Abstract
Combination strategies involving chemotherapy and monoclonal antibodies (mAb) are commonly used in attempts to produce better clinical outcomes. This practice has led to new and ongoing toxicities that may lead to reductions in dose or noncompliance, limiting the effectiveness of treatment. Viscum album L (VA) preparations are widely used in Europe as additive therapy and have been associated with reduced chemotherapy-related adverse reactions and increased health-related quality of life. Concomitant VA therapy might also reduce toxicity related to mAb. This retrospective study investigated the safety of combined treatment with VA and mAb in cancer patients. A total of 43 patients had combined therapy (474 exposures); 12 had VA without mAb (129 exposures), and 8 had mAb without VA (68 exposures). Most patients (89.3%) received concomitant chemotherapy or supportive therapies. A total of 34 patients (60.7%) experienced 142 adverse events (AEs). Leucopenia (14.1% of all events), acneiform rash (8.5%), and stomatitis (6.3%) occurred most frequently. Longitudinal logistic regression analysis suggested a nearly 5 times higher odds of experiencing an AE following treatment with mAb compared with mAb plus VA (95% CI = 1.53-16.14). Our results, together with theoretical consideration of potential botanical-drug interactions, suggest that combined treatment with VA and mAb is safe.
Keywords: adverse events, combination therapy, integrative oncology, Helixor, mistletoe, Viscum album L, safety, targeted therapy
Introduction
Monoclonal antibodies (mAb) have emerged as a promising approach to treating a range of tumor types. In contrast to conventional chemotherapy, which affects all rapidly dividing cells, mAb aim to inhibit specific molecular pathways that are necessary for tumor growth and maintenance.1 Among the most encouraging mAb is trastuzumab, which targets the human epidermal growth factor receptor 2 and is indicated in the treatment of breast cancer2; bevacizumab, which inhibits vascular endothelial growth factor and is indicated in the treatment of a range of diseases, including colorectal, lung, and ovarian cancer3; and cetuximab, which blocks the epidermal growth factor receptor and is indicated in the treatment of colorectal and lung cancer.4 Despite initial excitement over dramatic tumor regressions, however, mAb in general have achieved only limited improvements in overall survival because of primary resistance in some patients and the often rapid occurrence of secondary resistance in many responders.5 Combination strategies involving multiple mAb and chemotherapies have been increasingly used in an attempt to prevent or combat the emergence of secondary resistance. A number of recent studies have shown that combining 2 mAb may lead to an increase in median progression-free survival compared with monotherapy.6,7 There is also growing interest in whether immunotherapies that can harness endogenous anti-tumor immunity by modifying immune regulatory mechanisms can help consolidate impressive clinical responses from mAb into long-lasting clinical remissions.1,8,9 Although combination strategies seem to be delivering improved clinical outcomes, an important consideration when combining multiple therapies, especially cytotoxic drugs with narrow-therapeutic indexes, is the elevated risk of adverse drug-drug interactions.10 Despite being “targeted” therapies, these drugs affect multiple organ systems, and their chronic and combined use has led to the identification of new toxicities that can impair health-related quality of life (HRQL) and require long-term management.11 Importantly, ongoing toxicity caused by mAb can lead to reductions in dose or noncompliance, thereby, limiting the effectiveness of treatment. Supportive therapies that can relieve toxicity associated with mAb and chemotherapy or improve HRQL are, therefore, of significant interest.
Viscum album L (VA or European mistletoe) preparations are widely used as additive cancer therapy in Europe, especially in German-speaking countries, and have been associated with a reduction in chemotherapy-related adverse drug reactions and increased HRQL.12,13 Furthermore, it has been suggested that VA preparations can induce clinically beneficial immunomodulation through lectin-carbohydrate interactions, leading to enhancement of interleukin-12 secretion and natural killer cell function.14,15 Therefore, use of VA therapy preparations alongside mAb might help improve the balance of the innate immune system, thereby decreasing treatment-related toxicities and possibly even helping in overcoming tumor-induced immunosuppression and treatment resistance. Whereas at least 1 group has thoroughly investigated the safety of combined use of VA preparations and the chemotherapeutic agent, gemcitabine,16 we were unable to find information regarding the safety of combined use of VA preparations and mAb. The present study describes the combined use of intravenously administered Helixor VA preparations and a variety of mAb in cancer patients of the Havelhoehe Hospital in Berlin and investigates the safety of this combinatorial approach. Theoretical considerations regarding the potential for negative botanical-drug interactions between VA preparations and mAb are also discussed.
Methods
Study Design and Setting
A retrospective cohort study was conducted to assess the safety of combined (same-day) intravenous infusions of mAb and VA preparations manufactured by Helixor Heilmittel GmbH (Rosenfeld, Germany). The Network Oncology (NO), a joint clinical registry of German hospitals and outpatient practitioners specialized in integrative medicine, was used during this study.17 The NO database contains patient information and data on cancer diagnoses, therapies, adverse events (AEs), and disease progress extracted from patient files and recorded using the QuaDoSta (Quality management, Documentation, and Statistics) software that was developed at Havelhoehe Research Institute. Ethical approval for the NO project was obtained from the Medical Association Berlin.
