Abstract
A reduced number and/or reduced activity of natural killer (NK) cells, which are important for defense against a variety of cancers and viral infections, occur under various stress conditions and in patients with various diseases. In this article, we report that the 30% to 50% ethanol precipitate of oyster extract (EPOE50) dose-dependently enhanced the activity of mouse spleen NK cells in vitro and in vivo. The activity of EPOE50 was eluted with a molecular weight of about 2000 by gel filtration and was inactivated by periodate but not by proteinase K. The activity of highly purified NK cells was also augmented by EPOE50 but not by oligodeoxyribonucleotide 1585, which mimics bacterial DNA. Administration of EPOE50 to mice stimulated splenic NK cell activity without a change in splenic NK cell populations. Although the proliferation of B16 tumor cells in vitro was slightly stimulated by EPOE50, the growth of B16 melanoma in vivo was dose-dependently suppressed by administration of EPOE50. Taken together, our results indicate that EPOE50 augmented NK cell activity and that its administration to mice inhibited tumor growth presumably through the activation of NK cells and also suggest that the active substance is a sugar-containing oligomer or polymer and is not of bacterial origin.
Keywords: oyster, NK cell, activation, antitumor effect, melanoma
Introduction
Natural killer (NK) cells are part of the innate immune system and are important for defense against a variety of cancers and viral, bacterial, and parasitic infections.1-3 Evidence for important roles in immunity has been provided by animal models as well as patients deficient in NK cells.4 NK cells rapidly kill target cells without prior immunization or major histocompatibility complex restriction while leaving normal healthy cells unharmed. Their activation is regulated by a complex balance of activating and inhibitory signals. These signals are transmitted by activating receptors that bind ligands on tumors and pathogen-infected cells and by inhibitory receptors that bind class I major histocompatibility complex molecules.1,5 The latter receptors, which are dominant and prevent NK cell activation, explain self-tolerance and prevention of host cell killing. Other than surface receptors, cytokines, including interleukin (IL)-2, IL-12, IL-15, IL-18, and IL-21, have been shown to play a crucial role in activating NK cells and enhancing NK cytotoxicity against a tumor.1,6
Reduced NK cell number and/or reduced activity have been found to occur under various stress conditions and in patients with cancer, viral infection, chronic inflammation, and chronic fatigue syndrome.7-10 It is also known that some chemotherapy drugs and radiation therapy have side effects including suppression of NK cell activity.11-14 Such reduction removes the protective function of NK cells and would result in increased pathogenic viral infections and even cancer. Thus, it is important to restore the activity of NK cells for people who have low NK cell activity.
IL-2 has been the most commonly used cytokine for clinical trials to enhance the antitumor potential of NK cells. Systemic administration of IL-2 is now approved by the US Food and Drug Administration for use in malignant melanoma and metastatic renal cell carcinoma.15 However, since IL-2 perturbs complex regulatory pathways, serious side effects, including vascular leak syndrome, have been observed.15 Moreover, IL-2 also has a critical role in the generation and maintenance of regulatory T cells, which act to suppress a variety of immune responses.16 There is an obvious need for more specific NK cell modulators that lack wide-ranging side effects.
Foods have the advantage of being safe and easy to take. Some foods and their components have been reported to enhance NK cell activity. They include water-soluble extracts of Agaricus blazei Murill mushrooms, the lactic acid bacterium Lactobacillus plantarum HY7712, nucleotides, and vitamin E.17-21 We have investigated NK cell-stimulating activity in crude extracts of foods, especially vegetables and marine products. During our investigation using murine spleen cells in vitro, we found that an extract of oysters enhanced the cytotoxicity of NK cells. In this article, we show that the ethanol precipitate prepared from the extract of oysters potently augmented NK cell activity in spleen cells both in vitro and in vivo. We also describe the in vivo antitumor effect of the ethanol precipitate.
Materials and Methods
Reagents
RPMI-1640 medium, Phenol Red-free RPMI-1640 medium, propidium iodide, and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich, Inc (St Louis, MO). Calcein acetoxymethyl ester (calcein-AM) was purchased from Dojindo Laboratories (Kumamoto, Japan). Recombinant mouse IL-2 was obtained from Roche Applied Science (Indianapolis, IN). Oligodeoxynucleotide (ODN) 1585 and its negative control were purchased from InvivoGen (San Diego, CA). Mouse NK cell separation set-DM, mouse NK cell enrichment set-DM, and phycoerythrin (PE)-conjugated rat anti-mouse NK1.1, fluorescein isothiocyanate (FITC)-conjugated hamster anti-mouse CD3 ϵ chain, and rat anti-mouse CD16/CD32 monoclonal antibodies (mAbs) were obtained from BD Biosciences (San Jose, CA).
Preparation of Oyster Extracts and Ethanol Fractionation
One- or 2-year-old oysters, Crassostrea gigas, were obtained from a hanging culture bed in Seto Inland Sea of Hiroshima Prefecture and Okayama Prefecture, Japan. They were extracted with 1.5 volumes of water for 1.5 hours at 125°C and 2364 hPa, and the mixture was filtered. The filtrate was concentrated, sterilized, and spray-dried. The resulting powder (oyster extract [OE]) was dissolved in water and fractionated by graded precipitation at ethanol concentrations of 30% and 50% (v/v). The 50% ethanol supernatant was evaporated to dryness under reduced pressure. The 30% and 50% ethanol precipitates and the residue of 50% ethanol supernatant were then lyophilized, and the resulting powders were stored at 4°C. The powder weight percentages of the 30% ethanol precipitate, 50% ethanol precipitate, and 50% ethanol supernatant were 5.3 ± 0.3%, 2.6 ± 0.5%, and 92.1 ± 0.8%, respectively (mean ± SEM for 3 independent experiments). Since most of the NK cell-enhancing activity was retained in the 50% ethanol precipitate as described in the Results section (Figure 1A), the 50% ethanol precipitate, which is referred to as EPOE50, was used thereafter.
