Abstract
Objectives. This study aimed to identify the relationship between health-related quality of life (HRQoL) measured by the Functional Assessment Cancer Therapy–General (FACT-G) and survival in metastatic colorectal cancer (mCRC) patients. Methods. The clinical characteristics and FACT-G scores were retrospectively reviewed in mCRC patients who visited the Cancer Center of Korean Medicine. The overall survival (OS) was calculated and compared using the Kaplan-Meier method and log-rank test. Univariate and multivariate Cox regression analyses were performed based on clinical characteristics and FACT-G scores. To identify significant differences in answer frequency, χ2 tests and Fisher’s exact tests were used. Results. A total of 58 patients were reviewed. The proportion of patients who had an Eastern Cooperative Oncology Group–Performance Status ≥ 2 was 43.1%, multiple distant metastatic sites was 77.6%, liver metastases was 43.1%, been previously treated was 89.7%, and received more than the second-line chemotherapy was 75.5%. The mean total FACT-G score was 65.3 (median 65.6). The median OS was 7 months. There was no significant difference in OS between the 2 groups divided by the median values of FACT-G total and subscores. In univariate analyses, functional well-being (FWB) score had a significant impact on survival. In multivariate analyses, presence of liver metastasis, FACT-G total score, and FWB score were significant prognostic predictors of survival. No statistically different answer frequency was observed for any question regarding FWB. Conclusions. This study found that FACT-G total and FWB scores were potential prognostic factors for predicting OS in relapsed or refractory mCRC patients treated with Korean Medicine.
Keywords: colorectal neoplasm, neoplasm metastasis, quality of life, survival, Korean medicine
Introduction
Cancer is one of the leading causes of death worldwide. In clinical studies of cancer, response rate and overall survival (OS) have been widely used to assess the efficacy of a treatment modality.1 However, in metastatic cancer patients, health-related quality of life (HRQoL) is also important in deciding the type of treatment. The American Society of Clinical Oncology has suggested that patient outcomes, such as HRQoL, should receive more attention than response rates.2 Because HRQoL reflects the patient’s status in various aspects, it has been included as a treatment outcome in oncology clinical trials. HRQoL can represent the impact of chemotherapy response, but it can also be a predictor of survival.1 HRQoL is a known prognostic factor of survival according to previous studies.3-9 One review article about the quality of life in cancer patients revealed that most studies showed a positive relationship between HRQoL and survival in cancer patients.10 These results have encouraged doctors to recognize the importance of assessing the HRQoL status in cancer patients to evaluate their prognosis.
To assess the subjective HRQoL status in cancer patients, various measurement systems have been developed. One of the HRQoL questionnaires is the Functional Assessment Cancer Therapy–General (FACT-G), a validated and reliable oncology-specific HRQoL measurement system. The FACT-G consists of 4 domains: physical well-being (PWB), social/family well-being (SFWB), emotional well-being (EWB), and functional well-being (FWB).11 In most studies that have focused on the impact of HRQoL on patient survival using FACT-G, the baseline FACT-G total score was significantly associated with the survival of cancer patients.12-15 In colorectal cancer (CRC) patients, HRQoL scores also remained good prognostic values for survival. Many studies have used the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) as an HRQoL assessment tool16 and have reported that physical functioning and social functioning showed significant associations with survival.17-20 However, there have been few studies that have used the FACT-G as a HRQoL assessment tool in CRC patients. To determine the correlation between the EORTC QLQ-C30 and the FACT-G, one study investigated the equivalence on the basis of physical, emotional, and functional/role domains.21 However, based on empirical data, the EORTC QLQ-C30 and the FACT-G measure different aspects of HRQoL, so the results of EORTC QLQ-C30 cannot be a substitute for those of the FACT-G.22
Therefore, we investigated the association between HRQoL measured with the FACT-G and OS in metastatic colorectal cancer (mCRC) patients.
Methods
This study was designed as a retrospective cohort study, a type of observational study. It was performed with a cohort from the Cancer Center of Korean Medicine at Kyung Hee University Hospital at Gangdong from June 2006 to November 2014. The quality-of-life data and clinical data of mCRC patients were retrospectively reviewed under the approval from the institutional review board (IRB No. 2015-11010).