Study Participants
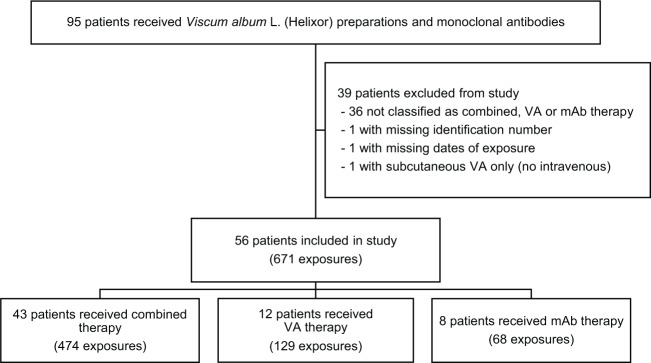
Data of consenting cancer patients treated between August 2005 and November 2014 were assessed between July 2014 and July 2015. We found 95 patients who had received both Helixor VA preparations and mAb at any time during their treatment. All patients had an ECOG performance status of 0 to 2, and no patient was ineligible based on comorbidities (ie, no evidence of refractory hypertension, internal bleeding, danger of perforation, or treatment with mAb within 4 weeks of surgery). Details of treatment with mAb or VA preparations, including dates, doses, routes of administration, concomitant medications, and associated AEs, along with demographic, diagnosis, and disease progression data were extracted. Patients were assigned to 1 or more of 3 treatment groups based on documented timing of treatment with mAb and VA preparations (Figure 1). Note that it was possible for patients to be assigned to different treatment groups at different times throughout their treatment. If a VA preparation and mAb therapy were received by a patient on the same day, the patient was described as receiving combined therapy. A patient was described as receiving VA therapy if they received one or more VA infusions, where they did not receive mAb for at least 1 month either side of the VA application. Likewise, mAb therapy refers to a patient not receiving a VA infusion for at least 1 month either side of treatment with mAb. A 1-month washout period was specified to eliminate any carryover effects and to avoid confusion over the causality of delayed or enduring AEs. Because of the complex nature of cancer treatment, some patients in all 3 groups received concomitant chemotherapy or supportive therapies (eg, antiemetic or pain medication). Patients were excluded from the study if they did not meet the criteria for at least 1 of the 3 treatment groups or if they had missing or invalid information regarding dates of treatment with mAb and VA preparations, specific drug names, concomitant medications, database identification number, date of birth, gender, date of diagnosis, or ICD-10 code. Although data for all routes of administration and types of VA preparations and targeted therapies were collected, our analysis was limited to intravenously administered Helixor VA preparations and intravenously administered mAb.
Figure 1.
Flowchart of participant selection from 95 patients whose medical records were reviewed.
Abbreviations: VA, Viscum album L; mAb, monoclonal antibodies.
Outcomes
The primary outcome was occurrence of an AE during or shortly after combined therapy, VA therapy, or mAb therapy. An AE was defined as “any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment”18(p 2). AEs were chosen as the primary outcome rather than adverse drug reactions (which are drug specific) because in situations where multiple medications are administered concomitantly (eg, a VA preparation, antiemetic medication, a mAb, and a chemotherapeutic agent), attributing causality of an AE to a single medication can be difficult. AEs that were clearly disease related or could be definitely attributed to a previous therapy were excluded. All other AEs (eg, could be related to mAb, VA, chemotherapy, or supportive therapies) were classified as Medical Dictionary for Regulatory Activities (MedDRA) 15.0 preferred terms and grouped by System Organ Class. Secondary outcomes were the type and frequency of specific AEs and the number of serious AEs.
Statistical Analysis
Patient characteristics and exposure to mAb, Helixor VA preparations, chemotherapy, and supportive therapies were compared between treatment groups. The relative frequency of patients experiencing 1 or more AEs, the number of exposures to which AEs occurred, the total number and types of AEs, the number of AEs per 100 exposures (crude incidence rates), and the number of AEs classified as serious were described for each treatment group. To assess which demographic and treatment factors were associated with AEs, a longitudinal logistic regression model was fit using the generalized estimating equations (GEE) method to account for the multiple observations per person. An exchangeable covariance structure was used to model the correlation of the repeated measurements over time. In the adjusted model, age at treatment, concomitant chemotherapy (no, yes), and concomitant supportive therapies (no, yes) were included as time-varying covariates. Use of time-varying covariates means that confounders can change over time. For example, a patient could be classified as having combined therapy on one occasion followed by mAb therapy on another occasion (as long as the washout period was satisfied by there being at least 1 month between different types of therapies). Baseline covariates included were gender (male, female) and Union for International Cancer Control stage at first treatment (UICC I-II, UICC III-IV, not applicable or unknown). Odds ratios (ORs), 95% CIs, and P values were calculated, and the level of significance (α) was set at .05. All analyses were conducted using R version 3.1.2.19 GEE models used the geepack package.20
Results
Out of 95 patients treated with Helixor VA preparations and mAb, 56 were selected for inclusion in this study (Figure 1). A total of 36 patients were excluded because they were only treated with VA and mAb on separate days but within 1 month of each other. One patient was excluded for not having a database identification number, which was necessary for acquiring basic patient information; 1 patient was excluded for not having a date of treatment; and another patient was excluded for only receiving subcutaneous VA despite intravenous VA being prescribed. Among the remaining 56 patients representing 671 exposures, 43 patients had 474 exposures to combined therapy, 12 patients had 129 exposures to VA therapy, and 8 patients had 68 exposures to mAb therapy. Seven patients were included in more than 1 treatment group: 2 patients had combined therapy and VA therapy at different times, whereas 5 patients had VA therapy and mAb therapy. The number of exposures per patient ranged from 1 to 44, with a median of 6 and interquartile range (IQR) of 3 to 18 for combined therapy, from 1 to 33 with a median of 8 (IQR = 2-17) for VA therapy, and from 1 to 25 with a median of 8 (IQR = 5-9) for mAb therapy. The total period of time during which patients received therapies ranged from 1 day to 2.4 years, with a median of 3.5 months (IQR = 0.8-10.8 months), for combined therapy; 1 day to 6.1 years, with a median of 7.9 months (IQR = 1.6-13.8 months), for VA therapy; and 1 day to 3.3 years, with a median of 4.9 months (IQR = 1.3-13.3 months), for mAb therapy.
