Abstract
Background:
Diabetic foot disease carries a high morbidity and is a leading cause of lower limb amputation. This may in part be due to the effect diabetes mellitus (DM) has on the microcirculation including in the skin.
Method:
We conducted a review of studies that have examined the relationship between microcirculatory function and wound healing in patients with DM. A search of the Medline, Embase, and Web of Science databases was performed coupled with a review of references for the period 1946 to March 2015.
Results:
Nineteen studies of diverse methodology and cohort selection were identified. Poor function of the microcirculation was related to poor outcome. Transcutaneous oxygen pressure (TcPO2) was the most commonly used method to measure the microcirculation and thresholds for poor outcome proposed ranged from 10 mmHg to <34 mmHg. Two studies reexamined microcirculatory function following revascularization. Both found an increase in TcPO2, however only 1 reached statistical significance. No significant difference in the results of microcirculation tests was found between diabetic and nondiabetic patients.
Conclusions:
While it is not possible to draw firm conclusions from the evidence currently available there are clear areas that warrant research. Good microcirculation unsurprisingly appears to associate with better wound healing. The influence of DM is not clear, and neither is the degree of improvement required to achieve healing. Studies that examine a clearly defined cohort both with and without DM are urgently required. Accurate quantitative assessment of microcirculation will aid prediction of wound healing identifying those at greatest risk of amputation.
Keywords: diabetic foot, healing, microcirculation, ulcer
Currently, 3.2 million people in the United Kingdom and 29.1 million people in the United States are diagnosed with diabetes mellitus (DM), accounting for 6% and 9.3% of the population respectively.1,2 It is estimated that the lifetime incidence of a foot ulcer may be as high as 25% among these patients with an associated increased risk of amputation.3 Diabetic ulceration and associated amputation also carry a significant cost burden to society, this will continue to increase along with the rising incidence of DM.4
DM is known to have a significant effect on the microvasculature, causing dysfunction of the arterioles and capillaries supplying the retina, kidneys and peripheral nerves,5 histological examination of capillaries has shown thickening of the basement membrane compared to nondiabetic patients.6-8 Different methods to quantifiably examine the microcirculation and its function have been developed; these include capillary microscopy (CM), transcutaneous oxygen pressure (TcPO2), and laser Doppler fluxmetry (LDF). How these measures of the microcirculation change with wound healing is not well described. The review that the International Working Group on the Diabetic Foot Guidance on prognosis is based on is a through and well performed systematic review.9 However the focus of the review is not on methods of assessing the microcirculation and while the discussion and conclusion consider TcPO2 the results that this conclusion is based on are not covered in the results. There is also no consideration of comparison to patients without DM or the role of repeated measures. The aim of this review was to examine the current evidence available on the relationship between the microcirculation in the ulcerated diabetic foot and wound healing. Specifically the ability to predict healing, how the results for those with DM compare to those without and how the results vary when repeated measurements are taken.
Methods
A search of the Medline, Embase, and Web of Science databases was performed. The search strategy consisted of the Medical Subject Headings (MESH) “microcirculation,” “wound healing,” “diabetic foot,” “skin ulcer,” “laser Doppler flowmetry,” “blood gas monitoring, transcutaneous,” “microscopic angioscopy,” “xenon radioisotopes.” In addition a key word search was performed. The terms used can be found in the appendix. Non-English-language and nonhuman studies were excluded, the date range for the search was 1946 to February 2015. Final inclusion in the review was dependant on meeting the criteria set out in Table 1; no limits were applied to length of follow-up or number of patients included.
Table 1.
Review Inclusion Criteria.
| Inclusion criteria |
|---|
| • English language article • At least one method of assessing the microcirculation • Patients with active tissue loss • Wound healing as an outcome measure • Results from patients with diabetes to be analyzed separately from patients without diabetes in 1 of the following 3 formats. ○ Patients with diabetes compared to patients without diabetes ○ Patients with diabetes who healed compared to patients with diabetes who did not heal ○ Repeated measurements from the same patient during the period of active diabetic ulceration being investigated |
One reviewer (DL) performed the search, reviewed abstracts and selected studies for inclusion. Any areas of uncertainty were reviewed by the senior author (AT) to provide a second opinion. The original intention was to perform a meta-analysis however there were insufficient numbers of high quality studies to be able to continue this plan and so a more descriptive approach was taken to reporting the data.