Patients
The medical records of CRC patients who answered the FACT-G questionnaire were reviewed. The patients answered the FACT-G questionnaire at the time of initiation of Korean Medicine (KM) treatment. We handed out the Korean version of the FACT-G questionnaire to patients, who then answered the questions by themselves at the hospital. Patients who did not agree to answer HRQoL questions or who were unable to answer the self-administered questionnaire because of their medical condition were not included in the study. Patients were mainly treated with herbal medicine, acupuncture, and moxibustion in our center. In Korea, some cancer patients choose KM treatment because they cannot be treated with conventional medicine as a result of old age, poor performance status, comorbidity, or adverse events of radical therapy. Therefore, the Western medical treatment history of patients was diverse, depending on the initial stage, response to previous treatment, and patient preference. To eliminate the influence of diverse treatments on HRQoL and to show the overall effects of KM treatment, we enrolled only recipients of KM treatment. Patients were eligible if they had histologically confirmed CRC. We reevaluated tumor-nodes-metastasis (TNM) stage based on medical records at the time of the survey. Only the patients who had distant metastasis at the time of survey were eligible. We excluded patients whose FACT-G scores could not be prorated because less than half of the items had been answered. Because we did not review the questionnaire after self-administration, unanswered items were present. We also excluded patients whose survival status was unclear. Survival information was obtained through the National Health Insurance Corporation. We could not identify survival status for patients who did not have National Health Insurance coverage. We also excluded patients who were undergoing concurrent anticancer treatment with conventional medicine (chemotherapy, radiotherapy) at the time of survey to eliminate the influence of concurrent therapy on HRQoL.
HRQoL Assessment
Patient HRQoL was assessed using the FACT-G, version 4, which was translated into Korean according to Functional Assessment of Chronic Illness Therapy (FACIT) methodology. The FACT-G consists of 27 questions and uses a 5-point Likert scale with responses that ranged from “Not at all” to “Very true.”11 We scored the FACT-G using the guideline of the FACT-G manual. The total FACT-G score, ranging from 0 to 108, is the sum of the scores of PWB, SFWB, EWB, and FWB. A higher total score indicates a better HRQoL. If the question had a negative meaning, we reversed the sign of the response before calculating the sum. If there were missing items, the subscale scores were prorated by multiplying the sum of the scores by the number of items in the subscale and then dividing the value by the number of items answered. Scores could be prorated only if more than half of the items were answered. Moreover, the total FACT-G response rate should be greater than 80%.11 Use of the FACT-G questionnaires of the FACIT system was also permitted according to FACIT.org (Licensor).
Measurements
All patients filled out the FACT-G questionnaire at the hospital at the start of KM treatment. Patient demographic and clinical variables (age; sex; body mass index; performance status; site of the primary tumor; number of distant metastatic sites; presence of liver metastasis; presence of stoma; prior treatment history, including surgery, radiotherapy, and chemotherapy; and the number of prior chemotherapy regimens) were investigated based on the time of the FACT-G survey. The performance status was measured using the Eastern Cooperative Oncology Group–Performance Status (ECOG-PS). The grade of the ECOG-PS ranges from 0 (normal activity) to 5 (death).23 A higher ECOG-PS score reflects a worse performance status. In this study, the ECOG-PS was dichotomized as ≤1 or ≥2. The site of the primary tumor was trichotomized as colon, rectum, or both colon and rectum. The number of distant metastatic sites was divided into 2 categories: 1 or >1. The presence of liver metastasis, previous treatment history, operation history, radiotherapy history, chemotherapy history, and presence of stoma were all dichotomized as no or yes. The number of prior chemotherapy regimens was divided into 2 categories: 1 or ≥2.
Treatment
The treatment modality applied for mCRC patients was KM, including herbal medication, acupuncture, and moxibustion. In Korea, both conventional Western medicine and KM are legally approved. The KM treatment modalities were initiated after each patient agreed to treatment with KM. The major anticancer agent was Rhus verniciflua Stokes (RVS) extract. The RVS extract originates from the lacquer tree, which grows in East Asia. In previous studies, RVS has been reported to have apoptotic activity and antiproliferative, anti–tumor migration, and antiangiogenic effects.24-26 The RVS extract was prepared using a standardized method of water extraction at 95°C, concentration, and lyophilization. After the toxic allergen urushiol was removed, the RVS extract was examined to determine the concentrations of the main compounds for quality control. Patients were administered 3 capsules of RVS extract for a total of 1500 mg/d. The median duration of treatment was 3 months, ranging from 0 to 33 months. The RVS extract can induce skin rash, but no adverse effect was reported in the patients included in this study.