Demographic and diagnosis information for all included patients are summarized in Table 1. Patients in the mAb therapy group were generally younger at the age of first treatment and more likely to be female and suffering from breast cancer; treatment more frequently began at an early UICC stage. Data for the time-varying factor—age at treatment—were skewed in both the individual therapy groups.
Table 1.
Characteristics of Patients Included in the Study.
| Patient Characteristics | All Patients (n = 56) | Combined Therapy (n = 43) | VA Therapy (n = 12) | mAb Therapy (n = 8) |
|---|---|---|---|---|
| Age at first exposure, median (IQR) | 59 (50-69) | 59 (50-70) | 58 (46-66) | 49 (48-63) |
| Age at exposure,a median (IQR) | 65 (51-72) | 69 (55-72) | 46 (45-74) | 51 (49-63) |
| Gender, n (%) | ||||
| Male | 22 (39.3) | 18 (41.9) | 6 (50.0) | 2 (25.0) |
| Female | 34 (60.7) | 25 (58.1) | 6 (50.0) | 6 (75.0) |
| Location of tumor, n (%) | ||||
| Breast | 15 (26.8) | 10 (23.2) | 2 (16.7) | 4 (50.0) |
| Digestive system | 32 (57.1) | 27 (62.8) | 6 (50.0) | 3 (37.5) |
| Hematological system | 3 (5.4) | 2 (4.7) | 2 (16.7) | 1 (12.5) |
| Respiratory system | 2 (3.6) | 1 (2.3) | 1 (8.3) | — |
| Urogenital system | 4 (7.1) | 3 (7.0) | 1 (8.3) | — |
| UICC stage at first treatment, n (%) | ||||
| I-II | 8 (14.3) | 5 (11.6) | 2 (16.7) | 3 (37.5) |
| III-IV | 38 (67.9) | 30 (69.8) | 7 (58.3) | 4 (50.0) |
| Not applicable or unknown | 10 (17.9) | 8 (18.6) | 3 (25.0) | 1 (12.5) |
Abbreviations: VA, Viscum album L; mAb, monoclonal antibodies; IQR, interquartile range; UICC, Union for International Cancer Control.
Median age calculated from all exposures allowing age of patients to increase over time.
The total number of exposures to different combinations of mAb and VA are presented in Table 2. Consecutive infusion of Helixor Abietis and cetuximab (8 patients, 104 exposures) was the most frequent combination, followed by Helixor Mali and bevacizumab (10 patients, 94 exposures) and Helixor Abietis and trastuzumab (8 patients, 94 exposures). Although 7 mAb were documented, 92% of all exposures to mAb were to bevacizumab, trastuzumab, or cetuximab.
Table 2.
Patient Exposure to Combinations of VA Preparations and mAb.a
| Number of Patients Exposed (Total Exposures) | Helixor A | Helixor M | Helixor P | mAb Therapy (No VA) | Total |
|---|---|---|---|---|---|
| Ado-trastuzumab emtansine | 2 (17) | — | — | — | 2 (17) |
| Bevacizumab | 19 (87) | 10 (94) | 2 (7) | 3 (18) | 32 (206) |
| Cetuximab | 8 (104) | 5 (31) | 1 (11) | 1 (4) | 13 (150) |
| Ofatumumab | — | — | — | 1 (3) | 1 (3) |
| Panitumumab | 1 (3) | 1 (9) | — | 1 (1) | 3 (13) |
| Rituximab | — | 1 (1) | 1 (3) | 1 (6) | 3 (10) |
| Trastuzumab | 8 (94) | 1 (13) | — | 3 (36) | 11 (143) |
| VA therapy (no mAb) | 6 (32) | 6 (55) | 3 (42) | — | 12 (129) |
| Total | 38 (337) | 20 (203) | 6 (63) | 8 (68) | 56 (671) |
Abbreviations: VA, Viscum album L; mAb, monoclonal antibodies.
Values represent the number of patients exposed, with the total number of exposures in parentheses.
Concomitant use of chemotherapeutic agents and supportive medications, such as antiemetic drugs, in addition to VA or mAb was reported at least once in 50 (89%) patients. Table 3 shows the number of exposures that involved concomitant chemotherapy only (11%, 4%, and 10%), supportive therapy only (16%, 2%, and 9%), chemotherapy and supportive therapy (50%, 16%, and 19%), or no concomitant therapy (23%, 78%, and 62%) for combined, VA, and mAb therapies, respectively. Dexamethasone, a corticosteroid used to counteract adverse reactions to chemotherapy agents, was the most frequently used concomitant therapy (20 patients, 139 exposures). The second and third most frequent premedication regimens consisted of dexamethasone together with dimethindene maleate (Fenistil), an antihistamine used to prevent hypersensitivity reactions (16 patients, 94 exposures) and dimethindene maleate alone (5 patients, 34 exposures). Of the total exposures to combined therapy, VA therapy, and mAb therapy, 61%, 20%, and 29% of exposures involved same-day chemotherapy (with or without supportive therapy), respectively. The most frequent chemotherapy regimen involved injections of folinic acid and fluorouracil, followed by slow infusion of fluorouracil through a Baxter pump (10 patients, 59 exposures). Other frequent chemotherapy regimens were as stated above but with the addition of oxaliplatin (FOLFOX: 12 patients, 52 exposures) or irinotecan (FOLFIRI: 5 patients, 48 exposures).
Table 3.
Patient Exposure to Concomitant Chemotherapy or Supportive Therapies.a
| Number of Patients Exposed (Total Exposures) | No Concomitant Therapies | Chemotherapyb | Supportive Therapiesc | Chemotherapy and Supportive Therapies |
|---|---|---|---|---|
| Combined therapy | 23 (108) | 12 (55) | 11 (75) | 33 (236) |
| VA therapy | 11 (101) | 2 (5) | 1 (3) | 5 (20) |
| mAb therapy | 5 (42) | 3 (7) | 2 (6) | 3 (13) |
| All patients | 35 (251) | 17 (67) | 14 (84) | 40 (269) |
Abbreviations: VA, Viscum album L; mAb, monoclonal antibodies.