Results
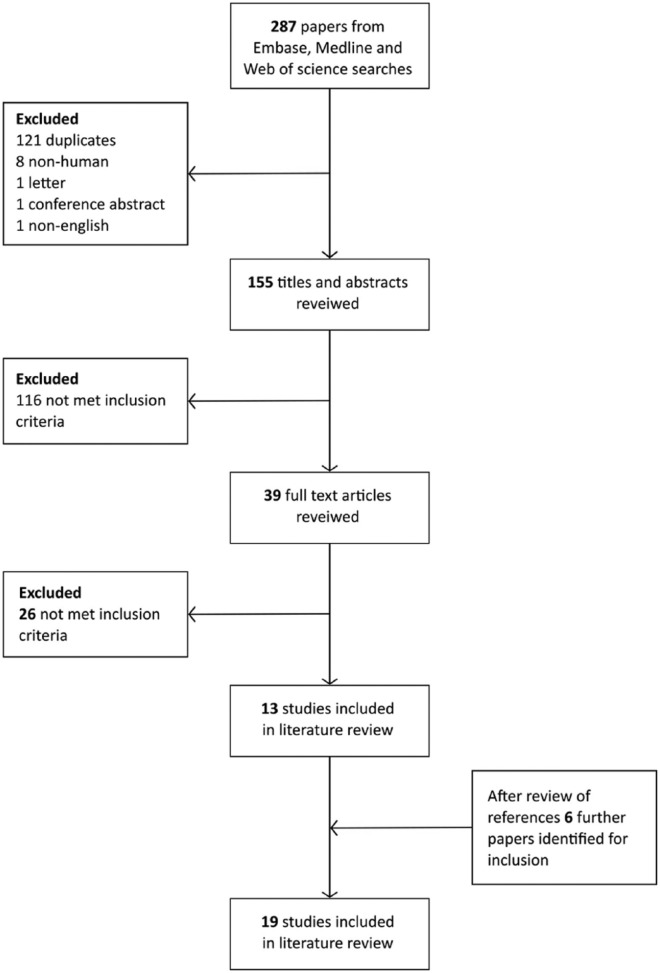
Two hundred eighty-seven articles were identified after searching the databases. Full text was obtained for all abstracts that met the inclusion criteria and all relevant data was extracted. After this assessment and review of references 19 studies were included in the final review (Figure 1).10-28 The date of publication ranged from 1985 to 2014, 2 studies were randomized controlled trials (RCTs), there were 3 pseudo-RCTs and the rest were observational studies (Table 2). Not all studies included all the comparisons considered below and some studies used more than 1 method to assess the microcirculation.
Figure 1.
Flow diagram illustrating study identification process.
Table 2.
Characteristics of Included Studies.
| Number of subjects |
||||||
|---|---|---|---|---|---|---|
| Author | Year | Country | Type of study | Microcirculation method | DM | Non-DM |
| Faris10 | 1985 | Australia | Cross-sectional | SPP using isotope washout | 64 | — |
| Karanfilian11 | 1986 | USA | Cohort | 1. LDF 2. TcPO2 |
34 | 22 |
| Pecoraro12 | 1991 | USA | Cross-sectional | 1. TcPO2
2. TcPCO2 |
46 | — |
| Jorneskog13 | 1993 | Sweden | Cross-sectional | 1. LDF-PORH 2. Capillary microscopy |
10 | — |
| Padberg14 | 1996 | USA | Case control | TcPO2 | 129 | 97 |
| Kalani15 | 1999 | Sweden | Cross-sectional | 1. TcPO2
2. TBP using LDF |
50 | — |
| Koblik16 | 2001 | Poland | Double blind RCT | LDF, PORH, and resting flow | 18 | — |
| Zimny17 | 2002 | Germany | Cross-sectional | TcPO2 | 31 | — |
| Fife18 | 2002 | USA | Cross-sectional (retrospective) | TcPO2 | 1144 | — |
| Newton19 | 2002 | UK | Cross-sectional | LDI | 5 | |
| Kalani20 | 2002 | Sweden | Pseudo-RCT | 1. TcPO2 + TcPCO2 during O2 inhalation 2. TBP using LDF |
38 | — |
| Klingel21 | 2003 | Germany | Cross-sectional | 1. TcPO2
2. LDF 3. Capillary microscopy |
8 | — |
| Petrofsky22 | 2007 | USA | Pseudo-RCT | LDI | 29 | — |
| Lawson23 | 2007 | USA | Pseudo-RCT | LDI | 10 | 10 |
| Ichioka24 | 2009 | Japan | Case control | TcPO2 | 31 | 22 |
| Petrofsky25 | 2010 | USA | RCT | LDI | 20 | — |
| Yang26 | 2013 | China | Cross-sectional | TcPO2 | 61 | — |
| Wang27 | 2014 | China | Cross-sectional | TcPO2 | 194 | — |
| Yotsu28 | 2014 | Japan | Cohort | 1. SPP using LDF 2. TcPO2 |
73 | — |
Using the Microcirculation to Predict Healing
Twelve studies out of 19 compared the microcirculation in patients with diabetes who healed to those who did not heal.10-12,15,18-21,24,26-28 Ten of these studies employed TcPO2,11,12,15,18,20,21,24,26-28 5 used LDF,11,15,20,21,28 1 used laser Doppler imaging (LDI),19 and 1 used isotope washout to measure skin perfusion pressure (SPP).10 These were all observational studies apart from 1 which randomized the first 14 of its participants but not the final 24.20 For 7 of the studies the participants received only standard therapy.10-12,15,26,27 Two studies examined the effects of HBO therapy: Kalani et al had 2 cohorts, 1 of which received standard therapy and the other which received HBO. The healed and unhealed groups in this study are made up of participants from either cohort.20 Fife et al performed a retrospective study of 1144 patients who received HBO therapy.18 Klingel et al reported the results of a very small pilot study (8 patients) all of whom received rheopheresis.21 Two studies treated their participants with dermal replacement therapy (Ichioka et al, bone marrow impregnated collagen; Newton et al, collagen containing glycosaminoglycans).19,24 Five studies only investigated patients with both diabetes and ischemia,11,15,18,20,21 3 studies excluded those with ischemia,12,19,24 in 1 study it was unclear,26 and 3 included a mix of patients.10,27,28 Only Yotsu et al divided the patients into groups depending in their etiology (neuropathic, ischemic, and neuro-ischemic).28
Transcutaneous oxygen pressure
Nine studies used TcPO2 to predict wound healing,11,12,15,20,21,24,26-28 the results are summarized in Table 3. Five studies found that those with a higher TcPO2 had a statistically significant higher chance of healing, with results ranging from 30 ± 4 mmHg to 61.11 ± 21.16 mmHg.11,15,21,26,27 Kalani et al and Yotsu et al failed to find a significant difference between the 2 groups.20,28 Pecoraro et al found a significant difference between those who had early healing and those who did not (56.3 ± 2.72 mmHg vs 26.9 ± 8.26 mmHg, P = .003) however was unable to demonstrate that the difference had persisted in those that healed overall (53.67 ± 2.99 mmHg vs 37.57 ± 11.02 mmHg, P = .126).12
Table 3.