Analysis
The OS time was measured from the date of initial KM treatment to the date of death resulting from any cause. We obtained the survival information through the Korean National Health Insurance Corporation. Survival curves were calculated with the Kaplan-Meier method and were compared using the log-rank test. Univariate analyses of Cox proportional hazards regression were performed on the HRQoL scores and potential prognostic factors. Before proceeding to multivariate analyses, we analyzed the Pearson’s correlations among the HRQoL scales to prevent multicollinearity that could influence the results of the multivariate analyses. Multivariate analyses were performed on the data that showed significance and borderline significance in univariate analyses. To identify answer differences on items of FACT-G between the 2 groups divided by median survival, χ2 tests or Fisher’s exact tests were carried out with subscale items that showed significance in multivariate analyses. A P value <.05 was considered statistically significant. We defined borderline significance as a P value <.10 but >.05. All data analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0 (SPSS, Inc, Chicago, IL).
Results
We analyzed 135 CRC patients who completed the FACT-G questionnaire and who visited the Cancer Center of Korean Medicine, Kyung Hee University Hospital at Gangdong from June 2006 to November 2014. Among the 135 patients, 26 were excluded because of ongoing conventional cancer treatment at the time of the survey; 25 were excluded because of no distant metastasis; and 7 were excluded because of unknown survival status. We could not identify survival status in 6 patients whose health insurance was suspended because of delayed payment and in 1 patient who was not of Korean nationality. Because the FACT-G total scores of 4 patients could not be prorated as a result of many missing answers on the questionnaire; these 4 patients were also excluded. Of 73 patients, 15 were also excluded because FACT-G was assessed after initiating KM treatment. Our final analysis included 58 mCRC patients (Figure 1). The proportion of patients who had a performance status of 2 or more was 43.1%, whereas 77.6% had multiple metastases. Liver metastasis was present in 43.1%. A total of 89.7% of the participants had been previously treated with conventional standard anticancer therapy. The proportion of patients who had undergone more than a second line of chemotherapy was 75.5%. The median times from initial cancer diagnosis and metastases diagnosis to FACT-G survey point were 23 months (0-125 months) and 11 months (0-56 months), respectively. A total of 6 patients had received no previous treatment. The median time from the last conventional treatment to KM treatment among 52 patients was 2 months (0-37 months). The median KM treatment duration of 58 patients was 3 months (0-33 months).
Figure 1.
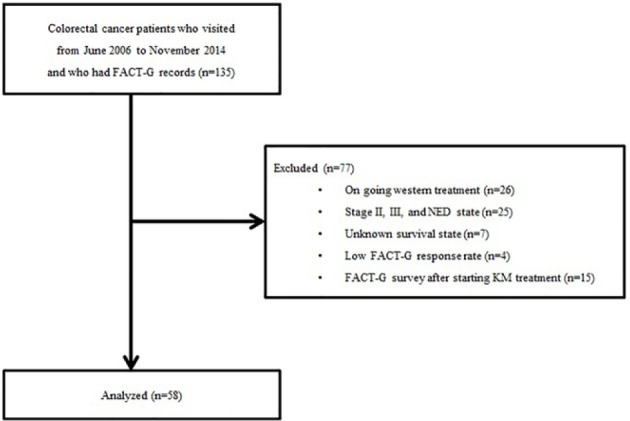
Flowchart of the study.
Abbreviations: FACT-G, Functional Assessment Cancer Therapy–General; NED, no evidence of disease; KM, Korean Medicine.