Values represent the number of patients exposed, with the total number of exposures in parentheses.
Chemotherapy agents included fluorouracil, bendamustine, capecitabine, carboplatin, cisplatin, cyclophosphamide, docetaxel, fludarabine, folinic acid, gemcitabine, irinotecan, oxaliplatin, paclitaxel, pemetrexed, and vinorelbine.
Supportive therapies included atropine, clemastine, dexamethasone, dimethindene maleate, paracetamol, and prednisolone.
Out of 56 patients included in the study, 34 (61%) experienced a total of 142 AEs on 85 exposures (Table 4). Among patients who experienced an AE, the median number of events per exposure was 1 (IQR = 1-2; range = 1-9) and the median number of events per patient overall was 3 (IQR = 1-6; range = 1-25). The highest incidence of AEs was in the mAb therapy group, with 5 out of 8 patients (63% of patients) experiencing a total of 22 events on 18 exposures (27% of exposures). This was followed by the combined therapy group, with 24 out of 43 patients (56%) experiencing 112 events on 61 exposures (13%), and the VA therapy group, with 5 out of 12 patients (42%) experiencing 8 events on 6 exposures (5%). Crude rates of AEs were calculated as 32 events occurring per 100 exposures to mAb therapy, 21 events per 100 exposures to combined therapy, and 6 events per 100 exposures to VA therapy. Apart from designation of AEs to 1 of the 3 therapy types based on timing of the event, specific causality was not attributed because many of the events could have been reactions to concomitantly applied mAb, VA, chemotherapeutic agents, and/or supportive therapies (Table 4). The most common events following exposure to combined therapy were leucopenia (16% of patients), stomatitis (14%), diarrhea and malaise (each 9%), skin reactions, rash, acne, nausea, chills, and palmar-plantar erythrodysesthesia (each 7%). For mAb therapy, common reactions were leucopenia and malaise (each 25%), neutropenia, weakness, acneiform rash, and anemia (each 14%), and for VA therapy, they were skin reaction or rash (25%), pruritus (17%), and urticaria (8%).
Table 4.
Frequency of Nonserious and Serious Adverse Events Classified as MedDRA-Preferred Terms and Grouped by System Organ Class.a
| System Organ Class | Preferred Term | All Patients, n = 56 (671) | Combined Therapy, n = 43 (474) | VA Therapy, n = 12 (129) | mAb Therapy, n = 8 (68) |
|---|---|---|---|---|---|
| Blood and lymphatic system | Anemia (m, c) | 1 | — | — | 1 |
| Granulocytopenia (m, c) | 1 | 1 | — | — | |
| Leucopenia (m, c) | 20 | 9 | — | 11 | |
| Neutropenia (m, c) | 2 | 1 | — | 1 | |
| Thrombocytopenia (m, c) | 2 | 2 | — | — | |
| Gastrointestinal | Colitis (m, c) | 1 | 1 | — | — |
| Diarrhea (m, c, v) | 6 | 6 | — | — | |
| Nausea/Vomiting (m, c, v) | 7 (5) | 7 (5) | — | — | |
| Stomatitis/Mucositis (m, c) | 9 | 9 | — | — | |
| General disorders and administration site | Asthenia (m, c) | 2 (1) | 1 (1) | — | 1 |
| Chills (m, v, c) | 4 | 4 | — | — | |
| Decreased appetite (m, c) | 1 | 1 | — | — | |
| Fatigue (m, v, c) | 2 | 2 | — | — | |
| Hyperhidrosis (m, c) | 1 | 1 | — | — | |
| Malaise (m, v, c) | 6 (3) | 4 (2) | — | 2 (1) | |
| Pain (m, v, c) | 1 | 1 | — | — | |
| Pyrexia (m, v, c) | 2 | 2 | — | — | |
| Swelling (m, v, c) | 1 | 1 | — | — | |
| Unspecified hospitalization (m, v, c) | 2 (2) | — | 1 (1) | 1 (1) | |
| Immune system | Hypersensitivity (m, v, c) | 2 | 2 | — | — |
| Infections and infestations | Abscess (c) | 2 (1) | 2 (1) | — | — |
| Eye infection (m, c) | 2 | 2 | — | — | |
| Fungal infection (m, c) | 1 | — | — | 1 | |
| Gingivitis (m, c) | 1 | 1 | — | — | |
| Oral herpes | 1 | 1 | — | — | |
| Pyelonephritis (m, c) | 1 (1) | 1 (1) | — | — | |
| Septic encephalopathy | 1 (1) | 1 (1) | — | — | |
| Skin infection (m, c) | 1 | 1 | — | — | |
| Stoma site infection (m, c) | 1 | — | — | 1 | |
| Urinary tract infection (m, c) | 3 | 3 | — | — | |
| Urosepsis (c) | 1 (1) | 1 (1) | — | — | |
| Musculoskeletal and connective tissue | Back pain (m, c) | 1 | 1 | — | — |
| Nervous system | Cognitive disorder (c) | 2 (2) | 2 (2) | — | — |
| Dizziness (m, c) | 3 (3) | 3 (3) | — | — | |
| Polyneuropathy (c) | 5 (1) | 4 (1) | — | 1 | |
| Somnolence (m, c) | 2 (2) | 2 (2) | — | — | |
| Renal and urinary | Bladder disorder (m, c) | 1 | 1 | — | — |
| Enuresis | 2 (2) | 2 (2) | — | — | |
| Respiratory, thoracic, and mediastinal | Dyspnea (m, c) | 1 | 1 | — | — |
| Rhinorrhea (m, v, c) | 1 | 1 | — | — | |
| Skin and subcutaneous | Acneiform rash (m) | 12 | 10 | — | 2 |
| Dry skin (m, c) | 3 | 3 | — | — | |
| Eczema (m, c) | 1 | 1 | — | — | |
| Generalized erythema (m, v, c) | 2 | 2 | — | — | |
| Palmar-plantar erythrodysesthesia (m, c) | 3 | 3 | — | — | |
| Pruritus (m, v, c) | 5 | 2 | 3 | — | |
| Rosacea | 1 | 1 | — | — | |
| Skin reaction/Rash (unspecified) (m, v, c) | 7 | 4 | 3 | — | |
| Urticaria (m, v, c) | 1 | — | — | — | |
| Vascular disorders | Hemorrhage (m, c) | 1 (1) | 1 (1) | — | — |
| Total | 142 (26 SAE) | 112 (23 SAE) | 8 (1 SAE) | 22 (2 SAE) |
Abbreviations: MeDRA, Medical Dictionary for Regulatory Activities; VA, Viscum album L; mAb, monoclonal antibodies; n, number of patients with the number of treatment exposures in parentheses; SAE, serious adverse events.