TcPO2 Results for Patients Who Healed Compared to Patients Who Did Not Heal.
| TcPO2 (mmHg) |
||||
|---|---|---|---|---|
| Author | Measurement/groups | Healed (n) | Unhealed (n) | P |
| Klingel21 | Mean change in TcPO2 weeks 0-12 Improved and deteriorated groups |
13.23 ± 9.57 (4) | −2.3 ± 6.65 (2) | <.05* |
| Kalani20 | Basal TcPO2, dorsum of foot All patients |
26 ± 10 (23) | 24 ± 10 (9) | ns |
| Karanfilian11 | Dorsum of foot All diabetic patients |
30 ± 4 (16) | 7 ± 2.5 (18) | <.05 |
| Yang26 | Dorsum of foot Group 1 (ulcers healed with intact skin) Group 3 (Ulcers that did not heal or deteriorated including requiring amputation) |
32 ± 10 (36) | 15 ± 12 (17) | <.001 |
| Ichioka24 | Peri-wound TcPO2
Diabetic subgroup (combination of treatment and conventional therapy group) |
34.5 ± 19.2 (32) | 26.4 ± 16.7 (10) | Not stated |
| Yotsu28a | Multiple measures from 2 areas on foot, lowest value recorded Contralateral foot used if extensive ulceration Ischemic group |
38, 12-40 (9) | 30, 3-45 (11) | ns |
| Neuro-ischemic group | 38, 22-51 (9) | 17, 16-32 (5) | ns | |
| Neuropathic group | 48, 40-56 (34) | 44, 43-50 (5) | ns | |
| Kalani15 | Dorsum of foot Healed with intact skin compared to impaired ulcer healing |
50 ± 20 (20) | 13 ± 14 (13) | <.001 |
| Pecoraro12 | Peri-wound TcPO2 overall healing | 53.67 ± 2.99 (39) | 37.57 ± 11.02 (7) | ns |
| Peri-wound TcPO2 early healing | 56.3 ± 2.72 (38) | 26.9 ± 8.26 (8) | .003 | |
| Wang27 | Site of measurement not stated Healing and nonhealing groups |
61.11 ± 21.16 (162) | 46.5 ± 18.06 (20) | <.01 |
All values are mean ± SD, unless otherwise noted.
Values are median, IQR.
Wilcoxon test for matched pairs for significance of change between week 0 and week 12 for each group separately.
Skin perfusion pressure
Two articles used SPP to compare the healed and unhealed groups.10,28 Faris et al in 1985 used an isotope washout method on 64 patients with diabetes and foot ulceration or gangrene. Those who healed had a mean SPP of 59 ± 16 mmHg compared to those who did not heal whose mean SPP was 35 ± 11 (P < .001).10 Yotsu et al in 2014 employed LDF instead of isotope washout to measure SPP on diabetic ulcers divided into the groups described above. They found that neuropathic ulcers had a higher SPP than both ischemic and neuro-ischemic ulcers, 65 ± 13.6 mmHg, 27 ± 14.1 mmHg and 34 ± 23.2 mmHg respectively (P < .001). However there was no significant difference between the healed and unhealed ulcers in each group (Table 4).28
Table 4.
Skin Perfusion Pressure Results for Patients Who Healed Compared to Patients Who Did Not Heal.28
| SPP (mmHg) |
|||
|---|---|---|---|
| Group | Healed (n) | Unhealed (n) | P |
| Neuropathic | 67, 57-75 (34) | 65, 40-69 (5) | .192 |
| Ischemic | 37, 17-43 (9) | 20, 15-37 (11) | .341 |
| Neuro-ischemic | 38, 22-51 (9) | 17, 16-32 (5) | .141 |
All values are median, IQR.
Laser Doppler
Karanfilian et al was the only article to use laser Doppler fluxmetry to compare between healed and unhealed patients. They demonstrated significantly higher skin blood flow velocity (LD-SBFV) and pulse wave amplitude (LD-PWA) results between those who healed and those who did not in both their study groups (Table 5).11
Table 5.
