Abstract
Occlusive vasculopathy due to the development and accumulation of granulomas at the level of intima of large vessels, as well as mediastinal lymph nodes and fibrosing mediastinitis secondary to sarcoidosis, causing extrinsic compression of mediastinal vascular structure are uncommon mechanisms of sarcoidosis-associated pulmonary hypertension. We present a case of a 62-year-old woman with a rare manifestation of sarcoidosis, which was misclassified and treated as chronic thromboembolic pulmonary hypertension for a long period. Fluorine-18-fluorodeoxyglucose positron emission tomography played a major role in accessing final diagnosis. Mechanisms that lead to development of pulmonary hypertension, the contribution of novel imaging modalities, and treatment options are discussed.
Keywords: sarcoidosis, pulmonary hypertension, fibrosing mediastinitis
Case description
A 62-year-old Caucasian woman with a previous diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) was admitted to our hospital for decompensation of right heart failure. She reported progressive exertional dyspnea in the past few months before admission and was in World Health Organization (WHO) functional class (FC) III. Physical examination revealed accentuated pulmonary component of the second heart sound, bilateral peripheral edema, and elevated jugular venous pressure. The patient denied chest pain, palpitations, and syncopal episodes. Past medical history included hypothyroidism, Meniere’s disease, and duodenal ulcer. Social and family history were unremarkable.
Seventeen years ago, the patient presented with dyspnea and fatigue. A lung ventilation/perfusion scan was performed and revealed zero uptake of radioactive material in the right upper lobe. There was also decreased perfusion in the following regions: middle lobe; anterior basal segment of right inferior lobe and apical segment of left superior lobe. Invasive pulmonary angiography findings were total occlusion of right superior trunk, as well as a linear occlusion of the right lower lobe pulmonary artery. Based on these findings, the diagnosis of chronic thromboembolic disease was established and the patient was put on anticoagulation therapy. Subsequently, she underwent balloon pulmonary angioplasty of the right lower lobe pulmonary artery. Three weeks later a stent was positioned due to restenosis. She remained relatively stable for a few years, until the time her clinical condition gradually deteriorated. Right heart catheterization (RHC) and pulmonary angiography were repeated. RHC showed mean pulmonary arterial pressure (mPAP) of 53 mmHg, right atrial pressure of 5 mmHg, pulmonary capillary wedge pressure (PCWP) of 8 mmHg, a cardiac index of 4.3 L/min−1, and a pulmonary vascular resistance (PVR) of 6.2 Wood Units. Pulmonary angiography revealed no pulmonary blood flow to the right upper lobe. The stent of the right lower lobe pulmonary artery remained patent. There was also a reduction in pulmonary blood flow due to a stenosis in the lateral basal segmental artery of the left pulmonary artery.