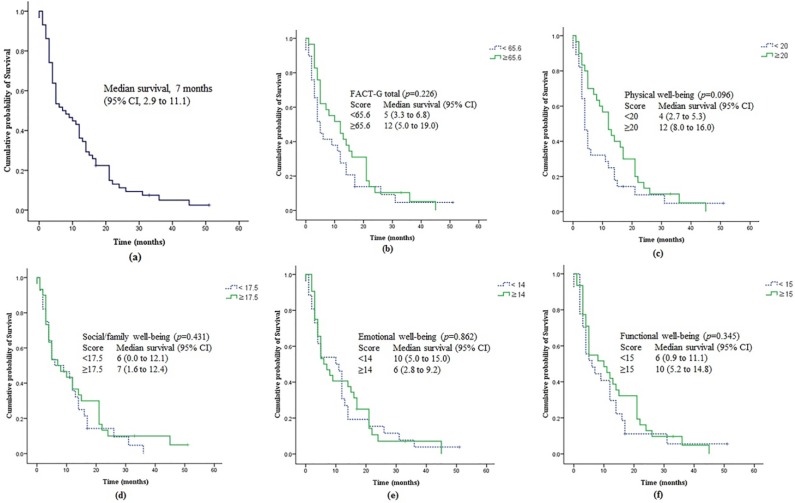
The mean scores of FACT-G were as follows: PWB, 18.5; SFWB, 17.3; EWB, 13.9; FWB, 15.6; and FACT-G total score, 65.3. The clinical characteristics and HRQoL characteristics of the patients are presented in Table 1. The median OS of 58 patients was 7 months (95% CI = 2.9-11.1; Figure 2), and 55 deaths were noted at the study point. The median survival times according to the FACT-G total and subscales are presented in Figure 2. The median survival time of patients with FACT-G score greater than or equal to the median was 12 months, whereas that of patients with FACT-G score below the median was only 5 months (P = .226; Figure 2). Among the HRQoL subscales, the median survival was not significantly different between the 2 groups (PWB, 12 vs 5 months, P = .096; SFWB, 6 vs 7 months, P = .431; EWB, 10 vs 6 months, P = .862; FWB, 6 vs 10 months, P = .345; Figure 2).
Table 1.
Patient Characteristics and HRQoL Scores (n = 58).
| n | Percentage | ||
|---|---|---|---|
| Sex | |||
| Male | 33 | 43.1 | |
| Female | 25 | 56.9 | |
| Age, years | |||
| Mean (range) | 56.3 (32-88) | ||
| BMI (kg/m2) | |||
| Mean (range) | 22.1 (15.8-29.1) | ||
| ECOG | |||
| ≤1 | 33 | 56.9 | |
| ≥2 | 25 | 43.1 | |
| Site of the primary tumor | |||
| Colon | 36 | 62.1 | |
| Rectum | 13 | 22.4 | |
| Both colon and rectum | 9 | 15.5 | |
| Number of distant metastatic sites | |||
| 1 | 13 | 22.4 | |
| >1 | 45 | 77.6 | |
| Presence of liver metastasis | |||
| No | 33 | 56.9 | |
| Yes | 25 | 43.1 | |
| Previous treatment history | |||
| Previously treated | 52 | 89.7 | |
| Not previously treated | 6 | 10.3 | |
| Previous treatment history | |||
| Surgery | 47 | 81.0 | |
| Chemotherapy | 49 | 84.5 | |
| Radiotherapy | 13 | 22.4 | |
| Number of chemotherapy regimens received | |||
| 1 | 12 | 24.5 | |
| ≥2 | 37 | 75.5 | |
| Presence of stoma | |||
| No | 51 | 87.9 | |
| Yes | 7 | 12.1 | |
| Scale | Mean ± SD | Median | Observed Range (Possible Range) |
| Physical well-being | 18.5 ± 5.9 | 20.0 | 6.0-28.0 (0-28.0) |
| Social/Family well-being | 17.3 ± 6.0 | 17.5 | 0-28.0 (0-28.0) |
| Emotional well-being | 13.9 ± 4.9 | 14.0 | 4.0-23.0 (0-24.0) |
| Functional well-being | 15.6 ± 6.0 | 15.0 | 3.0-28.0 (0-28.0) |
| FACT-G total score | 65.3 ± 16.3 | 65.6 | 29.0-101.0 (0-108.0) |
Abbreviations: HRQoL, health-related quality of life; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; FACT-G, Functional Assessment Cancer Therapy–General.
Figure 2.