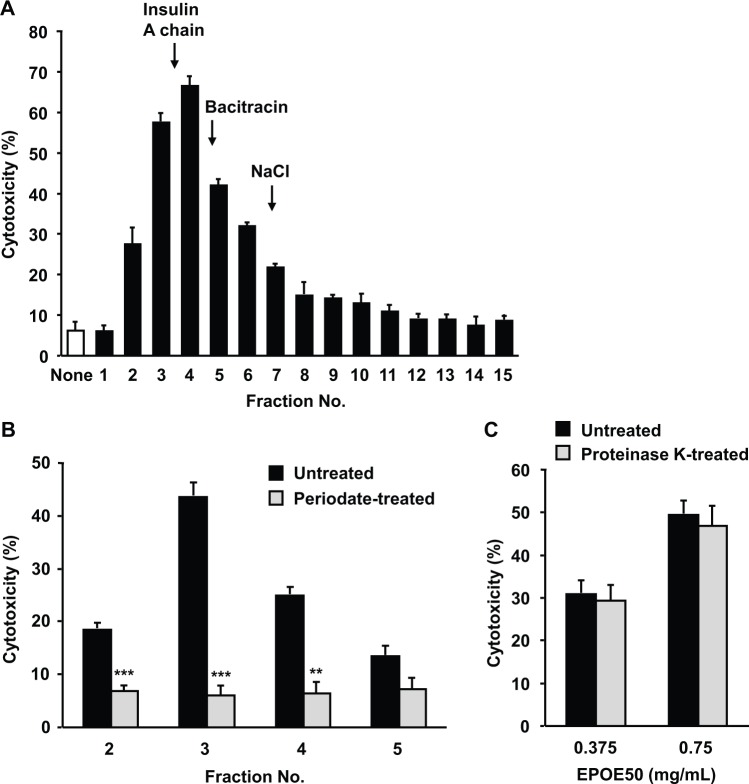
Figure 1.
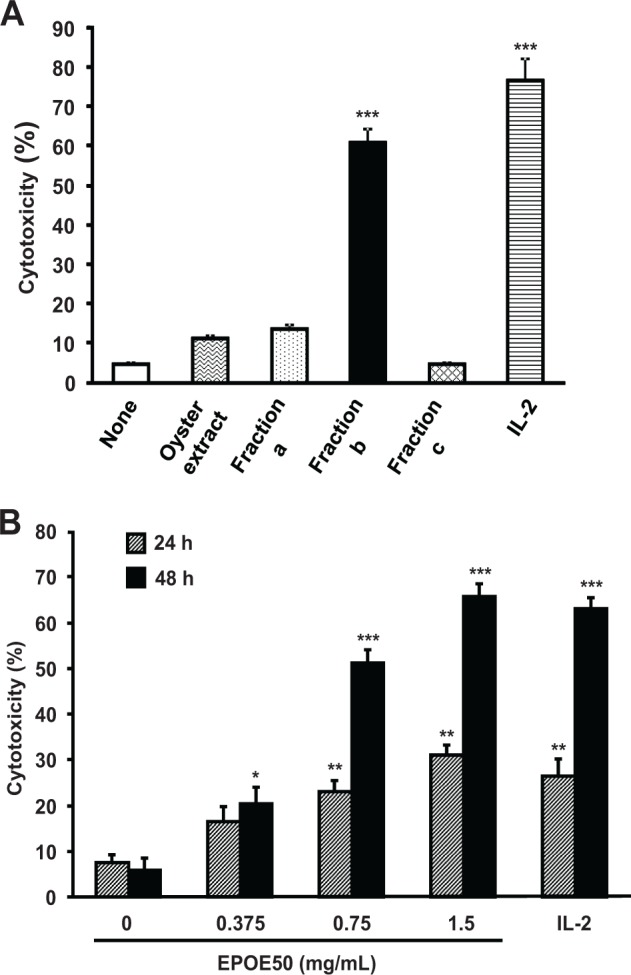
Enhancement of NK cell cytolytic activity in vitro by 30% to 50% ethanol precipitate of oyster extract (EPOE50). Spleen cells were incubated for 48 hours (A) and for 24 and 48 hours (B) with or without 1 mg/mL of oyster extract (OE) (A), 1 mg/mL of ethanol fractions a-c (A), indicated doses of EPOE50 (B), or 5 ng/mL of IL-2 (A and B). Fraction a, 30% ethanol precipitate of OE; Fraction b, 50% ethanol precipitate of OE; Fraction c, 50% ethanol supernatant of OE. The NK cell cytotoxic activity against YAC-1 tumor cells was then determined. The data are means ± SEM of 3 independent experiments. *P < .05, **P < .01, and ***P < .001, as compared with the values of respective control cultures incubated in the medium alone.
Mice
Female C57BL/6N mice, purchased from Charles River Japan (Yokohama, Japan) and Shandong University Laboratory Animal Center (Jinan, China), were maintained under specific pathogen-free conditions in the animal facilities of Okayama University (Okayama, Japan) and Jining Medical College (Rizhao, China) and were used between 7 and 12 weeks of age. Mouse experiments were conducted according to the Policy on the Care and Use of the Laboratory Animals, Okayama University, under protocols approved by the Animal Care and Use Committee, Okayama University.
Determination of OE Chemical Composition
The nitrogen content was determined by the Kjeldahl method22 and was multiplied by a factor of 6.25 to calculate the protein content. The glycogen content was determined by the Somogyi method after trichloroacetic acid extraction, ethanol precipitation, and hydrochloric acid hydrolysis.23 Taurine was measured as described previously.24 Direct dry ashing was done as described previously.25 The zinc content was determined with Hitachi Z-5000 atomic absorption spectrophotometer (Tokyo, Japan) at wavelength of 213.8 nm using air-acetylene flame after direct dry ashing.