TcPO2 and LDF Results in Patients With Diabetes Compared to Patients Without Diabetes.11
| Diabetes |
No diabetes |
|||
|---|---|---|---|---|
| Healed (16) | Unhealed (18) | Healed (15) | Unhealed (7) | |
| TcPO2 (mmHg) | 30 ± 4.0 | 7 ± 2.5* | 42 ± 3.5 | 2 ± 1.6* |
| LD-SBFV (mV) | 98 ± 13.0 | 50 ± 8.0* | 88 ± 15.0 | 37 ± 2.0* |
| LD-PWA (mV) | 14 ± 3.0 | 4 ± 0.5* | 8 ± 1.4 | 2 ± 0.3* |
Values are mean ± SE.
Significant difference between healed and unhealed groups (P < .05).
Prediction of healing
Three studies reported the accuracy of cut-off values for healing.10,15,18 Faris and Duncan found a SPP of less than 40 mmHg was an indicator of poor healing (sensitivity of 97%, specificity 80%, positive predictive value (PPV) 87% and negative predictive value (NPV) 95%).10 Kalani et al used a cutoff of 25 mmHg for TcPO2 and 30 mmHg for toe blood pressure (TBP) using LDF. For TcPO2 the sensitivity was 85%, specificity 92%, PPV 79% and NPV 94%. For TBP the sensitivity was 15%, specificity 97%, PPV 67% and NPV 77%.15 Fife et al tested multiple potential cut-offs for sea level TcPO2 as a predictor of failure of hyperbaric therapy. They found that 25 mmHg was the best cutoff with a 2.5 times greater chance of success. However the accuracy was still relatively poor with sensitivity of 67%, specificity 50%, PPV 35% and NPV 79%.18
Diabetes Compared to No Diabetes
Two out of 19 studies compared subjects both with DM and without DM.11,14 Both of these articles used TcPO2 to make their comparisons, in addition Karanfilian et al employed LDF.11
Padberg et al reported the predictive accuracy for healing of TcPO2 in critically ischemic wounds. In all, 204 wounds were stratified depending on the presence of DM, dialysis dependant chronic renal failure or neither disease. Probability of healing curves for each group were plotted and compared using multiple logistic regression. TcPO2 in DM patients had a predictive accuracy, sensitivity and specificity of 81%, for chronic renal failure these figures were 77%, 73%, and 82% respectively, and for neither disease 84%, 86%, and 82%.14
Only 1 study identified compared the mean results of microcirculatory tests for patients with diabetes and those without.11 The patients were all men with ulceration to the foot (34 with diabetes, 22 without). One-off measurements of TcPO2 and LDF (LD-SBFV and LD-PWA) and follow-up of at least 30 days was performed. The results are presented in Table 5. Patients without diabetes who did not heal had a lower TcPO2, LD-SBFV, and LD-PWA than patients with diabetes who did not heal. In the healed groups for the patients without diabetes the TcPO2 was higher than the patients with diabetes. However the LD-SBFV and LD-PWA were lower in the group without diabetes. The authors have not reported whether these differences are statistically significant.11
Multiple Measurements During Observation Period
Eight out of 19 studies reported the results of more than 1 measurement on the same group of patients.13,16,19,21-25 One study detected no change, 2 noted a decrease in reading, a further 2 noted an increase, and 3 noted a pattern of increasing then decreasing. Jorneskog et al used LDF and capillary microscopy to examine 10 patients with diabetes who received low molecular weight heparin for a period of 8 weeks. Measurements of the microcirculation (postocclusive reactive hyperemia (PORH), structural appearance of capillaries in the forefoot and toes) were undertaken 1-2 weeks prior to receiving heparin, after 4-7 weeks of treatment and 2 weeks after treatment was stopped. They found that there was no significant change in any of the laser Doppler parameters during or after treatment. It was however noted that 6 patients who had improved healing also had an improvement in their capillary stage, 3 others also improved clinically but 1 had no change in their capillaries, 1 initially improved but then deteriorated again, and in 1 it was not possible to determine their capillary stage. One patient deteriorated both clinically and on microscopic examination.13
Petrofsky et al published on electronic stimulation (ES) for diabetic foot ulcers in both 2007 and 2010.22,25 In 2007 the study groups were 10 patients who received global heating and ES, 9 who received local heating and ES, and 10 patients who received conventional therapy only. The measure of the microcirculation was blood flow using LDI (measured in arbitrary unit flux). The control group did not undergo LDI measurement, only wound area was measured. In 2010 the aim of the study was to examine the role that heating had compared to ES and heating. Ten patients received local heating only and a further 10 local heating and ES. The treatment period for both studies was 4 weeks. In both studies the blood flow around and in the ulcer had decreased by the end of the study. In the 2007 study the mean blood flow at baseline was reported for 1 cm from the ulcer (182.3 ± 26.1 increasing to 245.0 ± 28.5 with ES) and the edge of the ulcer (223.4 ± 34.1 increasing to 301.0 ± 29.3 with ES). The result for the center of the ulcer is reported as being similar and is illustrated in a graph but the actual values are not stated. At 4 weeks only the values for the center of the ulcer are stated (228 ± 36.2 increasing to 256.7 ± 46.3 with ES). The change in blood flow before and during ES at baseline and at 4 weeks is displayed in Table 6; there was a significant reduction in the increase at 4 weeks (<0.01). The results for the local heating group are illustrated in a graph and stated as being similar but of a smaller magnitude to the global group but the actual mean values are not quoted.22 In 2010 Petrofsky found that the mean resting blood flow from all 3 areas and both groups had reduced by 54.5 ± 22.3% after 4 weeks.25
Table 6.