The survival curves of (A) all 58 patients according to (B) FACT-G total score, (C) physical well-being score, (D) social/family well-being score, (E) emotional well-being score, and (F) functional well-being score dichotomized by the median value.
Abbreviation: FACT-G, Functional Assessment Cancer Therapy–General.
The patients’ clinical characteristics and HRQoL scores were evaluated in univariate analyses as potential prognostic factors of OS (Table 2). The FWB score was a statistically significant prognostic factor of survival in univariate analyses (P < .050). The presence of liver metastasis and FACT-G total score showed borderline significance as prognostic factors of survival (P < .10). All factors that were found to be significant or borderline significant in our univariate analyses were also analyzed using multivariate analyses.
Table 2.
Univariate Cox Proportional Hazard Regression of Clinical Data and HRQoL Scores.
| Variables | Unit of Increase | HR | 95% CI | P Value |
|---|---|---|---|---|
| ECOG | ECOG ≤1 | |||
| ≥2 | 1.436 | 0.837-2.463 | .189 | |
| Site of primary tumor | Colon | .115 | ||
| Rectum | 1.741 | 0.901-3.367 | .099 | |
| Both colon and rectum | 0.706 | 0.324-1.538 | .380 | |
| Number of distant metastatic sites >1 | Number of distant metastatic sites equal to 1 | 1.677 | 0.861-3.267 | .128 |
| Presence of liver metastasis | No presence of liver metastasis | 1.671 | 0.963-2.898 | .068 |
| Previous treatment history | No previous treatment history | 0.860 | 0.339-2.183 | .751 |
| Previous operation history | No previous operation history | 0.930 | 0.465-1.860 | .837 |
| Previous chemotherapy history | No previous chemotherapy history | 1.359 | 0.613-3.011 | .450 |
| Previous radiotherapy history | No previous radiotherapy history | 1.505 | 0.799-2.835 | .206 |
| Number of received chemotherapy regimens ≥2 | Number of received chemotherapy regimen equal to 1 | 1.217 | 0.626-2.369 | .562 |
| Presence of stoma | No stoma | 0.972 | 0.433-2.180 | .945 |
| FACT-G total score | 1 | 0.986 | 0.970-1.001 | .075 |
| Physical well-being score | 1 | 0.966 | 0.925-1.008 | .110 |
| Social/Family well-being score | 1 | 0.976 | 0.936-1.018 | .258 |
| Emotional well-being score | 1 | 0.990 | 0.939-1.045 | .721 |
| Functional well-being score | 1 | 0.953 | 0.912-0.996 | .034 |
Abbreviations: HRQoL, health-related quality of life; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; FACT-G, Functional Assessment Cancer Therapy–General.
To determine whether there was a correlation between the FACT-G total score and the FWB score, we performed Pearson’s correlation analyses. High correlation was identified between the 2 scores, as expected (r = 0.835; P < .001). Because of the high correlation, each HRQoL scale was examined separately with other clinical factors using the backward method of multivariate analyses. In multivariate analysis, the presence of liver metastasis, FACT-G total score, and FWB score were associated with survival and were all significant prognostic factors of OS (P < .050; Table 3). Considering that ECOG-PS is a known prognostic factor in mCRC, multivariate analyses after adjusting ECOG-PS were also performed. The result confirmed that the presence of liver metastasis increased the hazard of mortality, and high scores on FACT-G total and FWB were associated with prolonged survival time.
Table 3.
Multivariate Cox Proportional Hazard Regression Model of Clinical Data and HRQoL Scores.a
| Variables | Unit of Increase | HR | 95% CI | P Value |
|---|---|---|---|---|
| (a) | ||||
| Presence of liver metastasis | No liver metastasis as reference | 1.726 | 0.989-3.009 | .055 |
| FWB score | 1 | 0.951 | 0.909-0.995 | .028 |
| (b) | ||||
| Presence of liver metastasis | No liver metastasis as reference | 1.818 | 1.035-3.191 | .037 |
| FACT-G total score | 1 | 0.984 | 0.968-0.999 | .042 |
Abbreviations: HRQoL, health-related quality of life; HR, hazard ratio; FACT-G, Functional Assessment Cancer Therapy–General; FWB, functional well-being.