Preparation of Erythrocyte-Depleted Spleen Cells and Highly Purified NK Cells
Erythrocyte-depleted murine spleen cells were prepared from whole spleen cells by lysis of erythrocytes with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA, pH 7.2) and hereinafter are referred to as spleen cells. Highly purified NK cells were prepared from the spleen cells by negative selection using a mouse NK cell enrichment set-DM plus positive selection using a mouse NK cell separation set-DM according to the manufacturer’s protocol. The purity of recovered viable NK cells was more than 96% when the cells were stained with PE-conjugated anti-mouse NK1.1 mAb, FITC-conjugated anti-mouse CD3 ϵ chain mAb, and propidium iodide after preincubation of the cells with anti-mouse CD16/CD32 mAb and then analyzed by a flow cytometer (BD FACSCalibur, BD Biosciences) as described previously.26
NK Cell-Enhancing Activity
Spleen cells (1 × 106 cells/200 µL/well) or highly purified NK cells (1 × 105 cells/200 µL/well) were incubated for 48 hours, unless otherwise specified, with or without EPOE50 and other agents in a basal medium (Phenol Red-free RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum [FCS], 2 mM L-glutamine, 100 U/mL of penicillin G, and 100 µg/mL of streptomycin) containing 50 µM 2-mercaptoethanol at 37°C in an atmosphere containing 5% CO2 in triplicate in 96-well flat-bottom plates (Nunc, Roskilde, Denmark). The cells in each plate were then washed once with the basal medium lacking FCS, and the cytotoxic activity of NK cells was determined as described in the next section.
Cytotoxic Activity of NK Cells
The cytotoxic activity of NK cells was assayed as described previously.26 Briefly, YAC-1 cells (106/mL of the basal medium), obtained from Riken BioResource Center Cell Bank (Tsukuba, Japan), were pre-incubated with 15 µM calcein AM for 30 minutes at 37°C with occasional shaking and washed twice with the basal medium lacking FCS. The spleen cells or highly purified NK cells (effector cells), which had been pre-stimulated and washed as described in the previous section, or spleen cells (1 × 106 cells/well) from mice treated with EPOE50 or PBS were incubated for 4 hours with the YAC-1 cells (target cells, 2 × 104 cells) at an E:T ratio of 50:1 for spleen cells or with the YAC-1 cells (1 × 104 cells) at an E:T ratio of 10:1 for highly purified NK cells in triplicate in 200 µL/well of the basal medium. The plate containing cells was then centrifuged and washed twice with the basal medium lacking FCS. The plate was blotted dry, and 200 µL of 1% Triton X-100 in 25 mM borate buffer (pH 9.0) was added to each well. Fluorescence of individual wells was measured with a microplate fluorometer. The wavelengths of the filters used were excitation at 485 nm and emission at 527 nm. The percentage of cytotoxic activity was calculated as follows: cytotoxic activity (%) = (average fluorescence in wells incubated with target cells alone − fluorescence in wells of the experimental group)/(average fluorescence in wells incubated with target cells alone − average fluorescence in wells incubated with effector cells alone) × 100.
NK Cell Activity After Administration of EPOE50 In Vivo
Mice were intraperitoneally injected with phosphate-buffered saline (PBS) or EPOE50 dissolved in PBS (100, 200, and 300 mg/kg) for 3 consecutive days. Spleen cells (1 × 106 cells/200 µL/well) prepared from those mice 2 hours after the last injection were incubated with the target cells for the assay of cytotoxic activity of NK cells.
Flow Cytometric Analysis of NK Cells
NK cell populations in spleen cells were analyzed by flow cytometry as described previously.26 Briefly, spleen cells, prepared from mice treated with EPOE50 or PBS, were washed with and suspended in PBS containing 0.5% bovine serum albumin and 0.1% sodium azide (~1 × 106 cells/100 µL). The cells were then incubated with anti-mouse CD16/CD32 mAb (10 µg/mL) for 5 minutes on ice and stained with PE-conjugated rat anti-mouse NK1.1 mAb (4 µg/mL) plus FITC-conjugated anti-mouse CD3ϵ mAb (10 µg/mL) for 30 minutes on ice. After being washed with and suspended in PBS containing 0.5% bovine serum albumin and 0.1% sodium azide, the cells were stained with propidium iodide (2 µg/mL), and expression of NK 1.1 and CD3ϵ on viable cells was analyzed by a flow cytometer (Epics XL, Beckman Coulter). NK1.1+CD3ϵ− cells were regarded as NK cells.
Gel Filtration of EPOE50
EPOE50 dissolved in PBS (20 mg/mL, 2 mL) was applied to a column of Sephadex G-25 (1.45 × 5 cm) equilibrated with PBS and eluted with the same buffer at 4°C. Fractions of 1 mL were collected, and aliquots (50 µL) of each fraction were subjected to the assay of NK cell-enhancing activity with spleen cells.
Treatment of EPOE50 With Sodium Periodate
EPOE50 (50 mg/2.5 mL of PBS) was incubated with or without 40 mM sodium periodate for 48 hours at 4°C. After addition of 0.54 M sodium thiosulfate in 200 µL of PBS, 2 mL of the reaction mixture was applied to a column of Sephadex G-25 (1.45 × 5 cm) equilibrated with PBS and eluted with the same buffer. Fractions of 1 mL were collected, and aliquots (50 µL) of eluted fractions were subjected to the assay of NK cell-enhancing activity with spleen cells.
Treatment of EPOE50 With Proteinase K
EPOE50 (1.5 or 3 mg/mL of PBS) or PBS was incubated with or without proteinase K (0.25 mg/mL of PBS) for 30 minutes at room temperature. The reaction was stopped by heating the mixture for 15 minutes at 90°C. Aliquots (50 µL) of reaction mixtures were subjected to the assay of NK cell-enhancing activity with spleen cells.
