Abstract
Objective:
Physical activity variability is a risk factor for diabetic foot ulcers (DFU). Geographic context may influence variability. This study developed initial methods for monitoring location-specific physical activity in this population. Secondarily, preliminary comparisons in location-specific physical activity were made between patients at risk versus patients with active DFU.
Methods:
Five at-risk and 5 actively ulcerated patients were monitored continuously for 72 hours with physical activity and GPS monitors. A custom algorithm time synchronized the 2 devices’ data.
Results:
On average for all 10 subjects, 1.5 ± 2.1% of activity lacked a corresponding GPS location. 80 ± 11% of self-reported activity events per subject had a GPS identified location. The GPS identified locations were in agreement with the self-reported locations 98 ± 6% of the time. DFU participants’ weight-bearing activity was 188% higher at home than away from home. At-risk participants showed similar weight-bearing activity at home as active DFU participants, however, at-risk participants had 132% more weight-bearing activity away-from-home.
Conclusions:
Objectively monitoring location-specific physical activity proved feasible. Future studies using such methodology may enhance understanding of pathomechanics and treatment of DFU.
Keywords: diabetic foot ulcer, GPS, monitor, physical activity
Diabetic foot ulcers (DFU) commonly form due to physical stresses generated during weight-bearing activities.1,2 Physical activity (PA) variability appears to be more important to DFU development than the average daily level of PA.3-5 The locations visited by persons with or at-risk of DFU may significantly impact activity levels. At best, studies to date in this population have relied on self-report diaries for the locational context of PA.6,7 Self-report diaries are dependent on patients’ adherence to recording their activities and may be subject to both unintentional and intentional recall error. An objective means of continuously tracking the environmental context of PA could greatly enhance future studies regarding preventing/treating DFU as well as interventions to prevent them by helping elucidate variables that drive PA levels.
Although it remains a relatively new area of research, a number of researchers are looking to integrate GPS, geographic information systems, and accelerometry for enhanced PA monitoring.8-14 However, in the context of DFUs this previous work has had significant limitations. Previous research looking to pair accelerometry derived PA with GPS provided location have typically limited their monitoring to activity conducted outdoors8-13 where a GPS signal is typically available. However, past research in individuals at high risk of DFUs found that the participants actually took more steps in their homes than outside of them.15 Another limitation of previous research concerning merging PA data with GPS data is that the PA assessment is limited. Previous methodologies have often limited their data processing to moderate and vigorous PA.13,16 Considering patients that develop DFU have low daily PA levels4,17 and that proportionately much of this activity is done in the home, it is safe to say much of the weight bearing stress imparted on the feet of people at risk of or with active DFU will be done so at low PA intensity levels. Taking this concept further, previous studies have not monitored time spent standing. However prior research has highlighted the importance of standing time in regards to understanding the volume of daily stress applied to the feet of people at risk of DFU.18 The purpose of this preliminary investigation was to assess the feasibility of objectively, synchronously, and continuously monitoring finely detailed PA and its location of occurrence in individuals at-risk and with active DFU.
Methods
Subjects
Five patients at risk and 5 patients with active DFU were recruited (Table 1). All participants read and signed a local institutional review board approved informed consent form prior to participating. All participants were independently mobile (no use of wheelchair or similar device) community dwelling adults. At-risk participants had a history of previous DFU but were ulcer free for ≥4 weeks at time of enrollment. Active DFU participants utilized a variety of offloading devices for their wounds including removable cast walker (n = 1), diabetic shoes (n = 1), wedge forefoot offloading shoe (n = 1), soft cast with walking boot (n = 1), and an accommodative surgical shoe (n = 1).
Table 1.
Subject Demographics.
| At-risk (n = 5) | Active DFU (n = 5) | P value | ||
|---|---|---|---|---|
| Age (years) | 55 ± 11 | 55 ± 5 | .97 | |
| BMI (kg/m2) | 32.2 ± 7.4 | 30.0 ± 5.3 | .61 | |
| Sex | Male | 5 | 4 | 1.00 |
| Female | 0 | 1 | ||
BMI, body mass index. Age and BMI were compared between groups via independent t-tests. Sex was compared between groups via Fisher’s exact test.
Procedures
During the first visit participants were oriented with the PA monitor and GPS logger, as well as a digital watch with a voice recorder. The watch sounded an alarm every 2 hours between 10:00 and 20:00 each day. Participants were instructed to use the watch’s voice recorder to log their location and current PA each time the alarm sounded. After familiarization with the 3 devices, participants were sent home. Participants were monitored for a period of 72 hours before returning the devices at their second and final study visit.
