Abstract
Background:
Over the past generation, preclinical data have suggested that there is a potential physiologic benefit to applying oxygen topically to wounds. However, we are unaware of any studies in the literature that have robustly assessed whether this would lead to a higher proportion of healing in similarly treated people without oxygen. Therefore, the purpose of this study was to assess this in people being treated for chronic diabetic foot ulcers (DFUs).
Methods:
We enrolled and randomized 100 subjects with DFUs (79% male, aged 58.3 ± 12.1 years) to receive either active continuous diffusion of oxygen (CDO) therapy using an active CDO device, or an otherwise fully operational sham device that provided moist wound therapy (MWT) without delivering oxygen. Patients were followed until closure or 12 weeks, whichever was sooner. Patients, treating physicians and independent evaluators were blinded to the study arm. All patients received identical offloading, dressings and follow-up.
Results:
There were no significant differences in assessed descriptive characteristics between the treatment arms (P > .05 for all). A significantly higher proportion of people healed in the active arm compared to sham (46% vs 22%, P = .02). This relative effect became greater in more chronic wounds (42.5% vs 13.5%, P = .006). Patients randomized to the active device experienced significantly faster rates of closure relative to the sham (P < .001).
Conclusions:
The results of this study suggest that continuously diffused oxygen over a wound leads to significantly higher rates of closure, and faster time to closure, compared to similarly treated patients receiving standard therapy coupled with a sham device. Furthermore, the relative efficacy appears to improve the more the therapy may be needed (more chronic and larger wounds).
Keywords: closure rate, continuous diffusion of oxygen, diabetic foot ulcer, moist wound therapy, tissue oxygenation, wound healing
Diabetic foot ulcers (DFUs) and their resultant complications constitute a silent, sinister burden.1-3 Approximately half of DFUs will become infected. Once this occurs, some 20-30% will receive some form of amputation.1,4 Following amputation, five-year survival is worse than most forms of cancer.5,6 Furthermore, the direct costs associated with care of the diabetic lower extremity are greater than the five most expensive cancers in the United States alone.7 Treating the wounds to closure as rapidly and safely as possible, therefore, is a logical strategy to reduce morbidity and resources.
Oxygen has been shown to be an essential component in multiple mechanisms of action required for wound healing.8-10 Depressed levels of oxygen have been shown to be a rate-limiting step in these mechanisms. Conversely, increasing the amount of oxygen to levels higher than normal has been shown to result in increased, and often proportional, levels of activity. Aside from general cell metabolism and energy production, these mechanisms of action, and corresponding rates of action, affected by oxygen levels in the tissue include cell proliferation and reepithelialization,11,12 collagen synthesis and tensile strength,13-15 angiogenesis,16 antibacterial activity through respiratory burst,17,18 and growth factor signaling transduction.19,20 The damaged tissue in chronic wounds has increased oxygen demands and can achieve optimal healing through oxygen therapy.8,10 This need for supplemental oxygen can be exacerbated through the use of occlusive dressings which block or limit the amount of atmospheric oxygen available to the wound. This is compounded for patients with chronic wounds who often have compromised blood flow to the wound, thus further limiting tissue oxygenation and impairing healing.21
Enhancing tissue oxygenation can be achieved using different therapies. Traditionally, hyperbaric oxygen therapy has been used to achieve supersaturated levels of oxygen in the blood stream and tissues through high pressures and concentrations of inspired oxygen. However, this therapy is intermittent (90 minute exposures, 3-5 times per week), relies on circulation (which may be impaired) to bring the oxygen to the damaged tissue, and requires substantial time for the patient in terms of travel and preparation time. Topical oxygen therapy is a newer modality in which the affected tissue is placed within a chamber or bag and exposed to high concentrations of oxygen. However, this therapy is also intermittent (typically 90 minute exposures once a day) and the subject must remain immobile during the treatment. Yet topical oxygen can be applied in a wider variety of settings, including the patient’s home. The newest therapy, continuous diffusion of oxygen or continuously diffused oxygen (CDO) removes the above-mentioned limitations of being intermittent and immobilizing the patient during treatment. CDO uses pure, humidified oxygen to continuously treat a wound by supplying oxygen directly to the affected tissue within a MWT dressing. This allows for sustained delivery of oxygen to the tissue (24 hours a day, 7 days a week), full patient mobility during treatment, and application of the therapy in virtually any setting. Devices that supply CDO therapy are lightweight, silent, solid-state, and come in rechargeable or disposable versions.