Multivariate model of presence of liver metastasis and (a) FWB score and (b) FACT-G total score.
To identify the items showing significant difference in FWB between the 2 groups divided by median value of survival time, we performed χ2 tests or Fisher’s exact tests. Consequently, a borderline significant difference in answer frequency was identified at GF5 (“I am sleeping well,” P = .058) in FWB between the 2 groups distinguished by median survival.
Discussion
HRQoL can reflect various aspects of a patient’s life. Through the accurate assessment of HRQoL, we can understand how cancer and its treatment affect a patient’s life. Quality of life is as important as the tumor-related outcomes, particularly in metastatic cancer patients.2 Depending on the HRQoL status, doctors can choose the treatment strategies and be able to predict patient survival.10 Because quality of life is important in patients with advanced cancer, we decided to include metastatic cancer patients.
This study included a total of 58 mCRC patients. All patient characteristics were retrospectively reviewed at the time of the survey. The percentage of patients who had an ECOG grade ≥ 2 was 43.1%. ECOG is a well-known prognostic factor of survival in mCRC patients.27,28 Patients who had multiple metastatic sites, undergone treatment, or received more than a second regimen of chemotherapy made up large portions of our participants (77.6%, 89.7%, and 75.5%, respectively). Considering the severity of the disease burden of patients, the mean FACT-G score was expected to be lower than those in other published studies. In one study,29 the mean FACT-G total score of 20 patients who had distant metastases was 71.25. Compared with the study result, our mean FACT-G total score of 65.3 seemed to be low. In the present study, the mean scores of PWB, EWB, FWB, and FACT-G decreased when distant metastases were present. In the subgroup with distant metastasis, the mean scores of PWB, SFWB, EWB, and FWB were 17.30, 22.15, 16.85, and 14.95, respectively.29 Compared with previous study results, the total FACT-G mean score in our study was low as a result of the low mean scores of SFWB and EWB, not because of the mean scores of the PWB and FWB. In one Korean population study in which patients were mainly of stages I to III and performance status 0 to 1,30 SFWB mean score was consistently lower than the result of Ward et al.29 In their study, the authors reported that a possible reason for the low SFWB score could be cultural differences. Though the patients’ characteristics were different from those in our study, the contribution of cultural difference to low SFWB score is worth consideration. Moreover, the finding that most of the patients experienced failure of standard therapy could have had a negative impact on emotional state. Further studies that focus on the impact of cultural differences and failure of treatment on FACT-G score are needed.
The OS of the 58 patients included in this study was 7 months, and a total of 55 deaths were noted. The short survival time could have been caused by reasons mentioned previously, such as the large proportion of patients who received more than second-line chemotherapy. We should also consider the time interval from the initial diagnosis (or metastases) to the time when the FACT-G survey was conducted. In this study, the median duration from the initial diagnosis to the FACT-G survey was about 23 months, whereas the median duration from the metastases diagnosis to the FACT-G survey was about 11 months. When the duration of time from the metastases diagnosis and the number of previously received systemic therapy regimens are accounted for, the 7 months of OS in this study population of mCRC patients might not be considered a short survival time. However, it was hard to determine the effect of KM on survival because the median duration of treatment was short, at 3 months, and varied from 0 to 33 months.
For the comparison of survival time using the log-rank test, patients were grouped by the median value of the scores of the FACT-G total and subscales. The median OS of the group who had scores equal to or higher than the median FACT-G total score was 12 months, compared with 5 months in those having scores below the median (P = .226). The PWB score appeared to be a meaningful but not statistically significant factor of survival. The median survival in patients with score equal to or higher than the median score of PWB was 12 months, whereas that of patients with a score below the median of PWB was 4 months (P = .096). The difference in median survival between the 2 groups in other HRQoL subscales was not significant.
In univariate analyses, the FWB score was a statistically significant prognostic factor of survival. The presence of liver metastasis and FACT-G total score showed borderline significance as prognostic factors of survival.
The HRQoL scales were expected to have a high intercorrelation with the HRQoL subscales. Correlation analyses were used to identify any multicollinearity between these scales, and there were strong correlations between the FACT-G and FWB scores. To exclude any multicollinearity between HRQoL scales, multivariate analyses were performed separately for each HRQoL scale and clinical variable.