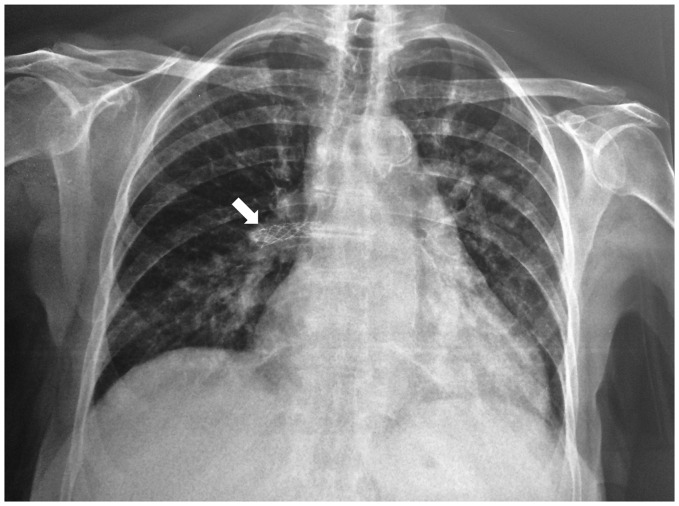
While hospitalized in the cardiology department, the patient was re-assessed and cardiopulmonary function was thoroughly re-evaluated with the prospect of referring her for pulmonary thromboendarterectomy. Laboratory tests revealed normal complete blood cell counts, electrolyte levels, liver function tests, and an elevated NT-proBNP value of 437 pg/mL. ECG on admission showed sinus rhythm and right ventricular hypertrophy. She achieved 450 m on 6-min walking test (6MWT). Findings of the chest radiograph included central pulmonary arterial dilatation and increased cardiothoracic ratio (Fig. 1). Pulmonary function tests revealed a mild restrictive ventilator defect with a forced expiratory volume in 1 s (FEV1) of 81% predicted, forced vital capacity (FVC) of 84% predicted, total lung capacity (TLC) of 78% predicted, and a reduced diffusing capacity of the lung for carbon monoxide of 51% predicted. Transthoracic echocardiography showed mild dilatation of the right ventricle with mildly reduced right ventricular systolic function. Tricuspid annular plane systolic excursion was 18 mm, S’ was 9 cm/s, fractional area change was 30%, and right ventricle free wall longitudinal strain was –20%. Right ventricular outflow acceleration time was suggestive of pulmonary hypertension (PH) and peak tricuspid regurgitation velocity was 3.8 m/s.
Fig. 1.
Chest radiograph of the patient showing central pulmonary arterial dilatation, increased cardiothoracic index, and loss of peripheral blood vessels in the right upper lobe. The stent of the right lower lobe pulmonary artery is prominent (arrow).
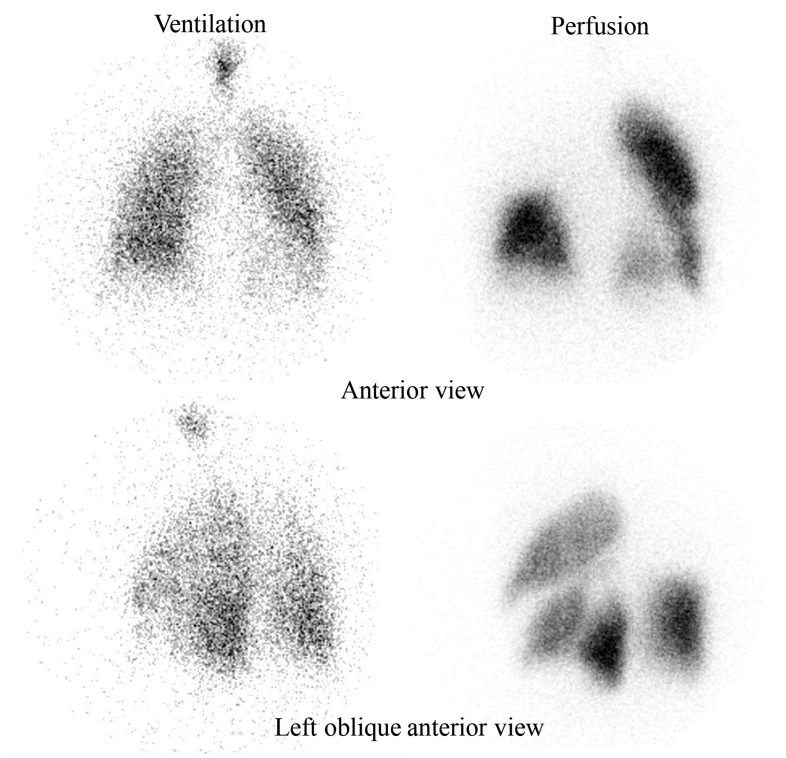
A new ventilation/perfusion scan was performed, which showed total absence of perfusion of the right upper lobe and absence of perfusion in the following regions: anteromedial basal segment of lower left lobe and superior basal segment of left lower lobe (Fig. 2); while contrast computed tomography (CT) angiography of the pulmonary artery showed dilatation of pulmonary artery trunk, complete obstruction of the right superior branch, and stenosis of the left lower lobe artery by tissue surrounding the vessel with poststenotic dilatation. High-resolution CT revealed lymph nodes and soft tissue in the mediastinum while there were no signs of pulmonary fibrosis. RHC, at that point, showed a mPAP of 44 mmHg, right atrial pressure of 9 mmHg, PCWP of 9 mmHg, PVR of 4.8 Wood Units, and a cardiac index of 4.2 L/min–1.