Physical Activity Monitoring
The tri-axial accelerometer based PA monitor had a 50 Hz sampling rate and battery life in excess of 5 days (PAMSys, Biosensics, Boston, MA, USA). It recorded step data and also continuously identified body posture (standing, sitting, lying side/prone/supine). Capturing standing time is important in this population as previous work has demonstrated the ratio of time spent with the feet loaded standing versus walking is approximately 2:1 in individuals at risk of DFU.18 The monitor was placed in a T-shirt with a foam padded pouch located next to the sternum. Participants were asked to wear the monitor/T-shirt at all times, except while bathing, during the 72-hour monitoring period.
GPS Monitoring
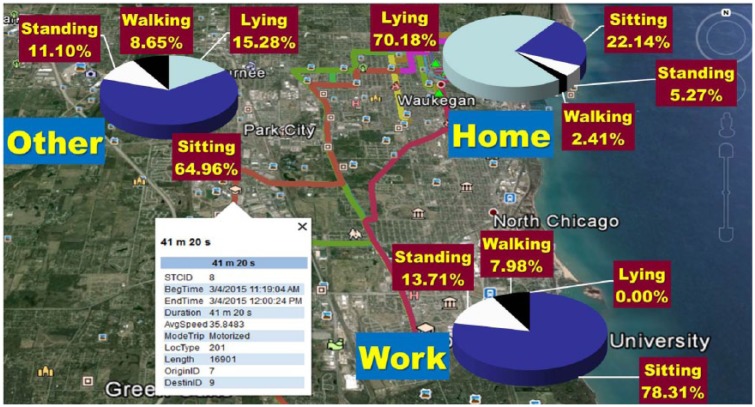
A small commercial GPS logger (QStarz Travel recorder XT, Taipei, Taiwan) was worn on a belt or carried in the pocket of each participant. Participants were asked to always keep the device close at hand and to charge it nightly. It was set to record location continuously at a 0.1 Hz sampling rate. An algorithm with episode detection rules based on spatial density was developed for processing and classifying raw GPS data.19 A staypoints/stops definition was created20 and all staypoints were classified as home, work or other (Figure 1). “Non-staypoint” GPS data was filtered and classified as moves (trips). Outliers representing a sudden jump in location, and imputable gaps where GPS data points were not recorded were treated prior to event detection. A gap was defined as a period where the time span between the “last fix” before signal loss and the “first fix” after signal loss was at least 3 min. in GPS trajectory data that record locations in temporal sequence. Gaps were filled differently depending on the distance and time duration between the last fix and the first fix. If the gap distance (the Euclidean distance between the last fix and the first fix) was small (≤300 meters) (ie, the location before and after a gap was spatially close), then a subject with a GPS device was estimated to be positioned at the location of the last fix for the time period of a gap minus 30 sec. to account for a warm start problem (signal loss when a subject exits a structure concealed from GPS signals). If the gap distance was large (>300 meters), the likely sequence of being positioned at the last fix and then moving to the first fix was estimated to fill the gap accordingly. If an initial gap fill resulted in a subject moving excessively slowly and speed did not increase later, the subject was estimated to be positioned at the last fix for a period of time prior to moving to the first fix.
Figure 1.
Representative GPS and physical activity data for 1 subject.
Data Synchronization Process and Diary Log Verification
The beginning and ending time of each stop and move episode detected from the GPS data was compared with the accelerometers’ activity epochs at 1-second intervals. This resulted in merged master files that contained a sequence of unique activity epochs with beginning time, ending time, time duration, location code, number of steps, and activity category. Processed and synchronized GPS and PA data was then compared to each individual’s self-reported daily logs of time, activity/posture, and location using a custom LabVIEW 2015 program (National instruments). Although the sample sizes were too small to justify statistically testing for group differences, Cohen’s d statistics21,22 were calculated to give perspective on the effect size of the differences.
Results
On average, 13.1 ± 20.4% of each subject’s monitoring period was missing a direct satellite provided GPS location. After processing the GPS data and synchronizing the processed data with PA data, 1.5 ± 2.1% of each participant’s 72-hour activity profile lacked a corresponding GPS location. Digital self-report diaries of activity were available for 9/10 subjects (1 subject failed to log activities with the watch). After identifying each self-reported PA event (standing, walking, etc) at the correct time within the objectively collected PA/GPS location combined data set, 80 ± 11% of self-reported activity events per subject had a GPS identified location. Of those self-reported activity events that had a corresponding GPS location identified, the self-reported and GPS identified locations were in agreement 98 ± 6% of the time. The 2% error was likely due to close proximity between Home and Work locations for 1 participant.