In multivariate analysis, presence of liver metastasis, FACT-G total score, and FWB score were strongly associated with survival and were significant prognostic factors of survival. The presence of liver metastasis increased the hazard of mortality; however, high scores of FACT-G total and FWB were significantly associated with prolonged survival time.
The importance of the presence of liver metastasis as a prognostic factor of survival has been shown in another study.27 However, none of the other clinical factors were shown to be significant prognostic factors. Among various clinical factors, performance status has been reported as one of the most important factors of survival in advanced CRC.31,32 However, in this study, the ECOG-PS was not a significant factor in patients’ OS, whereas patients’ self-reported HRQoL assessment and total FACT-G and FWB scores were significant prognostic factors of survival. These results imply the possibility that the self-reported quality-of-life score reflects the precise global status of the patient. The patient-reported HRQoL assessment using the FACT-G might also provide more useful information for survival than the ECOG-PS assessed by a doctor. This phenomenon was previously shown in a baseline HRQoL study using the EORTC QLQ-C30.17 Though the patient-reported HRQoL has a tendency to be important in predicting survival, a well-designed prospective study is required to confirm these results.
Among the HRQoL subscales, FACT-G total and FWB scores had a significant impact on mCRC patient survival. In previous studies on the relationship between HRQoL and survival in CRC patients, global HRQoL, role functioning, and ability to work were important prognostic factors of survival.17,33 Role functioning in EORTC QLQ-C30 has a correlation with the FWB in FACT-G because FWB assesses the capability to work, such as the statement, “I am able to work.”34 Therefore, the importance of FWB as a prognostic factor seems to be consistent with previous results. To determine the most important prognostic factor in FWB, we conducted χ2 tests or Fisher’s exact tests. There was no significant difference in answering frequency between the 2 groups divided by the median survival. Instead, a borderline significant difference was observed for item GF5 (“I am sleeping well”) of the FWB. Based on these results, we assume that the maintenance of all functions is more important than the maintenance of a portion of functions.
As a preliminary study for investigating the potential prognostic impacts of HRQoL, this study had limitations because of its small sample size and retrospective design, which could cause weakness in controlling for confounding factors. Despite these limitations, this study identified the association between FACT-G, a basic HRQoL assessment, and OS in mCRC patients treated with KM. In particular, the study focused on relapsed or refractory cancer patients, for whom the importance of HRQoL could not be overemphasized in deciding the treatment.
Conclusions
Based on the results of this study, the scores of FACT-G total and FWB could be useful factors in predicting the clinical outcome of survival and also an important indicator of HRQoL. Therefore, maintaining the functional capability of patients is an important goal of treatment in mCRC patients treated with KM. A prospective study with a large sample size is needed to verify these results.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Conroy T, Bleiberg H, Glimelius B. Quality of life in patients with advanced colorectal cancer: what has been learnt? Eur J Cancer. 2003;39:287-294. [DOI] [PubMed] [Google Scholar]
- 2. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines: American Society of Clinical Oncology. J Clin Oncol. 1996;14:671-679. [DOI] [PubMed] [Google Scholar]
- 3. Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423-431. [DOI] [PubMed] [Google Scholar]
- 4. Ringdal G, Götestam K, Kaasa S, Kvinnsland S, Ringdal K. Prognostic factors and survival in a heterogeneous sample of cancer patients. Br J Cancer. 1996;73:1594-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tamburini M, Brunelli C, Rosso S, Ventafridda V. Prognostic value of quality of life scores in terminal cancer patients. J Pain Symptom Manage. 1996;11:32-41. [DOI] [PubMed] [Google Scholar]
- 6. Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33:1025-1030. [DOI] [PubMed] [Google Scholar]