Fig. 2.
Ventilation/perfusion scan showing total absence of perfusion of the right upper lobe and absence of perfusion in anteromedial basal segment of lower left lobe and superior basal segment of left lower lobe.
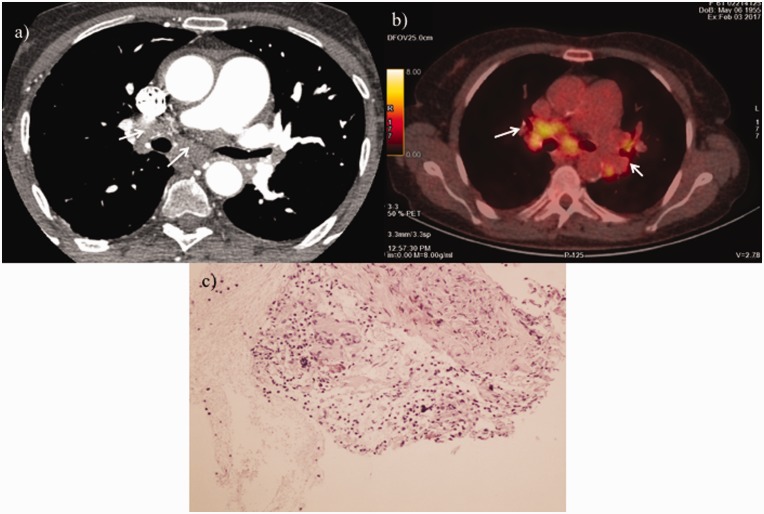
At that time, CTEPH was the most likely diagnosis and the patient was classified by the local surgical team as inoperable; thus, she was initiated on riociguat treatment. A second surgical opinion was acquired by another experienced surgical team, which evaluated the findings and suggested an alternate diagnosis. They suggested that the presence of mediastinal lymph nodes with increased bronchial artery perfusion without intraluminal filling defects in pulmonary artery branches—with the exception of right upper lobe artery—combined with the formation of soft tissue surrounding the ascending aorta and the left lower lobe artery (Fig. 3a) were not compatible with CTEPH diagnosis and indicated possible fibrosing mediastinitis. Vasculitis of the pulmonary artery and sarcoidosis were also included in the differential diagnosis. Of note, the patient reported no symptoms suggestive of large vessels vasculitis such as fever, fatigue, jaw, or upper limb claudication. There was also no history of persistent cough, recurrent chest infections, or hemoptysis. C-reactive protein and erythrocyte sedimentation rate were normal, anti-neutrophil cytoplasmic antibodies were negative, and anti-nuclear antibodies were positive with titre 1/640. Angiotensin converting enzyme was moderately elevated (109 iu/L with a normal range of 8–52). Fluorine-18-fluorodeoxyglucose positron emission tomography (FDG PET/CT) confirmed the presence of active lymph nodes in the mediastinum, lung hilum, and abdomen (Fig. 3b).
Fig. 3.
(a) Contrast-enhanced high-resolution CT of the chest showing lymph nodes and soft tissue in mediastinum (white arrows). (b) FDG-PET/CT axial slice at diagnosis showing active lymph nodes in mediastinum and lung hilum (white arrows). (c) Non-necrotizing granulomas consisting of epithelioid histiocytes, admixed with background of reactive lymphocytes and scattered plasma cells.