Participants with an active DFU had 187% more (2.61 ± 2.58 vs 0.91 ± 0.51 hrs/day, d = 1.1) weight-bearing activity (combined time standing and walking) at home relative to away from home. At-risk participants had similar weight-bearing activity at home as active DFU participants (2.53 ± 1.58 vs 2.61 ± 2.58 hrs/day), however, they had 131% more (2.10 ± 1.50 vs 0.91 ± 0.51 hrs/day, d = 1.2) weight-bearing activity outside of their homes relative to active DFU participants. Furthermore, active DFU participants’ total time (walking, standing, sitting and lying) away-from-home was only 7.7% (5.5 hours) of 72 hours monitored in contrast to 20.5% (14.8 hours) (d = 2.1) for at-risk participants.
Discussion
No methodologies for integrating accelerometer based PA data with GPS data have been previously evaluated in DFU populations. This study demonstrated the feasibility of combining the 2 technologies for continuously monitoring location-specific PA by both individuals at-risk and individuals with active DFU. While GPS has been evaluated for monitoring PA in other populations, the preliminary methodology utilized in this study has significant advantages relative to DFU. No consistent method of dealing with missing and inconsistent GPS data has previously existed in PA/GPS studies,11 therefore PA studies incorporating GPS have typically limited their monitoring to activity conducted outdoors where GPS signals are strongest.8,11,13 However, this study’s GPS processing algorithm filled time gaps of missing data based on the location prior to and after the gap. This resulted in only 1.5 ± 2.1% of each participant’s 72-hour activity profile lacking a corresponding GPS identified location of occurrence. Another advantage to the present study’s methodology was the continuous monitoring of all PA regardless of intensity. Previous studies, such as the work by Brown et al,13 have limited PA outcomes to moderate and vigorous PA. Considering DFU patients tend to exhibit below average daily levels of activity4,17 and the majority of this activity is conducted inside home, it is safe to say much of the weight-bearing stress imparted on the feet of people at risk of or with active DFU will be done so at low PA intensity levels.
From a research perspective, further refinement of this study’s methods to allow for finer categorization of environments PA is conducted in (home, work, commercial, recreational, etc) might provide insight into environmental factors driving the variability in PA that appears to be so important to the pathomechanics of DFU.3-5 From a patient care perspective, incorporating this monitoring into the daily lives of individuals at risk of DFU might enhance the capability to appropriately dose their activity4 through helping patients recognize how their activity varies in different locations. However, with greater utilization of such monitoring, careful thought will need to go into the ethical considerations of whom is granted access to individuals’ activity profiles and for what purposes.23
Moderate to strong effect sizes for group comparisons21,22 in the present study suggest at-risk patients may engage in more PA away from home compared to active DFU patients. One likely factor to contribute to the decrease in PA outside of the home by DFU patients is postural instability. Approximately 23% of people with diabetic peripheral neuropathy (a primary risk factor for DFU)24 report balance problems as being present either most or all of the time.25 Balance problems limit patients’ mobility and functioning26 and can be exasperated with the use of DFU offloading footwear.27,28 In addition, patients with active DFU may purposefully limit PA outside of the home with the intent of limiting physical stress on the wound and subsequently improved wound healing.
Conclusions
Pairing accelerometry based activity monitoring with GPS monitoring allowed for continuous and objective logging of location-specific PA profiles of both those at-risk and those with active DFU. Preliminary data showed a trend for those at-risk to engage in more away-from-home weight bearing PA.