Values represent the total number of documented adverse events, with the number of events classified as serious shown in parentheses. Causality was not attributed to adverse events because many of the events could have been reactions to concomitantly applied mAb (m), chemotherapeutic agents (c), VA (v), and/or supportive therapies (not indicated).
Of the total 142 AEs to 85 exposures, 26 events (18%) to 13 exposures (15%) were classified as serious because they required hospitalization, prolongation of existing hospitalization, or resulted in persistent or significant incapacity (Table 4). One patient (8%) in the VA therapy group experienced a serious AE (unspecified hospitalization), which was attributed to concomitant chemotherapy. One patient (13%) in the mAb group was hospitalized on 2 occasions: once following exposure to rituximab with concomitant paracetamol and dimethindene maleate (malaise) and once following exposure to ofatumumab with concomitant paracetamol and dimethindene maleate (unspecified hospitalization). Seven patients (16%) experienced 23 serious AEs to 10 exposures of combined therapy. Of these 10 exposures, 6 involved concomitant chemotherapies, and 9 involved concomitant supportive therapies, making attribution of causality difficult.
AE rates (ie, total exposures with ≥1 AE/total patient days of exposure) and results of the longitudinal analysis of AEs are shown in Table 5. Univariable analyses did not reveal any significant associations between demographic or treatment factors and AEs. A multivariable GEE model adjusted for treatment exposure, age at treatment, gender, UICC stage, concomitant chemotherapy, and concomitant supportive therapy indicated increased odds of experiencing an AE following mAb therapy compared with combined therapy (OR = 4.97; 95% CI = 1.53-16.14; P = .008). AE rates and the directions of ORs suggest that VA therapy and female gender were negatively associated with AEs, and stage III to IV cancer, concomitant chemotherapy, and supportive therapies were positively associated with AEs; however, none of these associations was statistically significant.
Table 5.
Incidence of AEs and Results of Univariable and Multivariable General Estimating Equations.a
| AE Rate (%) | Univariable |
Multivariable |
|||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | ||
| Combined therapy | 61/474 (12.9) | 1.00 | 1.00 | ||
| VA therapy | 6/129 (4.7) | 0.65 (0.21-2.01) | .457 | 0.77 (0.21-2.76) | .686 |
| mAb Therapy | 18/68 (26.5) | 2.80 (0.85-9.22) | .090 | 4.97 (1.53-16.14) | .008 |
| Age (mean ± SD) | 62.9 ± 10.0 | 1.01 (0.98-1.03) | .501 | 1.00 (0.97-1.03) | .903 |
| Male | 36/316 (11.4) | 1.00 | 1.00 | ||
| Female | 49/355 (13.8) | 1.15 (0.52-2.55) | .731 | 0.89 (0.43-1.88) | .768 |
| UICC I-II | 7/111 (6.3) | 1.00 | |||
| UICC III-IV | 70/457 (15.3) | 2.25 (0.74-6.82) | .151 | 3.42 (0.90-12.93) | .070 |
| NA/Unknown | 8/103 (7.8) | ||||
| Breast | 20/191 (10.5) | 1.00 | |||
| Digestive | 58/407 (14.3) | 1.30 (0.39-4.30) | .668 | ||
| Hematological | 3/15 (20.0) | 1.25 (0.22-7.23) | .800 | ||
| Respiratory | 1/19 (5.3) | 0.29 (0.06-1.30) | .107 | ||
| Urogenital | 3/39 (7.7) | 1.36 (0.18-10.07) | .767 | ||
| Chemotherapy = no | 32/335 (9.6) | 1.00 | 1.00 | ||
| Chemotherapy = yes | 53/336 (15.8) | 1.18 (0.71-1.98) | .522 | 1.09 (0.61-1.93) | .770 |
| Supportive therapy = no | 33/318 (10.4) | 1.00 | 1.00 | ||
| Supportive therapy = yes | 52/353 (14.7) | 1.18 (0.73-1.91) | .505 | 1.23 (0.69-2.20) | .474 |
Abbreviations: AE, adverse event; OR, odds ratio; VA, Viscum album L; mAb, monoclonal antibodies; UICC, Union for International Cancer Control.
AE rates are shown as the number of exposures with ≥1 adverse event over the total number of exposures (%).
Rates for serious AEs were similar for combined therapy (2%) and mAb therapy (3%) and were lower for VA therapy (0.8%). Significance tests were not performed for serious AEs because of the low number of events. The incidence rates of AEs according to mAb exposure are shown in Table 6. The highest incidence in the combined therapy group was for bevacizumab. Bevacizumab had an even higher incidence in the mAb group; however, the small sample size of the mAb group prevents an accurate assessment of incidence according to specific therapies.
Table 6.