Change in Blood Flow Associated With Electrical Stimulation at Baseline and at 4 Weeks (Global Heating Group Only).22
| Flux ± SD |
|||
|---|---|---|---|
| Position of measurement | Baseline (10) | Week 4 (10) | P value |
| 1 cm from ulcer | 63.5 ± 11.9 | 18.3 ± 10.8 | <.01 |
| Edge of ulcer | 77.6 ± 11.6 | 48.7 ± 9.6 | |
| Center of ulcer | 33.6 ± 3.1 | 28.4 ± 15.8 | |
Lawson et al as described above also investigated the effect of electrical stimulation on wound healing. They measured blood flow at the center and outside of the ulcer using LDI at baseline, 2 weeks, and 4 weeks. When looking at the outside of the ulcer the prestimulation results for the DM group showed larger increase in the blood flow than for the non-DM group (DM, 0-2 weeks 35%, 0-4 weeks 21%; non-DM, 0-2 weeks 0%, 0-4 weeks 18%). However at the center of the wound the non-DM had a greater increase (DM, 0-2 weeks 8%, 0-4 weeks 5%; non-DM, 0-2 weeks 22%, 0-4 weeks 38%). The statistical significance of these results is not reported.23
Koblik et al performed an RCT comparing optimization of insulin therapy and injection of an antithrombotic drug (sulodexide) with optimization of insulin therapy and placebo injections for 10 weeks. Measurements were taken at baseline and 8 weeks using LDF. The parameters measured were resting flux (RF), peak hyperemic flow (pLDF), time to peak hyperemic flow (tpLDF), and hyperemia duration (HD) after an occlusion of 30 seconds. These measures were repeated following a 60-second occlusion once the readings had stabilized. In the placebo group (6 patients) there was no significant change in the RF at 8 weeks (baseline: mean flux 11.6 ± standard error of mean 1.3; 8 weeks 12.3 ± 1.1. p = ns). The pLDF for both the 30- (51.7 ± 15.2 to 147.0 ± 16.2, P < .01) and 60-second occlusion (110.5 ± 13.0 to 164.8 ± 15.4, P < .01) significantly increased at 8 weeks.16
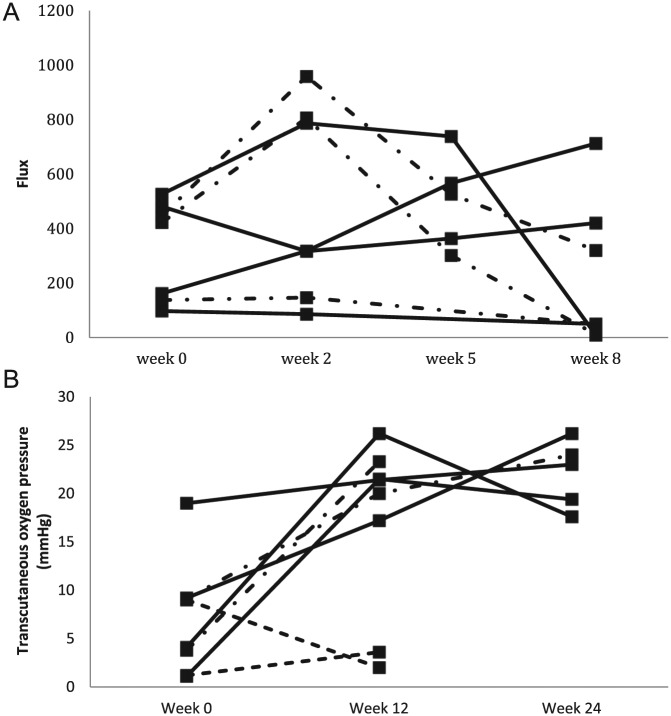
The results from 2 studies with small numbers are presented in graphical form in Figure 2. Newton et al’s 7 ulcers all healed or showed improvement at 8 weeks. Four measurements using LDI were performed at baseline, 2, 5, and 8 weeks. Four patients had an increase in blood flow over the first few weeks followed by a decrease to below baseline at 8 weeks. One increased throughout the measurement period. One decreased at 2 weeks, increased at weeks 5 and 8 but did not return to baseline level. One decreased throughout (Figure 2a). Those that had healed at 8 weeks, 2 increased then decreased, 1 increased throughout, and the other decreased throughout.19 Of Klingel’s 8 patients who received rheopheresis, 5 underwent TcPO2 at baseline, 12, and 24 weeks and 3 underwent TcPO2 at baseline and 12 weeks (due to minor amputation in 1 patient and major amputation in 2). Of the 4 patients who showed an improvement in their ulcer 2 had an increase in blood flow followed by a decrease, the other 2 increased throughout. In the patients whose ulcers were unchanged 1 increased TcPO2 at 12 weeks, the other increased at both 12 and 24 weeks. Of the 2 patients who deteriorated, 1 had a small increase at 12 weeks and the other had a small decrease (Figure 2b).21
Figure 2.