Antitumor Activity of EPOE50 In Vivo
B16 melanoma cells (5 × 105 cells) obtained from Riken BioResource Center Cell Bank were subcutaneously inoculated into the lower back of each C57BL/6 mouse. PBS or EPOE50 dissolved in PBS was intraperitoneally (100, 200, and 300 mg/kg) or orally (2 g/kg) administered to mice once a day for 17 days starting 3 days before tumor inoculation. The lengths of major and minor axes of the tumors were measured, and the tumor volume was calculated by using the following formula: (length of minor axes)2 × (length of major axes)/2.27
Antitumor Activity of EPOE50 In Vitro
B16 melanoma cells (1 × 103 cells/150 µL/well) were pre-incubated for 24 hours in RPMI 1640 medium, supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/mL penicillin G, and 100 µg/mL streptomycin, at 37°C in an atmosphere containing 5% CO2 in triplicate in 96-well flat-bottom plates (Nunc). Fifty microliters of the same fresh medium containing or not containing EPOE50 was added to wells, and the cultures were further incubated for 48 hours. After centrifugation of the plate, portions (160 µL) of the culture supernatant were removed. To each well of the plate, the same fresh medium (60 µL) was added, and MTT assay of viable cells was performed as described previously except that the incubation time with MTT was 2 hours.28
Statistical Analysis
Results are expressed as means and SEMs of 3 independent experiments or 5 to 9 mice. Data in 2 groups were analyzed by Student’s t test. Multiple comparison of the data was done by ANOVA followed by Tukey’s test. P values less than .05 were regarded as significant.
Results
Chemical Composition of OE
The contents of protein (%), glycogen (%), taurine (%), zinc (ppm), ash (%), and moisture (%) in OE were 33.6 ± 1.4, 26.5 ± 1.3, 5.4 ± 0.1, 330 ± 6, 12.9 ± 0.6, and 3.7 ± 0.2 (n = 3), respectively.
Augmentation of NK Cell Activity In Vitro by EPOE50
Spleen cells including NK cells were incubated in vitro for 48 hours with OE, graded ethanol precipitates, and the residue of 50% ethanol supernatant, and NK cell activity was then determined. Most of the NK cell-enhancing activity was retained in the 50% ethanol precipitate (fraction b), the activities of the 30% ethanol precipitate (fraction a), and the 50% ethanol supernatant (fraction c) being minimal (Figure 1A). Therefore, the 50% ethanol precipitate, referred to as EPOE50, was used in the subsequent experiments. NK cell activity was augmented in a dose-dependent manner by 0.375 to 1.5 mg/mL of EPOE50, the enhancement after 48-hour exposure being greater than that after 24-hour exposure (Figure 1B). Forty-eight-hour exposure to 1.5 mg/mL of EPOE50 resulted in a 10-fold increase in NK cell activity, which was as marked as the effect of 5 ng/mL of IL-2 (Figure 1B).
Enhancement of NK Cell Activity In Vivo by EPOE50
In order to examine the in vivo effect of EPOE50 on NK activity, mice were intraperitoneally administered the ethanol precipitate (100, 200, and 300 mg/kg) for 3 consecutive days, and NK activities of their spleen cells were determined. The splenic NK activity in mice treated with EPOE50 markedly increased in a dose-dependent manner (Figure 2A). In contrast, splenic NK cell populations did not significantly change in the mice treated with either dose of the ethanol precipitate, as determined by flow cytometry (Figure 2B).
Figure 2.
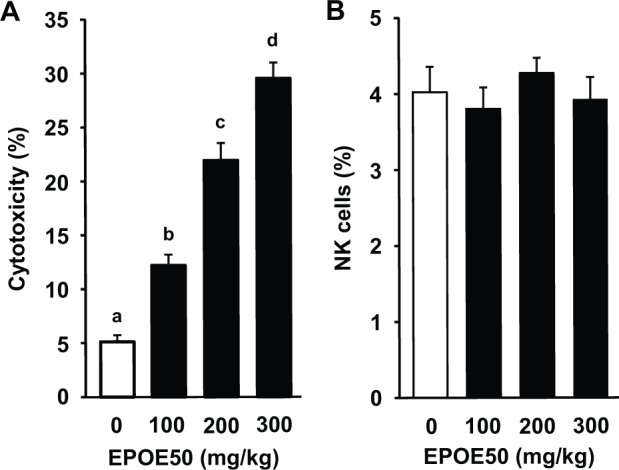
Enhancement of murine NK cell cytolytic activity in vivo by EPOE50. Mice were intraperitoneally injected with EPOE50 (100, 200, and 300 mg/kg) in 0.2 mL/head of PBS or PBS alone (control) for 3 consecutive days. NK cell cytotoxic activity of spleen cells prepared from those mice against YAC-1 tumor cells was then determined (A). Spleen cells were also stained with PE-conjugated NK1.1 mAb and FITC-conjugated CD3ϵ mAb and analyzed by a flow cytometer. The percentage of NK1.1+CD3ϵ− NK cell populations is shown (B). The data are means ± SEM of 9 mice. a-dDifferent superscript letters indicate significant difference (P < .05, P < .01, or P < .001).
Properties of an Active Substance in EPOE50
EPOE50 was subjected to gel filtration to determine the molecular weight of a substance with NK cell-enhancing activity. The active substance(s) was eluted in a peak with apparent molecular weight of about 2000 (Figure 3A). To test the chemical property of active substance(s), EPOE50 was treated with sodium periodate and proteinase K. Sodium periodate opens saccharide rings between vicinal diols leaving 2 aldehyde groups.29 After incubation of EPOE50 with or without sodium periodate, excess sodium periodate was inactivated by addition of the reducing agent sodium thiosulfate and removed by gel filtration. Enhancement of NK cell activity by eluted fractions was then assayed. The activity in fractions 2 to 4 of periodate-treated EPOE50 was reduced to the level of basal NK activity (Figure 3B). In contrast, enhancement of NK activity of EPOE50 was not influenced by treatment with proteinase K (Figure 3C).
