and meta-analysis. J Thromb Haemost 2015;13:1557–1567. [DOI] [PubMed] [Google Scholar]
- 13. Halvorsen S, Storey RF, Rocca B, Sibbing D, Ten Berg J, Grove EL, Weiss TW, Collet JP, Andreotti F, Gulba DC, Lip GYH, Husted S, Vilahur G, Morais J, Verheugt FWA, Lanas A, Al-Shahi Salman R, Steg PG, Huber K; Thrombosis ESCWGo. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2017;38:1455–1462. [DOI] [PubMed] [Google Scholar]
- 14. Chandra D, Gupta A, Grover V, Kumar Gupta V.. When should you restart anticoagulation in patients who suffer an intracranial bleed who also have a prosthetic valve? Interact Cardiovasc Thorac Surg 2013;16:520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AlKherayf F, Xu Y, Gandara E, Westwick H, Moldovan ID, Wells PS.. Timing of vitamin K antagonist re-initiation following intracranial hemorrhage in mechanical heart valves: systematic review and meta-analysis. Thromb Res 2016;144:152–157. [DOI] [PubMed] [Google Scholar]
- 16. Sembill JA, Gerner ST, Volbers B, Bobinger T, Lucking H, Kloska SP, Schwab S, Huttner HB, Kuramatsu JB.. Severity assessment in maximally treated ICH patients: the max-ICH score. Neurology 2017;89:423–431. [DOI] [PubMed] [Google Scholar]
- 17. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, Rami T, Israr S, Fontana G, de Knegt M, Fuchs A, Lyden P, Trento A, Bhatt DL, Leon MB, Makkar RR, Ramzy D, Cheng W, Siegel RJ, Thomson LM, Mangat G, Hariri B, Sawaya FJ, Iversen HK; RESOLVE, SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383–2392. [DOI] [PubMed] [Google Scholar]
- 18. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC, Tuhrim S.. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 19. Huttner HB, Steiner T, Hartmann M, Kohrmann M, Juettler E, Mueller S, Wikner J, Meyding-Lamade U, Schramm P, Schwab S, Schellinger PD.. Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke 2006;37:404–408. [DOI] [PubMed] [Google Scholar]
- 20. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J.. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5. [DOI] [PubMed] [Google Scholar]
- 21. Sherman DG, Albers GW, Bladin C, Fieschi C, Gabbai AA, Kase CS, O'Riordan W, Pineo GF; PREVAIL Investigators. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet 2007;369:1347–1355. [DOI] [PubMed] [Google Scholar]
- 22. Meurin P, Tabet JY, Weber H, Renaud N, Ben Driss A.. Low-molecular-weight heparin as a bridging anticoagulant early after mechanical heart valve replacement. Circulation 2006;113:564–569. [DOI] [PubMed] [Google Scholar]
- 23. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB.. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 24. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- 25. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–3069, 3069a-3069k. [DOI] [PubMed] [Google Scholar]
- 26. Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, Dawson J, Gandhi D, Ullman N, Mould WA, Mayo SW, Mendelow AD, Gregson B, Butcher K, Vespa P, Wright DW, Kase CS, Carhuapoma JR, Keyl PM, Diener-West M, Muschelli J, Betz JF, Thompson CB, Sugar EA, Yenokyan G, Janis S, John S, Harnof S, Lopez GA, Aldrich EF, Harrigan MR, Ansari S, Jallo J, Caron JL, LeDoux D, Adeoye O, Zuccarello M, Adams HP Jr, Rosenblum M, Thompson RE, Awad IA; CLEAR III Investigators. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017;389:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S.. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21:69–80. [DOI] [PubMed] [Google Scholar]
- 28. Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J.. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 29. Flaherty ML, Tao H, Haverbusch M, Sekar P, Kleindorfer D, Kissela B, Khatri P, Stettler B, Adeoye O, Moomaw CJ, Broderick JP, Woo D.. Warfarin use leads to larger intracerebral hematomas. Neurology 2008;71:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qureshi AI, Mendelow AD, Hanley DF.. Intracerebral haemorrhage. Lancet 2009;373:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandercock PA, Counsell C, Kane EJ.. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev 2015;CD000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–1581. [PubMed] [Google Scholar]
- 33. Crawley F, Bevan D, Wren D.. Management of intracranial bleeding associated with anticoagulation: balancing the risk of further bleeding against thromboembolism from prosthetic heart valves. J Neurol Neurosurg Psychiatry 2000;69:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. AlKherayf F, Xu Y, Westwick H, Moldovan ID, Wells PS.. Timing of anticoagulant re-initiation following intracerebral hemorrhage in mechanical heart valves: survey of neurosurgeons and thrombosis experts. Clin Neurol Neurosurg 2017;154:23–27. [DOI] [PubMed] [Google Scholar]
- 35. Cannegieter SC, Torn M, Rosendaal FR.. Oral anticoagulant treatment in patients with mechanical heart valves: how to reduce the risk of thromboembolic and bleeding complications. J Intern Med 1999;245:369–374. [DOI] [PubMed] [Google Scholar]
- 36. Le Tourneau T, Lim V, Inamo J, Miller FA, Mahoney DW, Schaff HV, Enriquez-Sarano M.. Achieved anticoagulation vs prosthesis selection for mitral mechanical valve replacement: a population-based outcome study. Chest 2009;136:1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laplace G, Lafitte S, Labeque JN, Perron JM, Baudet E, Deville C, Roques X, Roudaut R.. Clinical significance of early thrombosis after prosthetic mitral valve replacement: a postoperative monocentric study of 680 patients. J Am Coll Cardiol 2004;43:1283–1290. [DOI] [PubMed] [Google Scholar]
- 38. Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, Ayres AM, Elm J, Gurol ME, Greenberg SM, Viswanathan A, Anderson CD, Schwab S, Rosand J, Testai FD, Woo D, Huttner HB, Sheth KN.. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol 2017;82:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Korompoki E, Filippidis FT, Nielsen PB, Del Giudice A, Lip GYH, Kuramatsu JB, Huttner HB, Fang J, Schulman S, Marti-Fabregas J, Gathier CS, Viswanathan A, Biffi A, Poli D, Weimar C, Malzahn U, Heuschmann P, Veltkamp R.. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology 2017;89:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pennlert J, Overholser R, Asplund K, Carlberg B, Van Rompaye B, Wiklund PG, Eriksson M.. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 41. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 42. Flint AC, Lingamneni R, Rao VA, Chan SL, Ren X, Pombra J, Hemphill JC 3rd, Bonow RO.. Risks of thrombosis and rehemorrhage during early management of intracranial hemorrhage in patients with mechanical heart valves. J Am Coll Cardiol 2015;66:1738–1739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.