- 7. Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. Qual Life Res. 1997;6:151-158. [DOI] [PubMed] [Google Scholar]
- 8. Chang VT, Thaler HT, Polyak TA, Kornblith AB, Lepore JM, Portenoy RK. Quality of life and survival. Cancer. 1998;83:173-179. [DOI] [PubMed] [Google Scholar]
- 9. Lam P, Leung M, Tse C. Identifying prognostic factors for survival in advanced cancer patients: a prospective study. Hong Kong Med J. 2007;13:453-459. [PubMed] [Google Scholar]
- 10. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sehlen S, Lenk M, Hollenhorst H, et al. Quality of Life (QoL) as predictive mediator variable for survival in patients with intracerebral neoplasma during radiotherapy. Onkologie. 2003;26:38-43. [DOI] [PubMed] [Google Scholar]
- 13. Dharma-Wardene M, Au HJ, Hanson J, Dupere D, Hewitt J, Feeny D. Baseline FACT-G score is a predictor of survival for advanced lung cancer. Qual Life Res. 2004;13:1209-1216. [DOI] [PubMed] [Google Scholar]
- 14. Monk BJ, Huang HQ, Cella D, Long HJ. Quality of life outcomes from a randomized phase iii trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4617-4625. [DOI] [PubMed] [Google Scholar]
- 15. Fielding R, Wong WS. Quality of life as a predictor of cancer survival among Chinese liver and lung cancer patients. Eur J Cancer. 2007;43:1723-1730. [DOI] [PubMed] [Google Scholar]
- 16. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 17. Maisey NR, Norman A, Watson M, Allen MJ, Hill ME, Cunningham D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38:1351-1357. [DOI] [PubMed] [Google Scholar]
- 18. Efficace F, Bottomley A, Coens C, et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42:42-49. [DOI] [PubMed] [Google Scholar]
- 19. Efficace F, Innominato PF, Bjarnason G, et al. Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26:2020-2026. [DOI] [PubMed] [Google Scholar]
- 20. Braun DP, Gupta D, Grutsch JF, Staren ED. Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer. Health Qual Life Outcomes. 2011;9(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holzner B, Bode R, Hahn E, et al. Equating EORTC QLQ-C30 and FACT-G scores and its use in oncological research. Eur J Cancer. 2006;42:3169-3177. [DOI] [PubMed] [Google Scholar]
- 22. Kemmler G, Holzner B, Kopp M, et al. Comparison of two quality-of-life instruments for cancer patients: the functional assessment of cancer therapy-general and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30. J Clin Oncol. 1999;17:2932-2940. [DOI] [PubMed] [Google Scholar]
- 23. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] [Google Scholar]
- 24. Choi WC, Lee JH, Lee EO, Yoon SW, Ahn KS, Kim SH. Study on antiangiogenic and antitumor activities of processed Rhus verniciflua Stokes extract. Korea J Orient Physiol Pathol. 2006;20:825-829. [Google Scholar]
- 25. Kim JH, Kim HP, Jung CH, et al. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua Stokes in AGS gastric cancer cells. Int J Mol Med. 2006;18:201-208. [PubMed] [Google Scholar]
- 26. Park JH, Moon G. Effect of allergen removed Rhus verniciflua extract on inhibition of tumor metastasis. J Korean Tradit Oncol. 2010;15:47-61. [Google Scholar]
- 27. Köhne C-H, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308-317. [DOI] [PubMed] [Google Scholar]
- 28. Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [DOI] [PubMed] [Google Scholar]
- 29. Ward W, Hahn E, Mo F, Hernandez L, Tulsky D, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181-195. [DOI] [PubMed] [Google Scholar]
- 30. Yoo HJ, Kim JC, Eremenco S, Han OS. Quality of life in colorectal cancer patients with colectomy and the validation of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C), Version 4. J Pain Symptom Manage. 2005;30:24-32. [DOI] [PubMed] [Google Scholar]
- 31. Lavin P, Mittelman A, Douglass H, Jr, Engstrom P, Klaassen D. Survival and response to chemotherapy for advanced colorectal adenocarcinoma: an Eastern Cooperative Oncology Group report. Cancer. 1980;46:1536-1543. [DOI] [PubMed] [Google Scholar]
- 32. Kemeny N, Braun DW., Jr Prognostic factors in advanced colorectal carcinoma: importance of lactic dehydrogenase level, performance status, and white blood cell count. Am J Med. 1983;74:786-794. [DOI] [PubMed] [Google Scholar]
- 33. Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh T. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14:171-175. [DOI] [PubMed] [Google Scholar]
- 34. Luckett T, King M, Butow P, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. 2011;22:2179-2190. [DOI] [PubMed] [Google Scholar]