Trends during healing for LDI and TcPO2. (A) Adapted from Newton et al.19 Solid line, ulcers healed; alternating dashed line, ulcers improved. (B) Adapted from Klingel et al.21 Small dashed line, ulcers deteriorated.
Ichioka et al in their DM subgroup showed, in graphical form, a trend of increasing TcPO2 in the healed group and a decrease at 4 days in the unhealed group. The mean TcPO2 values at 4 and 14 days are not reported, however logistic regression analysis showed the results at these time point contributed significantly to the prediction of outcome (P < .001 and .002 respectively).24
Discussion
Within this group of studies the most commonly used method to assess the microcirculation was TcPO2 (n = 12), followed by LDF (n = 7), LDI (n = 4), capillary microscopy (n = 2), and isotope washout (n = 1). These proportions are probably representative of the current state of clinical usage of these methods with TcPO2 and LDF being the most common.
Within this group of studies, a variety of methods for examining the microcirculation have been used. Some of these methods have now fallen out of favor as technology has developed less invasive methods. This includes Xe clearance and SPP using isotope washout. LDF, TcPO2 and capillary microscopy remain in regular use. LDF is relatively underrepresented in this cohort, which is surprising considering that its utility in evaluating patients with critical limb ischemia is well-documented.29-31 One reason for this may be the relative age of many of the studies included (only 3 since 2000 and going back as far as 1978). TcPO2 was the most commonly used method in this review, which fits with its presence in the literature on critical limb ischemia and diabetic foot disease as a whole.
There is disagreement on how to carry out each of the methods of assessing the microcirculation, including positioning of the probes and in the case of TcPO2 the skin temperature that recordings were made at. Probes were most commonly positioned on the dorsum of the foot,11,12,15,17,20,26,27 but they are also positioned peri-wound12,18,21,24 and in 1 case it was not stated.14 A possible explanation for Yotsu et al not detecting a significant difference is their method of measurement.28 Multiple measurements were taken in 2 areas of the foot and the lowest result recorded. Of particular note, the contra-lateral foot was used if there was extensive ulceration, this may well have skewed their results. TcPO2 was most commonly measured at 44°C12,15,18,20,21,24 but also at 45°C11,14 or not stated.17,26-28
Due to the variety of countries and inclusion/exclusion criteria, the cohorts differed across the studies. For example, Yang et al26 and Lawson et al23 excluded patients with evidence of osteomyelitis whereas most of the other studies did not.
Unsurprisingly, the overall trend from the results is that if the microcirculation is functioning poorly then wound healing is poorer and outcomes are worse. Due to the larger number of studies, TcPO2 best demonstrates this. Most studies demonstrated a significantly higher TcPO2 in those patients who healed. What is less clear is the threshold at which healing occurs. The TcPO2 thresholds quoted for successful outcome in this review range from 10 mmHg to 34 mmHg. Karanfilian quotes sensitivity of 100% and specificity of 88% for healing if the TcPO2 is >10 mmHg.11 Pecoraro et al found that a TcPO2 of <20 mmHg was associated with a 39 fold increased risk of early healing failure.12 Both Kalani et al and Yang et al used the threshold of <25 mmHg and quoted sensitivities and specificities of 85% Vs 92% and 88.6% Vs 82.4% respectively.15,26 This threshold, when looking at the collated results in the healed and unhealed groups in Table 3, appear to hold true when considering the healed groups, all the mean results are above 25 mmHg. However it is worth observing that the mean TcPO2 is also higher than 25 mmHg in 6 of the unhealed groups.12,24,27,28 The current consensus among experts is that patients with a SPP ≥40 mmHg, TBP ≥45 mmHg or TcPO2 ≥25 mmHg are more likely to heal than their counterparts with poorer perfusion and that a TBP <30 mmHg or TcPO2 <25 mmHg is an indication for urgent vascular imaging.9,32 This is based on a recent review examining the utility of prognostic markers in diabetic foot disease in which the authors faced similar difficulties to us in identifying studies of sufficient quality to draw conclusions from.9 Eventually 11 studies involving 5890 patients were included however there was still significant heterogeneity and difference in the measures used. Their conclusions were based predominantly on 3 articles of acceptable rather than high quality (Quality in Prognosis Studies Tool).10,15,33
Only 1 study in this current review truly compared the results of testing the microcirculation in patients with DM and those without.11 Karanfilian et al found that the DM patients who healed had a lower TcPO2 than non-DM patients who healed. Conversely the LDF results were higher in the DM healed group. In the unhealed groups the opposite is true. The accuracy of TcPO2 for predicting healing is shown to be reasonable in those with DM, slightly poorer than those without DM but better than those with CRF. It is hard to explain this pattern however cohort selection may offer an explanation as the non-DM cohort had significant PVD whereas the DM cohort was made up of a mix of patients with diabetic foot disease, with and without PVD.11
The results from the repeated measures suggest that there is a change in the microcirculation during healing but the true trend and how it relates to healing has not yet been identified.