Figure 3.
Properties of an active substance with a NK cell-enhancing activity in EPOE50. (A) Gel filtration profile of the NK cell-enhancing activity. Two milliliters of EPOE50 (20 mg/mL) was applied to a Sephadex G-25 column, and 1-mL fractions were collected. Aliquots (50 µL) of each fraction were subjected to the assay of NK cell-enhancing activity using spleen cells. Molecular weight markers used were insulin A chain (2531), bacitracin (1423), and NaCl (58.4). The data are representative of 3 independent experiments with similar results and expressed as means ± SEM of triplicate cultures. (B) Inactivation of the NK cell-enhancing activity in EPOE50 treated with periodate. After being incubated with or without periodate and subsequent removal of periodate by gel filtration of the reaction mixture, aliquots (50 µL) of each eluted fraction were subjected to the assay of NK cell-enhancing activity using spleen cells. Cytotoxicity of spleen cells alone was 7.1 ± 1.8%. The data are means ± SEM of 3 independent experiments. **P < .01, and ***P < .001, as compared with the values of control cultures incubated with the eluates of untreated EPOE50. (C) The NK cell-enhancing activity in EPOE50 is resistant to proteinase K treatment. After being incubated with or without proteinase K and subsequent heating, aliquots (50 µL) of each reaction mixture were subjected to the assay of NK cell-enhancing activity using spleen cells. Cytotoxicities of spleen cells incubated with and without heated proteinase K alone were 9.3 ± 0.8% and 8.2 ± 0.9%, respectively. The data are means ± SEM of 3 independent experiments.
An Active Substance in EPOE50 Is Different From ODN Containing Unmethylated Deoxycytidine-Phosphate-Deoxyguanosine Dinucleotides
Since oysters filter a large volume of seawater during their feeding activities, they frequently accumulate bacteria from their environment, the DNA of which has a much higher frequency of unmethylated deoxycytidine-phosphate-deoxyguanosine dinucleotides (CpG motif) than that in vertebrate DNA.30,31 NK cells in human and murine lymphocytes respond to and are activated by bacterial DNA and ODNs containing an unmethylated CpG motif.32-34 This activation of NK cells is not observed when the highly purified NK cell preparation is used and thus is caused indirectly by inducing the secretion of IL-12, type I interferons, and tumor necrosis factor-α presumably from dendritic cells and macrophages.32 In order to test the possibility that the active substance(s) in EPOE50 with a molecular weight of about 2000 is an unmethylated CpG motif-containing oligodeoxynucleotide of bacterial origin, we determined whether or not highly purified NK cells are activated by EPOE50. As shown in Figure 4, NK cells in splenocytes but not highly purified NK cells were activated by oligodeoxynucleotide 1585 containing an unmethylated CpG motif. In contrast, EPOE50 markedly induced activation of not only NK cells in splenocytes but also highly purified NK cells as did IL-2.
Figure 4.
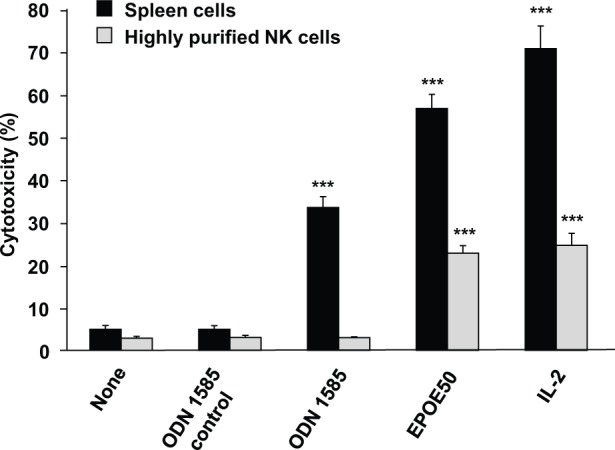
Activation of highly purified NK cells by EPOE50 but not by ODN 1585. Spleen cells and NK cells purified by negative plus positive selection of spleen cells were incubated for 48 hours with or without 0.5 mg/mL of EPOE50, 1 µM ODN 1585, 1 µM ODN 1585 control, or 5 ng/mL of IL-2. The NK cell cytotoxic activity against YAC-1 tumor cells was then determined. The data are means ± SEM of 3 independent experiments. ***P < .001, as compared with the values of respective control cultures incubated in the medium alone.