This study focuses on an FDA cleared device which supplies CDO therapy, the TransCu O2® System (EO2 Concepts, San Antonio, TX). The device uses fuel cell technology to continuously generate pure, humidified oxygen at flow rates of 3-15 ml/hr and deliver it directly to the wound bed environment within the MWT dressing system via tubing. CDO therapy can be simply described as MWT plus oxygen. This CDO device differentiates itself from other continuous oxygen delivery devices by being the only system to employ sensors which monitor and control not only the amount of oxygen being delivered, yet also monitor and control the pressure within the wound bed. The oxygen control system compensates for environmental fluctuations that can affect oxygen output, thus ensuring consistent oxygen delivery. The pressure control system ensures that there is no blockage of oxygen flow and that the oxygen pressure in the wound bed does not exceed capillary collapse pressure. Excessive localized pressures could collapse capillaries and impair delivery of blood and nutrients to the affected tissues during wound repair. The unit continuously monitors and controls for these variables, and warns the patient/physician if the flow of oxygen is impaired or if the pressure in the wound bed is too high. The TransCu O2 System is intended to treat various ulcers, including DFUs, venous leg ulcers, pressure ulcers and other skin wounds.
Previous studies have demonstrated the efficacy of CDO using various devices that provide CDO therapy. A retrospective analysis on the impact of CDO in chronic toe ulcer healing for 20 patients showed an overall success rate (full closure) of 74% on wounds that were unresponsive to other therapies.22 The author highlighted a chief benefit being that of high patient compliance (95%), which he attributed to the device’s ease of use, the noticeability of improvement within a short period of time, and the reduction of pain, which has also been reported elsewhere.23 Another retrospective analysis of 25 patients in a Veteran’s Healthcare Administration environment showed 68% full closure, both as a stand-alone and adjunctive therapy.24 The author found that CDO improves wound healing potential, including in wounds receiving advanced tissue/skin substitute applications. A prospective, randomized clinical trial of CDO versus MWT followed 17 patients (9 CDO, 8 MWT) for 4 weeks and found significant differences in wound volume reduction.25 The CDO group had an average volume reduction of 87%, whereas the MWT group had an average volume reduction of 46% (P < .05). Significant differences in the healing rate of CDO as compared to MWT were recently demonstrated in a prospective, randomized pilot clinical trial with 9 patients receiving MWT and 9 receiving CDO.26 The study focused on smaller DFUs (approx. 1.5 cm2), UT Grade I-III, over an 8-week period. CDO was shown to close 90% of the wounds by the end of the study, whereas the MWT group experienced 30% closure. The authors also noted significantly faster wound closure rates in the CDO arm and more noticeable differences from CDO in the more advanced ulcers (Grades II and III).
In a double-blind preclinical study using a sham as the control arm, similar to the design discussed herein, wherein all wounds received MWT dressings and a CDO device, significant results were found for both the rate of reepithelialization and amount of full closure achieved.11 Full closure was 57% in the active CDO arm and 25% in the sham arm (P = .008). The rate of reepithelialization was increased in the active CDO arm by 130% relative to the sham arm (P = .006) and full closure was achieved. The authors also noted that histologically the repair tissue showed more advanced wound remodeling and organized collagen in the active CDO arm. Similarly, a nonsham blinded preclinical trial showed significant results with each animal as its own control. The rate of reepithelialization was increased in the CDO arm by 156% relative to the control arm (P = .01).27
Pain reduction associated with CDO therapy has also been reported. For a patient who served as her own control during CDO therapy treatment, her pain levels were reported as high as 8/10 on a visual analogue score (VAS), with pain medications taken as needed, during the 5-month duration of the ulcer prior to CDO therapy.28 After 20 days of CDO therapy, the patient reported a pain level of 2/10 and was no longer taking pain medications. At this time, CDO therapy was temporarily discontinued since the patient was leaving town for a holiday. Six days later the patient returned to the clinic with a pain level of 10/10 and reported difficulty sleeping. CDO therapy was reapplied and, within three days, the patient’s pain level was controlled (VAS 2/10) and she ceased taking narcotics. In an uncontrolled, nonrandomized study of 10 patients with venous ulcers, CDO therapy was reported to significantly (P < .009) reduce pain in a six-week period.29 The corresponding mean reduction in wound size was 58.9%. In a case series review, four patients with severe, very painful wounds were successfully closed and the pain was significantly reduced in all cases.30 Similarly, a six-patient case review of CDO in patients with diabetes and chronic lower extremity wounds reported significant pain reduction.31
This report focuses on the per protocol analysis of the effect of CDO on the primary outcome of full wound closure by 12 weeks, as well as the secondary outcome of rate of wound closure. The effects of baseline wound size and run-in wound closure rate, which are measures of how severe and chronic the wound is, are also reported.