Conclusions
Due to the heterogeneity of the cohorts and the data presented it is not possible to draw any firm conclusions from a review of the current literature. We can, however, surmise that good microcirculation associates with better wound healing. The influence of DM and associated neuropathy is not clear, and neither is the degree of improvement required to achieve healing. Studies that examine a clearly defined cohort both with and without DM are urgently required. Accurate quantitative assessment of microcirculation will greatly aid predicting feet at risk, of predicting wound healing with and without surgery, and for identifying those at greatest risk of amputation.
Appendix
Search Strategy
| MESH search | |
|---|---|
| Search | Search terms |
| 1 | microcirculation |
| 2 | wound healing |
| 3 | diabetic foot |
| 4 | skin ulcer |
| 5 | laser Doppler flowmetry |
| 6 | blood gas monitoring, transcutaneous |
| 7 | Microscopic angioscopy |
| 8 | Xenon radioisotopes |
| 9 | 3 or 4 |
| 10 | 5 or 6 or 7 or 8 |
| 11 | 1 and 2 and 9 and 10 |
| 12 | 11 limited to English and humans |
| Keyword search | |
| Search | Search terms |
| 1 | capillar* or venule* or arteriole* or small adj2 vessels or skin microcirculation or skin blood supply or skin blood flow or microangiopath* or microcircula* disturbance* |
| 2 | transcutaneous adj3 oxygen* or transcutaneous PO2 or transcutaneous oximetry or transcutaneous adj3 carbon dioxide or TcPO2 or TcPCO2 |
| 3 | laser Doppler* or laser Doppler fluxmetry or laser Doppler Imaging or laser Doppler velocimetry or laser Doppler flux or LDF or LDI or post occlusive reactive hyperemia or PORH |
| 4 | capillary microscopy or capillary pressure or capillaroscopy |
| 5 | skin adj2 pressure or skin adj2 perfusion |
| 6 | xenon clearance or isotope clearance or hemodynamic test* or venoarteriolar response |
| 7 | 2 or 3 or 4 or 5 or 6 |
| 8 | wound* or ulcer* or ulcer healing or tissue loss or healing or wound complication* or non-healing or nonhealing or granulation tissue or amputat* |
| 9 | 1 and 7 and 8 |
| 10 | 9 limited to English and humans only |
Footnotes
Abbreviations: CM, capillary microscopy; DM, diabetes mellitus; ES, electrical stimulation; HBO, hyperbaric oxygen therapy; HD, hyperemia duration; HTN, hypertension; LDF, laser Doppler fluxmetry; LDI, laser Doppler imager; LD-PWA, laser Doppler pulse wave amplitude; LD-SBFV, laser Doppler skin blood flow velocity; MESH, medical subject headings; NPV, negative predictive value; pLDF, peak hyperemic flow; PORH, postocclusive reactive hyperemia; PPV, positive predictive value; RCT, randomized control trial; RF, resting flux; SBF, skin blood flow; SPP, skin perfusion pressure; SVR, skin vascular resistance; TBP, toe blood pressure; TcPO2, transcutaneous oxygen pressure; tpLDF, time to peak hyperemic flow.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Health & Social Care Information Centre. Quality and outcomes framework achievement, prevalence and exceptions data, 2012/13. Available at: http://www.hscic.gov.uk/catalogue/PUB12262/qual-outc-fram-12-13-rep.pdf.
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. J Am Med Assoc. 2005;293:217-228. [DOI] [PubMed] [Google Scholar]
- 4. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, J. A. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [DOI] [PubMed] [Google Scholar]
- 5. Akbari CM, LoGerfo FW. Diabetes and peripheral vascular disease. J Vasc Surg. 1999;30:373-384. [DOI] [PubMed] [Google Scholar]
- 6. LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes: implications for foot care. N Engl J Med. 1984;311(25):1615-1619. [DOI] [PubMed] [Google Scholar]
- 7. Malik RA, Newrick PG, Sharma AK, et al. Microangiopathy in human diabetic neuropathy: relationship between abnormalities and the severity of neuropathy. Diabetologia. 1989;32:92-102. [DOI] [PubMed] [Google Scholar]
- 8. Rayman G, Malik RA, Sharma AK, Day JL. Microvascular response to tissue injury and capillary ultrastructure in the foot skin of type 1 diabetic patients. Clin Sci. 1995;89:467-474. [DOI] [PubMed] [Google Scholar]
- 9. Brownrigg JR, Hinchliffe RJ, Apelqvist J, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(S1):128-135. [DOI] [PubMed] [Google Scholar]
- 10. Faris I, Duncan H. Skin perfusion pressure in the prediction of healing in diabetic patients with ulcers or gangrene of the foot. J Vasc Surg. 1985;2(4):536-540. [DOI] [PubMed] [Google Scholar]
- 11. Karanfilian RG, Lynch TG, Zirul VT. The value of laser Doppler velocimetry and transcutaneous oxygen tension determination in predicting healing of ischemic forefoot ulcerations and amputations in diabetic and nondiabetic patients. J Vasc Surg. 1986;4(5):511-516. [PubMed] [Google Scholar]