Incidence of AEs According to Monoclonal Antibody Exposure.
| Monoclonal Antibodies | Combined Therapy |
mAb Therapy |
||
|---|---|---|---|---|
| Exposures | Exposures With ≥1 AE (%) | Exposures | Exposures With ≥1 AE (%) | |
| Ado-trastuzumab emtansine | 17 | 1 (5.8) | — | — |
| Bevacizumab | 188 | 32 (17.0) | 18 | 9 (50.0) |
| Cetuximab | 146 | 20 (13.7) | 4 | 2 (50.0) |
| Ofatumumab | — | — | 3 | 2 (66.7) |
| Panitumumab | 12 | 1 (8.3) | 1 | 1 (100.0) |
| Rituximab | 4 | 0 | 6 | 1 (16.7) |
| Trastuzumab | 107 | 7 (6.5) | 36 | 3 (8.3) |
Abbreviations: AE, adverse event; mAb, monoclonal antibodies.
Discussion
In this retrospective cohort study investigating the safety of combined therapy with Helixor VA preparations and mAb, the highest incidence of AEs occurred after exposure to mAb therapy. A multivariable GEE model indicated that the odds for patients experiencing an AE following mAb therapy were nearly 5 times higher compared with that for mAb plus VA. Whereas AE rates suggested that concomitant chemotherapy was positively associated with AEs (16% of all exposures with chemotherapy vs 10% without chemotherapy), this association was not statistically significant. This is most likely a result of sample size limitations. Furthermore, chemotherapy AEs might be underreported compared with AEs related to mAb or VA because of the well-known and expected toxicity profiles of chemotherapeutic agents. The rate of serious AEs did not appear to differ between combined and mAb therapies and was lower for VA therapy.
The most frequent AEs related to combined therapy were development of an acneiform rash, leucopenia, and stomatitis/mucositis, none of which were serious. Serious AEs related to combined therapy were mostly nervous system related, such as dizziness, nausea and vomiting, infections, or malaise. The toxicity profile of the combined therapy group was similar to that of the targeted therapy group, for which leucopenia, acneiform rash, and malaise were the most frequent events. Apart from an unspecified hospitalization attributed to chemotherapy, all AEs in the VA therapy group were skin-related reactions.
It is important to acknowledge a number of limitations of our study. First, of the 95 medical records initially reviewed, 36 patients were excluded for not satisfying the 1-month washout period. Specification of a washout period was important for this study because many of the observed AEs can have a delayed onset or be long-lasting (eg, leucopenia, acneiform rash). However, this resulted in small sample sizes for both the individual therapy groups and a consequent decrease in statistical power. Because of the retrospective and nonrandomized nature of this study, patient characteristics and diagnoses were not balanced across treatment groups. Factors considered as potential confounders (ie, age, gender, UICC stage, concomitant chemotherapy, and supportive therapies) were included in multivariable analysis; however, it is likely that unmeasured confounders, such as previous surgery or unmeasured comorbidity, exist. Furthermore, although mechanistically it would make sense to assess different mAb separately and to also consider the role of therapy dose, small sample sizes and heterogeneity prevented us from doing this. Another limitation related to the use of retrospective data is that AE descriptions were often not detailed enough to enable grading in terms of severity. Some descriptions were vague, such as “unspecified hospitalization” or “skin reaction,” and some mild, expected AEs such as fatigue or headaches were probably underreported. Importantly, however, we were able to identify all serious AEs based on hospitalization and AE management data.
Overall, although our results must be interpreted with care, they provide a first picture of the current application and toxicity profile of a combination strategy involving VA and mAb. Furthermore, to our knowledge, this is the first attempt to investigate the safety of such a strategy. The types and frequencies of AEs and serious AEs observed in the combined and mAb groups in our study are consistent with adverse drug reactions to mAb and chemotherapeutic agents described in the literature.21-27 In a phase II trial treating metastatic colorectal cancer patients with FOLFOX and cetuximab, 76% of patients had an AE (grades 3-4).28 The most frequent events were neutropenia (30%), skin reactions (18%), rash (11%), and diarrhea (8%). In another study, a phase III trial comparing FOLFIRI plus cetuximab with FOLFIRI plus bevacizumab in colorectal cancer, 71% of patients in the cetuximab group and 64% of patients in the bevacizumab group had AEs (grades 3-4), with the most frequent events being hematotoxicity (cetuximab, 25%; bevacizumab, 21%), skin reactions (26% and 2%), and diarrhea (11% and 14%).29 Additionally, common grade 1 to 2 events included acneiform rash (61%), nausea (45%), and stomatitis (38%). In our study, 55.8% of patients exposed to combined therapy (VA plus mAb with or without chemotherapy) reported AEs. This incidence is for events of any grade; however, low-grade events were most likely underreported. The most frequent events for combined therapy were leucopenia (16% of patients); stomatitis (14%); skin reactions, including rash and dry skin (12%); diarrhea; and malaise (each 9%). The rates of AEs observed in our study were lower than those reported for clinical trials involving similar medications. Nevertheless, the types of AEs are similar, and there is no evidence to suggest that VA increased the frequency or severity of the known toxicities of the applied mAb or chemotherapeutic agents.
The types of AEs observed in the VA therapy group (eg, pruritus, skin reaction, and urticaria) are consistent with adverse drug reactions to intravenously administered VA preparations, as previously reported.30 The relative frequency for patients experiencing an AE in the VA therapy group (42% of patients) is much higher than what we have published previously for intravenous VA (5%). This difference is because our previous study focused on VA-related AEs only, whereas the current study includes AEs caused by VA and by concomitant chemotherapy (42% of patients in the VA therapy group had concomitant chemotherapy plus supportive therapy and 17% of patients had concomitant chemotherapy only).