Suppression of In Vivo Tumor Growth by EPOE50
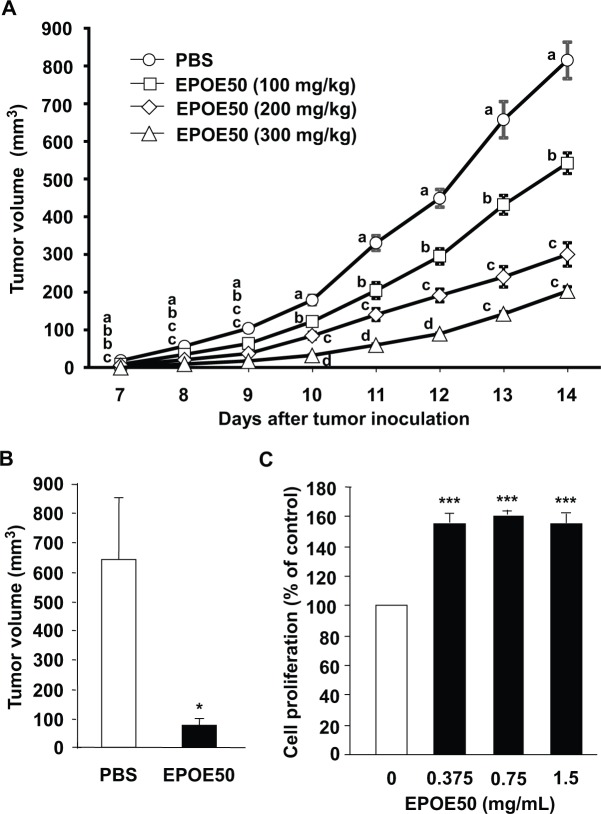
NK cells have well-established roles in the host’s immune defense against a variety of spontaneous and induced cancer. Since NK cell activity in mice was augmented by treatment with EPOE50, impaired growth of transplanted tumors in those animals is expected. We therefore examined the effect of EPOE50 treatment in vivo on the growth of NK cell-sensitive B16 melanoma in mice, a well-established and widely used tumor model in which treatment is extremely difficult. B16 cells were subcutaneously inoculated into the lower backs of mice, and 3 different doses (100, 200, and 300 mg/kg) of EPOE50 was intraperitoneally administered to mice once a day for 17 days starting 3 days before tumor inoculation. Figure 5A shows curves of tumor growth in treated and untreated mice. Administration of the ethanol precipitate dose-dependently suppressed tumor growth on day 7 and thereafter. In the experiment, the final body weights of untreated, 100 mg/kg EPOE50 treated, 200 mg/kg EPOE50 treated, and 300 mg/kg EPOE50 treated mice were 17.4 ± 0.3 g, 17.3 ± 0.3 g, 16.9 ± 0.3 g, and 16.8 ± 0.2 g, respectively, and the body weight gains of untreated, 100 mg/kg EPOE50 treated, 200 mg/kg EPOE50 treated, 300 mg/kg EPOE50 treated mice were 0.71 ± 0.03 g, 0.64 ± 0.04 g, 0.58 ± 0.03 g, and 0.55 ± 0.05 g, respectively. No significant difference was observed between untreated mice and mice treated with either dose of EPOE50 in either body weight parameters except significant difference between untreated mice and 300 mg/kg EPOE50-treated mice (P < .05) in the body weight gains. An inhibitory effect of EPOE50 on tumor growth in mice was also observed after its oral administration (Figure 5B). On the other hand, the proliferation of B16 tumor cells in vitro was not inhibited but rather was slightly stimulated by EPOE50 (Figure 5C).
Figure 5.
EPOE50 inhibits growth of B16 melanoma in vivo but not in vitro. (A and B) EPOE50 or PBS was intraperitoneally (100, 200, and 300 mg/kg, A) or orally (2 g/kg, B) administered to mice once a day for 17 days starting 3 days before B16 melanoma inoculation. The lengths of major and minor axes of tumors were measured, and the tumor volumes were calculated from these measurements. The data are means ± SEM of 9 mice (both PBS and EPOE50) (A) and 5 mice (both PBS and EPOE50) (B). a-dDifferent superscript letters on the same day indicate significant difference (P < .05, P < .01, or P < .001). *P < .05, as compared with the values of control mice treated with PBS alone. (C) EPOE50 does not inhibit the proliferation of melanoma in vitro but instead promotes its proliferation. B16 melanoma cells were incubated for 48 hours with or without the indicated doses of EPOE50 and subjected to the MTT assay. The data are expressed as percentage of the values of control cultures incubated in the medium alone and are means ± SEM of 3 independent experiments. ***P < .001, as compared with the values of control cultures incubated in the medium alone.
Discussion
We demonstrated in this study that NK cell cytolytic activity was markedly increased by in vitro treatment of spleen cells and highly purified NK cells with the 30% to 50% ethanol precipitate of oyster extract EPOE50. Intraperitoneal administration of EPOE50 to mice also enhanced splenic NK cell activity without a change in splenic NK cell populations. These results suggest that enhancement of NK cell cytotoxicity induced by administration of EPOE50 is largely dependent on activation of but not on proliferation or accumulation of NK cells in the spleen. Since the cytolytic activity of highly purified NK cells was augmented by EPOE50 (Figure 4), it seems likely that EPOE50 directly, not indirectly, activated NK cells. Augmentation of NK cell activity in vitro after 48-hour treatment with EPOE50 was greater than that after 24-hour treatment with the same fraction, suggesting that its enhancing process needs many hours. This time course was not different from that of IL-2-induced enhancement of NK cell activity (Figure 1B), which shows a gradual increase as reported previously.35
Wang et al reported that oligopeptide-rich oyster hydrolysates had antitumor activity and NK cell-enhancing activity in vivo.36 The oyster hydrolysates they used were enzymatically produced by proteolysis of oyster proteins with the use of Bacillus sp. SM98011 protease in vitro. EPOE50 used in our study was prepared from the extract of untreated oysters without any enzymatic treatment. Thus, to our knowledge, this is the first report on the stimulatory effects of an oyster preparation with no enzymatic modification on NK cell activity. Moreover, an active substance in EPOE50 has the properties of a sugar-containing oligomer or polymer, which is different from oligopeptides as discussed below.
The NK cell-stimulating activity of EPOE50 was completely inactivated by treatment with periodate but not with proteinase K. Periodate cleaves bonds between adjacent carbon atoms that contain hydroxyl groups in carbohydrates, creating 2 aldehyde groups.29 Proteinase K exhibits broad substrate specificity and thus degrades many proteins and peptides even in the native state.37,38 The results of gel filtration of EPOE50 indicated that the activity was eluted with an apparent molecular weight of 2000. Therefore, the active substance seems to be a sugar-containing oligomer or polymer such as an oligosaccharide, polysaccharide, or oligonucleotide but not an oligopeptide. The in vitro NK cell-stimulating effects of some oligosaccharides, polysaccharides, and ODNs from plants, fungi, and bacteria have been reported. Fructooligosaccharides of Asparagus recemosus with a polymerization degree of 7 to 8, a polysaccharide with a molecular mass of 47 kDa isolated from Litchi chinensis, polysaccharides from Trametes hirsuta and Pseudostellaria heterophylla, and ODNs from bacteria enhanced the cytotoxicity of NK cells.32,39-42 Nigerooligosaccharides, which are enzymatically synthesized and mainly composed of nigerosyl maltose and nigerosyl glucose, had a stimulatory effect on NK cells.43 Moreover, a synthetic heparin-type oligosaccharide that interacts with the natural cytotoxicity receptor NKp44, one of the activating receptors of NK, enhanced anti-NKp44-induced release of interferon-γ from an NKp44-expressing NK-92 cell line, indicating induction of NKp44-mediated NK activation.44 To date, however, we know of no report concerning polysaccharides or oligosaccharides of animal origin with an in vitro NK cell-stimulating activity.