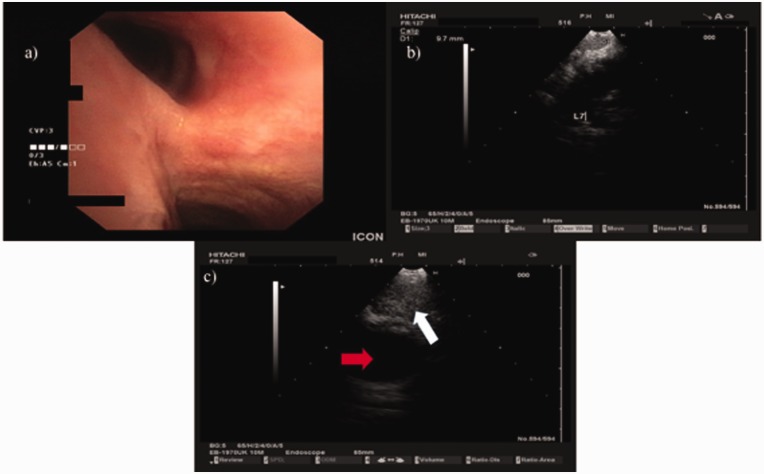
Endobronchial ultrasound-guided lymph node biopsy (Fig. 4) was performed and lymph node histology revealed multiple lymphocytes and epithelioid cells both scattered and forming granulomas establishing the diagnosis of stage I sarcoidosis, with mediastinal lymph node enlargement and absence of parenchymal lung disease (Fig. 3c). Cardiac MRI with gadolinium was also performed and excluded cardiac involvement. Upon establishment of sarcoidosis diagnosis, discontinuation of riociguat was decided and treatment with prednisolone 1 mg/kg/day and azathioprine 50 mg b.i.d. was initiated. The patient was re-evaluated six months after corticosteroid therapy and reported mild relief of symptoms. Hemodynamic assessment was performed and revealed a reduction in mPAP (mPAP was 34 mmHg), a lower mean right atrial pressure of 2 mmHg, and a decrease in cardiac output which was 4L/min–1 as well as an increase in PVR that was now 8 Wood Units. This hemodynamic change was attributed to the discontinuation of pulmonary vasodilator therapy. A new CT scan showed a decrease in the size of the mediastinal lymph nodes (Fig. 5).
Fig. 4.
EBUS procedure. The patient was intubated with a STORZ rigid bronchoscope 12-mm outer rim and 11-mm inner rim diameter. The Pentax EB-1970UK endoscope was inserted through the working channel and multiple biopsies were taken from lymph node stations 7 (subcarinal) and 4 right with a 22-G EBUS needle. No endobrochial lesion was observed. (a) Camera of EB-1970UK EBUS showing the carina. (b) Ultrasound picture of EB-1970UK EBUS from EUB-6500HV Hitachi ultrasound source showing lymph node station 7 (subcarinal). (c) Ultrasound picture of EB-1970UK EBUS from EUB-6500HV Hitachi ultrasound source showing lymph node station 4 right, red arrow showing the superior vena cava and white arrow showing lymph node 4 right.
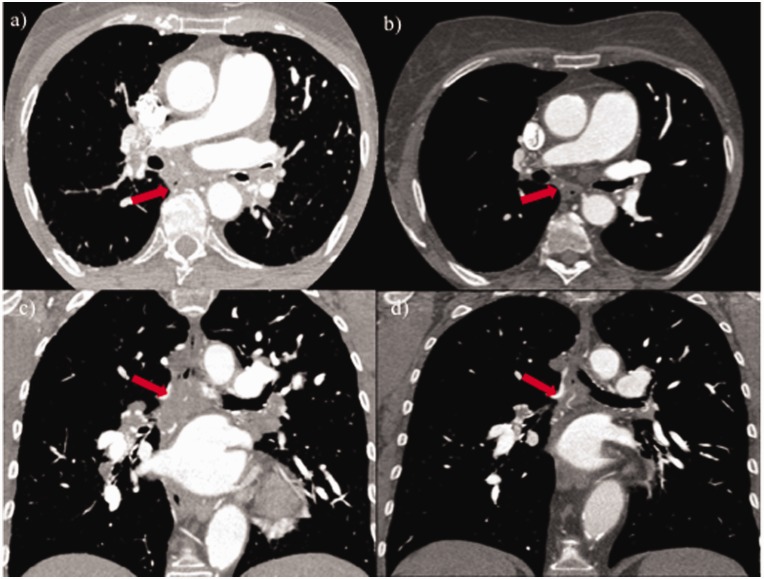
Fig. 5.