Although we did not find any comparable studies in the literature, a number of groups have investigated whether VA preparations interact with traditional chemotherapy agents. Mansky et al16,31 have shown that combined therapy involving subcutaneous injections of VA (Helixor Abietis) and intravenously administered gemcitabine is well tolerated, and clinical response was similar to that for gemcitabine alone. Importantly, the addition of VA did not affect the pharmacokinetics of gemcitabine at any of the VA dose levels tested (1-250 mg) and no botanical-drug interactions were observed. Our results differed from those reported by Mansky et al in that we did not observe a high incidence of local reactions or febrile and flu-like reactions, which are characteristic of subcutaneous administration of VA.32 As expected, because of the known toxicity profiles of cetuximab and panitumumab, we observed a higher incidence of skin disorders such as acneiform rash. The hematological toxicity profile (leucopenia, anemia, and thrombocytopenia) was similar across all treatment groups (except for VA therapy) in both studies and is most likely a result of concomitant chemotherapy. A recent in vitro study investigated the effect of VA preparations on several commonly used chemotherapeutic agents in different cancer cell lines.33 It was found that VA preparations (Iscador Ltd) did not inhibit chemotherapy-induced cytostasis or cytotoxicity and showed an additive inhibitory effect at higher concentrations of VA.
Because of the complex and nonstandardized nature of botanical drugs, prediction and identification of botanical-drug interactions can be difficult. Adverse botanical-drug interactions often involve botanical-mediated induction or inhibition of hepatic and intestinal drug-metabolizing enzymes, such as cytochrome P450s (CYPs).34 However, previous in vitro investigations have shown that VA preparations have either no or minor effects on a range of CYPs, suggesting that VA-drug interactions based on drug metabolism are unlikely.35,36 Furthermore, mAb do not undergo hepatic metabolism but undergo proteolytic catabolism throughout the body.37,38 As a result, it is generally believed that they are not subject to significant pharmacokinetic-type drug interactions.39
Although pharmacokinetic interactions may be less of a concern regarding combined VA and mAb, potential pharmacodynamic interactions are important to consider. For example, vascular endothelial growth factor mAb, such as bevacizumab, are associated with causing hypertension, bleeding episodes, and thrombotic events and, therefore, should not be used with anticoagulants or following surgery.40,41 A potential drug interaction of VA might be an additive hypotensive effect when combined with antihypertensive drugs.42 Also, because application of VA can induce an innate immune response mediated by monocyte activation with a specific T-cell response,43,44 it has been suggested that interactions be considered in coadministration with immunosuppressive drugs. Theoretically, none of these properties should negatively interact with the mAb included in our study; and in fact, a more active immune system, along with potential additive antitumor properties, might be beneficial to established anticancer protocols involving mAb and chemotherapy agents. VA preparations have been shown to inhibit the transmembrane efflux pump P-glycoprotein in vitro, an effect that in tissues exposed to chemotherapy agents, might result in an increased cytoplasmic drug concentration and cytotoxicity.45,46 Furthermore, a range of clinical studies reported a reduction in adverse drug reactions to conventional anticancer therapy and improved HRQL when VA therapy was given concomitantly.12,13 The mechanisms involved in these possible benefits of VA are not well understood but may include upregulation of endorphins, stimulation of the immune system, stabilization of DNA, or psychological factors.12 Although we observed a lower incidence of AEs in the VA plus mAb group compared with mAb alone, whether this was a result of VA is not clear from this study.
Conclusions
Results from our observational study found a reduced incidence of AEs following exposure to mAb with VA compared to mAb without VA and no unexpected AEs. These results are in line with the prediction, based on theoretical considerations, of no significant interactions occurring between VA and mAb. Future research should focus on investigating combined use of VA with specific mAb, in larger and more homogeneous patient groups in order to further elucidate the safety and effectiveness of this approach. Overall, the current results suggest that combined therapy with VA and mAb is safe.
Acknowledgments
The authors would like to thank Antje Happe for her involvement in project management and administration and Evelyn Hartanto who entered data into the database.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Network Oncology was partially funded by Helixor Heilmittel GmbH & Co. KG. By contract, researchers were independent from the funder. Matthias Kröz received an honorarium for a lecture given at a Helixor meeting and received a fee for a single consultation.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munagala R, Aqil F, Gupta RC. Promising molecular targeted therapies in breast cancer. Indian J Pharmacol. 2011;43:236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [DOI] [PubMed] [Google Scholar]
- 5. Corso S, Giordano S. Targeted therapies in cancer and mechanisms of resistance. J Mol Med (Berl). 2014;92:677-679. [DOI] [PubMed] [Google Scholar]
- 6. Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kümler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014;40:259-270. [DOI] [PubMed] [Google Scholar]
- 8. Ghirelli C, Hagemann T. Targeting immunosuppression for cancer therapy. J Clin Invest. 2013;123:2355-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blower P, De Wit R, Goodin S, Aapro M. Drug-drug interactions in oncology: why are they important and can they be minimized? Crit Rev Oncol Hematol. 2005;55:117-142. [DOI] [PubMed] [Google Scholar]
- 11. Appleby L, Morrissey S, Bellmunt J, Rosenberg J. Management of treatment-related toxicity with targeted therapies for renal cell carcinoma: evidence-based practice and best practices. Hematol Oncol Clin North Am. 2011;25:893-915. [DOI] [PubMed] [Google Scholar]
- 12. Kienle GS, Kiene H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142-157. [DOI] [PubMed] [Google Scholar]
- 13. Melzer J, Hostanska K, Felix I, Saller R. Evidence-based integrative medicine as exemplified by adjuvant mistletoe treatment. Eur J Integr Med. 2008;1:10-11. [Google Scholar]
- 14. Hajtó T, Adámy A, Langmár Z, et al. Enhanced effectiveness of conventional oncotherapy with plant immunomodulators: overview of recent advances. Adv Med Plant Res. 2013;1:56-65. [Google Scholar]
- 15. Hajtó T, Fodor K, Perjési P, Németh P. Difficulties and perspectives of immunomodulatory therapy with mistletoe lectins and standardized mistletoe extracts in evidence-based medicine. Evid Based Complement Alternat Med. 2011;2011:298972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansky PJ, Wallerstedt DB, Sannes TS, et al. NCCAM/NCI phase 1 study of mistletoe extract and gemcitabine in patients with advanced solid tumors. Evid Based Complement Alternat Med. 2013;2013:964592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schad F, Axtner J, Happe A, et al. Network Oncology (NO): a clinical cancer register for health services research and the evaluation of integrative therapeutic interventions in anthroposophic medicine. Forsch Komplementarmed. 2013;20:353-360. [DOI] [PubMed] [Google Scholar]
- 18. ICH Harmonized Tripartite Guideline. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. October 1994. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf. Accessed November 28, 2016.