- 12. Pecoraro RE, Ahroni JH, Boyko EJ, Stensel VL. Chronology and determinants of tissue repair in diabetic lower-extremity ulcers. Diabetes. 1991;40:1305-1313. [DOI] [PubMed] [Google Scholar]
- 13. Jorneskog G, Brismar K, Fagrell B. Low molecular weight heparin seems to improve local capillary circulation and healing of chronic foot ulcers in diabetic patients. Vasa. 1993;22(2):137-142. [PubMed] [Google Scholar]
- 14. Padberg JFT, Back TL, Thompson PN, Hobson Ii RW. Transcutaneous oxygen (TcPO2) estimates probability of healing in the ischemic extremity. J Surg Res. 1996;60(2):365-369. [DOI] [PubMed] [Google Scholar]
- 15. Kalani M, Brismar K, Fagrell B, Ostergren J, Jorneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22(1):147-151. [DOI] [PubMed] [Google Scholar]
- 16. Koblik T, Sieradzki J, Sendur R, et al. The effect of insulin and sulodexide (Vessel Due F) on diabetic foot syndrome—pilot study in elderly patients. J Diabetes Complications. 2001;15(2):69-74. [DOI] [PubMed] [Google Scholar]
- 17. Zimny S, Schatz H, Pfohl M. Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complications. 2002;16:327-332. [DOI] [PubMed] [Google Scholar]
- 18. Fife CE, Buyukcakir C, Otto GH, et al. The predictive value of transcutaneous oxygen tension measurement in diabetic lower extremity ulcers treated with hyperbaric oxygen therapy: a retrospective analysis of 1144 patients. Wound Repair Regen. 2002;10(4):198-207. [DOI] [PubMed] [Google Scholar]
- 19. Newton DJ, Khan F, Belch JJF, Mitchell MR, Leese GP. Blood flow changes in diabetic foot ulcers treated with dermal replacement therapy. J Foot Ankle Surg. 2002;41(4):233-237. [DOI] [PubMed] [Google Scholar]
- 20. Kalani M, Jorneskog G, Naderi N, Lind F, Brismar K. Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers—long-term follow-up. J Diabetes Complications. 2002;16(2):153-158. [DOI] [PubMed] [Google Scholar]
- 21. Klingel R, Mumme C, Fassbender T, et al. Rheopheresis in patients with ischemic diabetic foot syndrome: results of an open label prospective pilot trial. Ther Apher Dial. 2003;7(4):444-455. [DOI] [PubMed] [Google Scholar]
- 22. Petrofsky JS, Lawson D, Suh HJ, et al. The influence of local versus global heat on the healing of chronic wounds in patients with diabetes. Diabetes Technol Ther. 2007;9(6):535-544. [DOI] [PubMed] [Google Scholar]
- 23. Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Med Sci Monit. 2007;13(6):CR258-CR263. [PubMed] [Google Scholar]
- 24. Ichioka S, Yokogawa H, Sekiya N, et al. Determinants of wound healing in bone marrow-impregnated collagen matrix treatment: impact of microcirculatory response to surgical debridement. Wound Repair Regen. 2009;17(4):492-497. [DOI] [PubMed] [Google Scholar]
- 25. Petrofsky JS, Lawson D, Berk L, Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes. 2010;2(1):41-46. [DOI] [PubMed] [Google Scholar]
- 26. Yang C, Weng H, Chen L, et al. Transcutaneous oxygen pressure measurement in diabetic foot ulcers: mean values and cut-point for wound healing. J Wound Ostomy Continence Nurs. 2013;40(6):585-589. [DOI] [PubMed] [Google Scholar]
- 27. Wang A, Sun X, Wang W, Jiang K. A study of prognostic factors in Chinese patients with diabetic foot ulcers. Diabet Foot Ankle. 2014;5:22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yotsu RR, Pham NM, Oe M, et al. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications. 2014;28(4):528-535. [DOI] [PubMed] [Google Scholar]
- 29. Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34(7):373-384. [DOI] [PubMed] [Google Scholar]
- 30. Kluz J, Malecki R, Adamiec R. Practical importance and modern methods of the evaluation of skin microcirculation during chronic lower limb ischemia in patients with peripheral arterial occlusive disease and/or diabetes. Int Angiol. 2013;32(1):42-51. [PubMed] [Google Scholar]
- 31. Klomp HM, Wittens CHA, van Urk H. Transcutaneous oximetry, laser Doppler fluxmetry, and capillary microscopy: variability in patients with advanced atherosclerotic disease of the lower extremity. Vasc Endovascular Surg. 2000;34(3):231-243. [Google Scholar]
- 32. Lipsky BA, Aragon-Sanchez J, Diggle M, et al. IWGDF Guidance on the Diagnosis and Management of Foot Infections in Persons With Diabetes. Prevention and Management of Foot Problems in Diabetes: Guidance Documents and Recommendations. The Hague, Netherlands: International Working Group on the Diabetic Foot; 2015. [Google Scholar]
- 33. Holstein P, Lassen NA. Healing of ulcers on the feet correlated with distal blood pressure measurements in occlusive arterial disease. Acta Orthop Scand. 1980;51(6):995-1006. [DOI] [PubMed] [Google Scholar]