Axial and coronal slices of high-resolution CT of the chest before (a, c) and six months after (b, d) corticosteroid therapy showing improvement and shrinking of nodular infiltrates (red arrows).
Discussion
Several mechanisms may lead to the development of precapillary PH in sarcoidosis, the most common being pulmonary fibrosis. However, Hunes et al. reported that invasion of vessel walls by granulomas, often combined with intimal fibrosis, medial proliferation, and inflammatory changes is another phenotype of sarcoidosis-associated PH, often in the absence of pulmonary fibrosis.1 Granulomatous fibrosing mediastinitis and presence of mediastinal lymph nodes leading to extrinsic compression of pulmonary arteries have been described as additional possible mechanisms leading to PH.2,3 Similar to what occurred in our patient, these cases may be often misclassified as CTEPH.4 Radiology findings such as segmental ventilation/perfusion mismatch may also be present in this clinical situation and high-resolution CT as well as pulmonary angiography are necessary for establishing the right diagnosis.5,6 Obviously FDG-PET/CT played a major role in accessing the final diagnosis emphasizing the utility of novel imaging modalities in the diagnostic workout of such complicated cases. For example, in our case, FDG-PET/CT scan ruled out the possibility of isolated pulmonary vasculitis and indicated the specific area for ultrasound-guided biopsy.7 Additionally, FDG-PET/CT has been suggested as a useful tool for assessing the inflammatory activity of sarcoidosis, especially in less common cases of extra-pulmonary involvement,8 although in our patient no other organ damage was found. Given the complexity of such cases, it is pivotal that, when controversial findings are present, a second opinion by an expert center, with a multi-professional team, should be obtained.
Only two cases with sarcoidosis-associated fibrosing mediastinitis and PH have been reported so far.7,9 However, our patient has specific characteristics that differentiate her from the previous cases. First, she was diagnosed with CTEPH several years ago and she was relatively stable for a long period of time. This observation may underline the benign nature of her disease on both pulmonary vascular and systemic grounds. It is also worth noting that her sarcoidosis remained silent for many years and, apart from the slow evolution of the disease, no major parenchymal pulmonary or other organ disease was developed.
As far as treatment options with pulmonary vasodilators are concerned, sildenafil, bosentan, and epoprostenol may have a beneficial effect on WHO FC, exercise capacity, and hemodynamics in these patients.10 Tadalafil failed to show improvement in 6MWT distance at 24 months in a small number of patients with sarcoidosis-associated PH.11 Bonham et al. showed that the use of intravenous prostacyclin therapy and oral vasodilators led to clinical and hemodynamic improvement in their cohort.12 However, until today, PH-specific agents have not been established as a treatment option for sarcoidosis-associated PH. In addition, endovascular stent placement has been proposed and has resulted in improvement in highly selected patients although there is an increased mortality risk with these procedures.13,14 Condado et al. reported a case series of three out of five patients with pulmonary vascular compression due to sarcoidosis, who underwent pulmonary artery or vein angioplasty and stenting, resulting in improvement of symptoms. While this procedure may provide clinical benefit in selected patients, stenting of the pulmonary vascular tree is not routinely recommended in sarcoidosis-associated PH or CTEPH by current guidelines.16 The use of systemic corticosteroids in extrinsic compression of pulmonary arteries has not been well established, although it has been shown to relieve symptoms and lead to regression of mediastinal soft tissue and reduction of pulmonary artery pressure in a few cases.6,7,9 Boucly et al. reported that two patients in their registry, with hyperactive mediastinal lymph nodes causing extrinsic compression of pulmonary arteries, responded to immunosuppressive therapy, highlighting the possible reversibility of the inflammatory process.17
In conclusion, extrinsic compression of pulmonary arteries by mediastinal lymph nodes, as a complication of sarcoidosis, is a rare clinical entity and mechanism for sarcoidosis-associated PH.18 This phenotype of sarcoidosis may lead to misclassification of PH under CTEPH, with all the consequences that this may have on the management of these patients. The interesting aspect in our case was that there was a change in the original diagnosis after several years of presumed chronic thromboembolic disease. This led to the modification of the administered therapy and hopefully to a better clinical outcome of the patient.