Although oysters frequently accumulate seawater bacteria whose DNA has the ability to activate murine NK cells, the following evidence supports the notion that the active substance of EPOE50 is different from ODNs containing an unmethylated CpG motif. First, EPOE50 caused activation of highly purified NK cells, while unmethylated CpG motif-containing ODN 1585 did not. Second, activity of EPOE50 was sensitive to periodate treatment that cleaves bonds between hydroxyl group–containing adjacent carbon atoms in carbohydrates as described above, but the deoxyribose moiety of unmethylated CpG motif-containing ODNs has no such adjacent carbon atoms containing hydroxyl groups.
The growth of B16 melanoma in vivo was significantly suppressed by treatment with EPOE50. The following lines of evidence indicate that the antitumor effect of EPOE50 in vivo may be mediated through the activation of NK cells. First, NK cell activity was enhanced by injection of EPOE50 in vivo (Figure 2A). Both EPOE50-caused NK cell activation and tumor growth inhibition showed the same dose dependence. Second, B16 melanoma cells are a target cell type of NK cells and are killed by NK cells.45,46 Third, EPOE50 did not inhibit but promoted the in vitro proliferation of B16 melanoma cells. If the active substances with B16 proliferation-promoting activity could be separated from that with NK cell-enhancing activity, more potent suppression of tumor growth in vivo would be obtained, although it is not known whether the active substances with each activity are different or not. An in vivo antitumor effect was observed by not only intraperitoneal injection but also oral administration of EPOE50.
Oysters are cultivated and eaten worldwide and have the advantage of being safe. In fact, consecutive administration of EPOE50 prepared from oyster extract to mice had minimal effect on body weight gain. The slight decrease in body weight gains observed in tumor-bearing mice after 17-day treatment with EPOE50 may reflect a decrease in tumor volumes rather than body weight loss. A recent study by Cheng et al has shown that oysters have polysaccharides that enhance antigen-specific T helper 1 (Th1) immunity in vitro and in vivo.47 Thus, oysters have components that augment not only innate immunity but also acquired immunity and are potential immunostimulatory agents for defense against cancers and viral infection.
Conclusion
Our results showed that the ethanol precipitate of oyster extract augmented NK cell activity in vitro and in vivo and that its administration to mice inhibited tumor growth presumably through the activation of NK cells. The results also suggest that the active substance is a sugar-containing oligomer or polymer and is not of bacterial origin. Oysters might serve as an immunostimulatory food, and it is worthwhile to identify the active ingredient(s) of oysters.
Footnotes
Authors’ Note: Authors Kaito Sakaguchi, Ming Zhong, and Saeko Kawai contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a grant from the Shandong Provincial Natural Science Foundation, China (ZR2014HM038).
References
- 1. Bodduluru LN, Kasala ER, Madhana RMR, Sriram CS. Natural killer cells: the journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015;357:454-467. [DOI] [PubMed] [Google Scholar]
- 2. Brandstadter J, Yang Y. Natural killer cell responses to viral infection. J Innate Immun. 2011;3:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41-49. [DOI] [PubMed] [Google Scholar]
- 4. Shibuya A. Development and functions of natural killer cells. Int J Hematol. 2003;78:1-6. [DOI] [PubMed] [Google Scholar]
- 5. Kirwan SE, Burshtyn DN. Regulation of natural killer cell activity. Curr Opin Immunol. 2007;19:46-54. [DOI] [PubMed] [Google Scholar]
- 6. Marçais A, Viel S, Grau M, Henry T, Marvel J, Walzer T. Regulation of mouse NK cell development and function by cytokines. Front Immunol. 2013;4:450. doi: 10.3389/fimmu.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22:307-318. [DOI] [PubMed] [Google Scholar]
- 8. Mandal A, Viswanathan C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 2015;8:47-55. [DOI] [PubMed] [Google Scholar]
- 9. Marketon JIW, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16-26. [DOI] [PubMed] [Google Scholar]
- 10. Meeus M, Mistiaen W, Lambrecht L, Nijs J. Immunological similarities between cancer and chronic fatigue syndrome: the common link to fatigue? Anticancer Res. 2009;29:4717-4726. [PubMed] [Google Scholar]
- 11. Komada Y, Zhang SL, Zhou YW, et al. Cellular immunosuppression in children with acute lymphoblastic leukemia: effect of consolidation chemotherapy. Cancer Immunol Immunother. 1992;35:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGinnes K, Florence J, Penny R. The effect of radiotherapy on the natural killer (NK)-cell activity of cancer patients. J Clin Immunol. 1987;7:210-217. [DOI] [PubMed] [Google Scholar]
- 13. Wei G, Moss J, Yuan CS. Opioid-induced immunosuppression: is it centrally mediated or peripherally mediated? Biochem Pharmacol. 2003;65:1761-1766. [DOI] [PubMed] [Google Scholar]
- 14. Weintzen ML, Bonavida B. Mechanism of inhibition of human natural killer activity by ultraviolet radiation. J Immunol. 1984;133:3128-3132. [PubMed] [Google Scholar]
- 15. Skrombolas D, Frelinger JG. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev Clin Immunol. 2014;10:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105-1111. [DOI] [PubMed] [Google Scholar]