Footnotes
Abbreviations: DFU, diabetic foot ulcer; GPS, global positioning system; PA, physical activity.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a joint institutional grant from Rosalind Franklin University of Medicine and Science and DePaul University. This project was also partially supported by grant 2T35DK074390 from the National Institute of Diabetes and Digestive and Kidney Disease. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
References
- 1. Brand PW. The diabetic foot. In: Ellenberg M, Rifkin H, eds. Diabetes Mellitus, Theory and Practice. 3rd ed. New York, NY: Medical Examination Publishing; 1983:803-828. [Google Scholar]
- 2. Brand PW. The insensitive foot (including leprosy). In: Jahss M, ed. Disorders of the Foot and Ankle. 2nd ed. Philadelphia, PA: Saunders; 1991:2170-2175. [Google Scholar]
- 3. Maluf KS, Mueller MJ. Novel Award 2002. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech. 2003;18:567-575. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DG, Lavery LA, Holtz-Neiderer K, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27:1980-1984. [DOI] [PubMed] [Google Scholar]
- 5. Lott DJ, Maluf KS, Sinacore DR, Mueller MJ. Relationship between changes in activity and plantar ulcer recurrence in a patient with diabetes mellitus. Phys Ther. 2005;85:579-588. [PubMed] [Google Scholar]
- 6. Bus SA, Waaijman R, Nollet F. New monitoring technology to objectively assess adherence to prescribed footwear and assistive devices during ambulatory activity. Arch Phys Med Rehabil. 2012;93:2075-2079. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451-455. [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez DA, Cho GH, Elder JP, et al. Identifying walking trips from GPS and accelerometer data in adolescent females. J Phys Activity Health. 2012;9:421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez DA, Cho GH, Evenson KR, et al. Out and about: association of the built environment with physical activity behaviors of adolescent females. Health Place. 2012;18:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krenn PJ, Titze S, Oja P, Jones A, Ogilvie D. Use of global positioning systems to study physical activity and the environment: a systematic review. Am J Prev Med. 2011;41:508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCrorie PR, Fenton C, Ellaway A. Combining GPS, GIS, and accelerometry to explore the physical activity and environment relationship in children and young people—a review. Int J Behav Nutr Phys Act. 2014;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez DA, Merlin L, Prato CG, et al. Influence of the built environment on pedestrian route choices of adolescent girls. Environment Behavior. 2015;47:359-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown BB, Wilson L, Tribby CP, et al. Adding maps (GPS) to accelerometry data to improve study participants’ recall of physical activity: a methodological advance in physical activity research. Br J Sports Med. 2014;48:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper AR, Page AS, Wheeler BW, Hillsdon M, Griew P, Jago R. Patterns of GPS measured time outdoors after school and objective physical activity in English children: the PEACH project. Int J Behav Nutr Phys Act. 2010;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJM. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451-455. [DOI] [PubMed] [Google Scholar]
- 16. Oliver M, Parker K, Witten K, et al. Children’s out-of-school independently mobile trips, active travel, and physical activity: a cross-sectional examination from the kids in the city study. J Phys Act Health. 2015; 13(3):318-324. [DOI] [PubMed] [Google Scholar]
- 17. Lemaster JW, Reiber GE, Smith DG, Heagerty PJ, Wallace C. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093-1099. [DOI] [PubMed] [Google Scholar]
- 18. Najafi B, Crews RT, Wrobel JS. Importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care. 2010;33:2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hwang S, Hanke T, Evans C. Automated extraction of community mobility measures from GPS stream data using temporal DBSCAN. In: Murgante B, Misra S, Carlini M, Torre C, Nguyen HQ, Taniar D, Apduhan B, Gervasi O, eds. Computational Science and Its Applications—ICCSA 2013. Berlin, Germany: Springer; 2013:86-98. [Google Scholar]
- 20. Spaccapietra S, Parent C, Damiani ML, de Macedo JA, Porto F, Vangenot C. A conceptual view on trajectories. Data Knowledge Engineer. 2008;65:126-146. [Google Scholar]
- 21. Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [DOI] [PubMed] [Google Scholar]
- 22. Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Professional Psychol Res Pract. 2009;40:532. [Google Scholar]
- 23. Crews RT, Bowling FL, Boulton AJ. Controversies in off-loading: should big brother be watching? Curr Diab Rep. 2009;9:417-419. [DOI] [PubMed] [Google Scholar]
- 24. Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol. 2014;70:e1-18; quiz 19-20. [DOI] [PubMed] [Google Scholar]
- 25. Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28:2378-2383. [DOI] [PubMed] [Google Scholar]
- 26. Vileikyte L, Gonzalez JS. Recognition and management of psychosocial issues in diabetic neuropathy. Handbook Clin Neurol. 2014;126:195-209. [DOI] [PubMed] [Google Scholar]
- 27. Goodworth AD, Kunsman M, DePietro V, LaPenta G, Miles K, Murphy J. Characterization of how a walking boot affects balance. J Prosthet Orthot. 2014;26:6. [Google Scholar]
- 28. Crews RT, Sayeed F, Najafi B. Impact of strut height on offloading capacity of removable cast walkers. Clin Biomech. 2012;27:725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]