Methods
Study Design, Primary and Secondary Outcomes
This is a planned analysis of a randomized, balanced, double blind, sham-controlled, parallel group clinical trial evaluating use of the CDO device for DFUs. Both arms received identical treatment (device, dressings, etc) and the devices were functional in both arms with the exception that the oxygen did not flow to the wound in the sham arm. Prior to assignment of a device, all patients were subjected to a run-in period during which they received MWT and were evaluated for wound size and rate of wound closure to ensure that the wounds were indeed chronic in nature. Patients, treating physicians and independent analysts were blinded to the treatment arm, thereby eliminating the placebo effect. The total sample size was 100. The primary efficacy outcome was complete wound closure (yes, no), defined as complete reepithelialization with no drainage as assessed by the treating clinician and confirmed by a blinded observer. Eligible subjects were those who were confirmed to meet all inclusion and none of the exclusion criteria. Full criteria and details on participating sites are available on ClinicalTrials.gov under the Identifier NCT01645891.
As published previously,32 the effects of initial or baseline wound size and initial or run-in rate of wound closure were investigated. These were defined as:
Baseline wound size: the wound area as determined by digital planimetric analysis at the randomization visit.
Run-in wound closure rate: the percentage of wound closure (percentage wound area reduction, or PWAR) during the run-in period prior to the placement of the device. All subjects received MWT during this period.
The basis for assessing effect of wound size is that smaller wounds are more likely to heal within the 12-week study timeframe. In theory, if the Active arm enhances healing rates, the relative effect between the Active and Sham arms should be similar or greater in larger wounds than in smaller wounds. With regard to the run-in wound closure rate, it has been shown that wounds related to DFUs which exhibit a relatively high rate of closure early in a treatment regimen, measured as PWAR, are significantly more likely to close without intervention of advanced therapies.33 Sheehan et al found that wounds experiencing less than 50% PWAR in 4 weeks were significantly less likely to close than those experiencing greater than or equal to 50% PWAR.34 Similarly, Lavery et al found that wounds experiencing less than 60% PWAR in 4 weeks were significantly less likely to close than those experiencing greater than or equal to 60% PWAR.35 In other words, a high PWAR indicates wounds that are easy to close, whereas a lower PWAR is indicative of wounds that are harder to close. Therefore, wounds that exhibit lower run-in rates of closure (indicating that they are more chronic) should be more responsive to the use of advanced modalities such as CDO.
On the basis of early results from this study (published in Wound Medicine),32 the protocol was amended to change the minimum baseline wound size and run-in rate of wound closure inclusion/exclusion criteria mentioned above. Subjects that failed these criteria were removed from the study.
Screening, Randomization, and Treatment
Those with a DFU present for a minimum of 30 days, yet not more than a year, were eligible for enrollment. Ages were limited to between 30 and 90 years with wound sizes ranging from 1.5 cm2 to 10 cm2, as measured by planimetric analysis from photos taken after wound debridement. Subjects were recruited from a total of 34 sites in the continental United States. All enrolled subjects received a standard MWT regimen consisting of wound cleansing, moist wound care, off-loading and, as appropriate, aggressive debridement. After initial screening for eligibility and obtaining informed consent, a patient history and baseline assessment were obtained by the study clinician. Variables assessed included ankle-brachial index, wound duration, location and size, patient age, race, gender, and HbA1c. All wounds were classified according to the University of Texas classification for diabetic wounds by a wound specialist based on clinical and laboratory data.36 All wounds were surgically debrided to a bleeding base as necessary; the number of debridements was not limited but usually debridements were performed once a week before treatment commenced.
Prior to enrollment, subjects went through a run-in period to screen out nonchronic wounds that would be easy to close without advanced intervention. The study inclusion/exclusion criteria required wounds experiencing high run-in wound closure rates, measured as PWAR, during the run-in period to be excluded from the study. The intent was to find a balance between a short run-in period and robust screening criteria to help ensure that nonchronic wounds were not included in the study. As detailed in an interim analysis of the data,32 the closure rate during the run-in period had an effect on the sensitivity of the analysis. Subjects were excluded who experienced:
wounds that closed more than 30% in either week of the first two weeks, or
wounds that closed more than 50% in the first two weeks.
This is referred to below as a run-in wound closure rate of 30%/50% PWAR. Since the PWAR assessment relied on planimetric analysis of wound photos, some subjects were initially placed on a device at the conclusion of the run-in period and subsequently found to be not eligible for failing study inclusion/exclusion criteria. These subjects were removed as not eligible and are not included in the randomized number shown on Figure 1.
Figure 1.