Conflict of interest
Authors indicate no conflict of interest related to the content of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006; 61: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schowengerdt CG, Suyemoto R, Main FB. Granulomatous and fibrous mediastinitis. A review and analysis of 180 cases. J Thorac Cardiovasc Surg 1969; 57: 365–379. [PubMed] [Google Scholar]
- 3.Damuth TE, Bower JS, Cho K, et al. Major pulmonary artery stenosis causing pulmonary hypertension. Chest 1980; 78: 888–891. [DOI] [PubMed] [Google Scholar]
- 4.Huitema MP, Grutters JC, Rensing BJ, et al. Pulmonary hypertension complicating pulmonary sarcoidosis. Neth Heart J 2016; 24: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K, Ohno S, Takada M, et al. Sarcoidosis complicated with major pulmonary artery obstruction and stenosis. Intern Med 2012; 51: 2775–2780. [DOI] [PubMed] [Google Scholar]
- 6.Toonkel RL, Borczuk AC, Pearson GD, et al. Sarcoidosis-associated fibrosing mediastinitis with resultant pulmonary hypertension: a case report and review of the literature. Respiration 2010; 79: 341–345. [DOI] [PubMed] [Google Scholar]
- 7.Riancho-Zarrabelita L, Zurbano F, Gomez-Roman J, et al. Isolated pulmonary vasculitis: case report and literature review. Semin Arthritis Rheum 2015; 44: 514–517. [DOI] [PubMed] [Google Scholar]
- 8.Robin P, Benigni P, Feger B, et al. An atypical sarcoidosis involvement in FDG PET/CT: A case report. Medicine (Baltimore) 2016; 95: e5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yangui F, Battesti JP, Valeyre D, et al. Fibrosing mediastinitis as a rare mechanism of pulmonary oedema in sarcoidosis. Eur Respir J 2010; 35: 455–456. [DOI] [PubMed] [Google Scholar]
- 10.Barnett CF, Bonura EJ, Nathan SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest 2009; 135: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford HJ, Baughman RP, Aris R, et al. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ 2016; 6: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonham CA, Oldham JM, Gomberg-Maitland M, et al. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest 2015; 148: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton-Craig CR, Slaughter R, McNeil K, et al. Improvement after angioplasty and stenting of pulmonary arteries due to sarcoid mediastinal fibrosis. Heart Lung Circ 2009; 18: 222–225. [DOI] [PubMed] [Google Scholar]
- 14.Doyle TP, Loyd JE, Robbins IM. Percutaneous pulmonary artery and vein stenting: a novel treatment for mediastinal fibrosis. Am J Respir Crit Care Med 2001; 164: 657–660. [DOI] [PubMed] [Google Scholar]
- 15.Condado JF, Babaliaros V, Henry TS, et al. Pulmonary stenting for the treatment of sarcoid induced pulmonary vascular stenosis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 281–287. [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 17.Boucly A, Cottin V, Nunes H, et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017; 50: 1700465. [DOI] [PubMed] [Google Scholar]
- 18.Seferian A, Steriade A, Jais X, et al. Pulmonary hypertension complicating fibrosing mediastinitis. Medicine (Baltimore) 2015; 94: e1800. [DOI] [PMC free article] [PubMed] [Google Scholar]