- 17. Carver JD. Dietary nucleotides: cellular immune, intestinal and hepatic system effects. J Nutr. 1994;124:144S-148S. [DOI] [PubMed] [Google Scholar]
- 18. Hanson MGV, Özenci V, Carlsten MCV, et al. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunol Immunother. 2007;56:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jang SE, Joh EH, Lee HY, et al. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J Microbiol Biotechnol. 2013;23:414-421. [DOI] [PubMed] [Google Scholar]
- 20. Kim YS, Sayers TJ, Colburn NH, Milner JA, Young HA. Impact of dietary components on NK and Treg cell function for cancer prevention. Mol Carcinog. 2015;54:669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin JG, Fan MJ, Tang NY, et al. An extract of Agaricus blazei Murill administered orally promotes immune responses in murine leukemia BALB/c mice in vivo. Integr Cancer Ther. 2012;11:29-36. [DOI] [PubMed] [Google Scholar]
- 22. Nozawa S, Hakoda A, Sakaida K, Suzuki T, Yasui A. Method performance study of the determination of total nitrogen in soy sauce by the Kjeldahl method. Anal Sci. 2005;21:1129-1132. [DOI] [PubMed] [Google Scholar]
- 23. Somogyi M. A reagent for the copper-iodometric determination of very small amounts of sugar. J Biol Chem. 1937;117:771-776. [Google Scholar]
- 24. James LB. Taurine levels in cat plasma. J Chromatogr. 1981;209:479-483. [DOI] [PubMed] [Google Scholar]
- 25. National Standards of the People’s Republic of China. Determination of Ash in Foods: GB 5009.4-2010. Beijing, People’s Republic of China: National Standards of the People’s Republic of China; 2010. [Google Scholar]
- 26. Zhong M, Kadota Y, Shimizu Y, Gohda E. Induction of cytolytic activity and interferon-γ production in murine natural killer cells by polymyxins B and E. Int Immunopharmacol. 2008;8:508-513. [DOI] [PubMed] [Google Scholar]
- 27. Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim (NY). 2013;42:217-224. [DOI] [PubMed] [Google Scholar]
- 28. Kawamoto T, Gohda E, Iji H, Fujiwara M, Yamamoto I. SKW 6.4 cell differentiation induced by interleukin 6 is stimulated by butyrate. Immunopharmacology. 1998;40:119-130. [DOI] [PubMed] [Google Scholar]
- 29. Perlin AS. Glycol-cleavage oxidation. Adv Carbohy Chem Biochem. 2006;60:183-250. [DOI] [PubMed] [Google Scholar]
- 30. Bird AP. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 1987;3:342-347. [Google Scholar]
- 31. Son NT, Fleet GH. Behavior of pathogenic bacteria in the oyster, Crassostrea commercialis, during depuration, re-laying, and storage. Appl Environ Microbiol. 1980;40:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840-1845. [PubMed] [Google Scholar]
- 33. Shimada S, Yano O, Tokunaga T. In vivo augmentation of natural killer cell activity with a deoxyribonucleic acid fraction of BCG. Jpn J Cancer Res. 1986;77:808-816. [PubMed] [Google Scholar]
- 34. Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072-4076. [PubMed] [Google Scholar]
- 35. Saxena RK, Saxena QB, Adler WH. Interleukin-2-induced activation of natural killer activity in spleen cells from old and young mice. Immunology. 1984;51:719-726. [PMC free article] [PubMed] [Google Scholar]
- 36. Wang YK, He HL, Wang GF, et al. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar Drugs. 2010;8:255-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ebeling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974;47:91-97. [DOI] [PubMed] [Google Scholar]
- 38. Hilz H, Wiegers U, Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of ‘masked’ proteins. Eur J Biochem. 1975;56:103-108. [DOI] [PubMed] [Google Scholar]
- 39. Jing Y, Huang L, Lv W, et al. Structural characterization of a novel polysaccharide from pulp tissues of Litchi chinensis and its immunomodulatory activity. J Agric Food Chem. 2014;62:902-911. [DOI] [PubMed] [Google Scholar]
- 40. Shenbhagaraman R, Premalatha MK, Jenefar S, et al. Immunopotentiating properties of extracellular polysaccharide from Trametes hirsuta strain VKESR. Carbohydr Polym. 2014;106:299-304. [DOI] [PubMed] [Google Scholar]
- 41. Thakur M, Connellan P, Deseo MA, et al. Characterization and in vitro immunomodulatory screening of fructo-oligosaccharides of Asparagus racemosus Willd. Int J Biol Macromol. 2012;50:77-81. [DOI] [PubMed] [Google Scholar]
- 42. Wong CK, Leung KN, Fung KP, Choy YM. The immunostimulating activities of anti-tumor polysaccharides from Pseudostellaria heterophylla. Immunopharmacology. 1994;28:47-54. [DOI] [PubMed] [Google Scholar]
- 43. Murosak S, Muroyama K, Yamamoto Y, Liu T, Yoshikai Y. Nigerooligosaccharides augments natural killer activity of hepatic mononuclear cells in mice. Int Immunopharmacol. 2002;2:151-159. [DOI] [PubMed] [Google Scholar]
- 44. Hecht ML, Rosental B, Horlacher T, et al. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8:712-720. [DOI] [PubMed] [Google Scholar]
- 45. Lakshmikanth T, Burke S, Ali TH, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palmieri G, Morrone S, Lollini PL, et al. TNF impairs in vivo and in vitro natural killer (NK) susceptibility of B16 melanoma cells. Scand J Immunol. 1992;35:279-287. [DOI] [PubMed] [Google Scholar]
- 47. Cheng JY, Ng LT, Lin CL, Jan TR. Pacific oyster-derived polysaccharides enhance antigen-specific T helper (Th)1 immunity in vitro and in vivo. Immunopharmacol Immunotoxicol. 2013;35:235-240. [DOI] [PubMed] [Google Scholar]