- 19. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 20. Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:1-11. [Google Scholar]
- 21. Smith IE, Pierga JY, Biganzoli L, et al. First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2251 patients. Ann Oncol. 2011;22:595-602. [DOI] [PubMed] [Google Scholar]
- 22. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [DOI] [PubMed] [Google Scholar]
- 23. Bossi P, Kornek G, Lanzetta G, et al. Safety and feasibility of every-other-week maintenance cetuximab after first-line chemotherapy in patients with recurrent or metastatic head and neck squamous cell cancer. Head Neck. 2013;35:1471-1474. [DOI] [PubMed] [Google Scholar]
- 24. Cohen MH, Chen H, Shord S, et al. Approval summary: cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer. Oncologist. 2013;18:460-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmucker C, Ehlken C, Agostini HT, et al. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus goldstandard. PLoS One. 2012;7:e42701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32. [DOI] [PubMed] [Google Scholar]
- 27. Gebbia V, Del Prete S, Borsellino N, et al. Efficacy and safety of cetuximab/irinotecan in chemotherapy-refractory metastatic colorectal adenocarcinomas: a clinical practice setting, multicenter experience. Clin Colorectal Cancer. 2006;5:422-428. [DOI] [PubMed] [Google Scholar]
- 28. Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [DOI] [PubMed] [Google Scholar]
- 29. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [DOI] [PubMed] [Google Scholar]
- 30. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Safety of intravenous application of mistletoe (Viscum album L.) preparations in oncology: an observational study. Evid Based Complement Alternat Med. 2014;2014:236310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mansky PJ, Grem J, Wallerstedt DB, Monahan BP, Blackman MR. Mistletoe and gemcitabine in patients with advanced cancer: a model for the phase I study of botanicals and botanical-drug interactions in cancer therapy. Integr Cancer Ther. 2003;2:345-352. [DOI] [PubMed] [Google Scholar]
- 32. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Adverse drug reactions and expected effects to therapy with subcutaneous mistletoe extracts (Viscum album L.) in cancer patients. Evid Based Complement Alternat Med. 2014;2014:724258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weissenstein U, Kunz M, Urech K, Baumgartner S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement Altern Med. 2014;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fimognari C, Ferruzzi L, Turrini E, et al. Metabolic and toxicological considerations of botanicals in anticancer therapy. Expert Opin Drug Metab Toxicol. 2012;8:819-832. [DOI] [PubMed] [Google Scholar]
- 35. Engdal S, Nilsen OG. In vitro inhibition of CYP3A4 by herbal remedies frequently used by cancer patients. Phytother Res. 2009;23:906-912. [DOI] [PubMed] [Google Scholar]
- 36. Doehmer J, Eisenbraun J. Assessment of extracts from mistletoe (Viscum album) for herb-drug interaction by inhibition and induction of cytochrome P450 activities. Phytother Res. 2012;26:11-17. [DOI] [PubMed] [Google Scholar]
- 37. Panoilia E, Schindler E, Samantas E, et al. A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother Pharmacol. 2015;75:791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52:83-124. [DOI] [PubMed] [Google Scholar]
- 39. Prueksaritanont T, Tang C. ADME of biologics: what have we learned from small molecules? AAPS J. 2012;14:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother. 2004;38:1258-1264. [DOI] [PubMed] [Google Scholar]
- 41. Ignoffo RJ. Overview of bevacizumab: a new cancer therapeutic strategy targeting vascular endothelial growth factor. Am J Health Syst Pharm. 2004;61(21, suppl 5):S21-S26. [DOI] [PubMed] [Google Scholar]
- 42. Cassileth BR, Yeung KS, Gubili J. Herb-Drug Interactions in Oncology Second Edition. New York, NY: PMPH-USA; 2010. [Google Scholar]
- 43. Son GS, Ryu WS, Kim HY, Woo SU, Park KH, Bae JW. Immunologic response to mistletoe extract (Viscum album L) after conventional treatment in patients with operable breast cancer. J Breast Cancer. 2010;13:14-18. [Google Scholar]
- 44. Heinzerling L, Von Baehr V, Liebenthal C, Von Baehr R, Volk HD. Immunologic effector mechanisms of a standardized mistletoe extract on the function of human monocytes and lymphocytes in vitro, ex vivo, and in vivo. J Clin Immunol. 2006;26:347-359. [DOI] [PubMed] [Google Scholar]
- 45. Matthes H, Friedel WE, Bock PR, Zänker KS. Molecular mistletoe therapy: friend or foe in established anti-tumor protocols? A multicenter, controlled, retrospective pharmaco-epidemiological study in pancreas cancer. Curr Mol Med. 2010;10:430-439. [DOI] [PubMed] [Google Scholar]
- 46. Engdal S, Nilsen OG. Inhibition of P-glycoprotein in Caco-2 cells: effects of herbal remedies frequently used by cancer patients. Xenobiotica. 2008;38:559-573. [DOI] [PubMed] [Google Scholar]