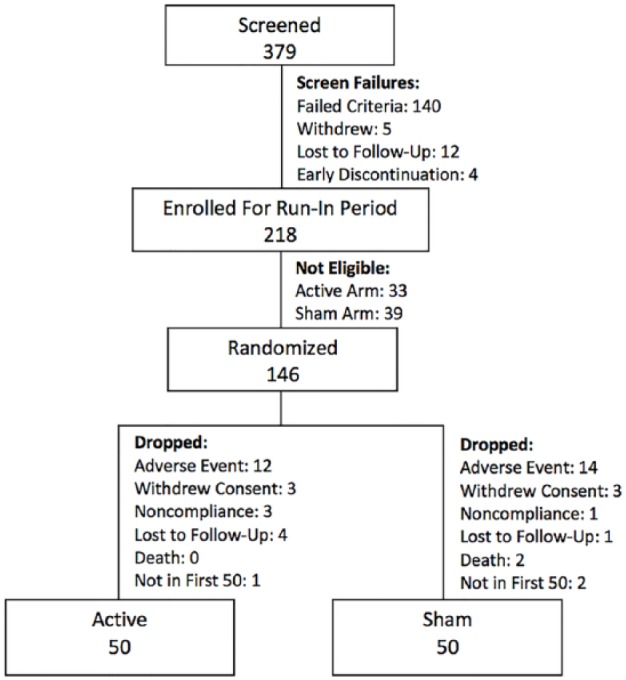
Consort diagram.
Subjects were randomized to either the Treatment arm (hereafter referred to as Active arm) or the Control arm (hereafter referred to as Sham arm). All subjects in the Active arm received CDO therapy in addition to standard of MWT during the Treatment Period and all of those in the Sham arm received sham units (functioning CDO device with no oxygen going to the wound) and standard of care MWT during the Treatment Period. All CDO devices were set to the minimum flow rate of 3 ml/hr, which is the manufacturer’s recommended oxygen flow rate for the wound sizes in this study.
All patients were followed for the Treatment Period of 12 weeks, or until the wound closed, whichever event occurred first. During the Treatment Period, weekly assessments were made of wound size (width, height, and depth) via planimetric analysis, degree of reepithelialization of the wound, pain, quality of life, and the development of granulation tissue. Between visits, dressings were changed as needed by the subjects themselves or a relative/friend for the vast majority of subjects (>99%).
To evaluate whether there was an effect of wound size on the primary outcome of wound closure, the effect on the primary outcome was reported by wound size in 0.5 cm2 segments from all wounds greater than 1.5 cm2 up to a minimum wound size of 4.0 cm2. Higher minimum wound sizes were not reported since the sample sizes became too small (n < 30). To evaluate whether there was an effect of run-in wound closure rate on the primary outcome of wound closure, results were reported by excluding subjects at two lower rates of PWAR, each corresponding to more chronic wounds. The lowest rate analyzed was chosen to match that established by Lavery et al as defining a chronic wound which would benefit from advanced intervention: less than 60% in 4 weeks.35 This in turn corresponds to less than 30% in 2 weeks, the run-in period used in this study. The intermediate rate of 40% in 2 weeks was also analyzed. Since each 2-week period analyzed had a 10% change from the original 50%, the corresponding 1-week period rate of change was 5%. Therefore, the analysis eliminated wounds that experienced more than 25% PWAR in either week of the first two weeks or 40% PWAR in the first two weeks (referred to as an run-in wound closure rate of 25%/40% PWAR), as well as 20% PWAR in either week of the first two weeks or 30% PWAR in the first two weeks (referred to as an run-in wound closure rate of 20%/30% PWAR). We defined relative performance as the ratio of the proportion of subjects in the Active arm reaching full closure divided by the proportion of subjects in the Sham arm reaching full closure, expressed as a percentage.
Statistical Methods
This study was planned to follow a group sequential design with one interim analysis at the midpoint and one at the endpoint of the study with Obrien-Fleming stopping bounds. We assumed that 82.4% of treated and 45.5% of controls would experience wound closure; with two-sided testing, an interim analysis when 50% and 100% of Subjects complete the treatment phase, Obrien-Fleming stopping bounds, and an overall significance level of 5%, then this study would achieve 90% power with 41 Subjects per treatment arm.
The study failed to cross the boundary at midpoint; at the interim analysis of primary outcome based on n = 42 subjects (n = 21 in each arm) 52.4% of Active and 38.1% of Sham subjects experienced wound closure (P = .54).32 In a conditional power calculation, we concluded that if the efficacy specified in the protocol was experienced in the remainder of the study, then the total sample size required to reach 90% power would be 100, or n = 50 per arm.
Subjects who failed to meet eligibility criteria, withdrew for any reason, or who completed but were not among the first 50% to complete in each arm were excluded. Continuously distributed outcomes were summarized with the sample size, mean, and standard deviation and categorical outcomes were summarized with frequencies and percentages. When contrasting treatment arms with regard to binary outcomes, we report the relative risk (RR) and its 95% confidence interval (CI). The statistical significance of the relation between binary outcomes and treatment arm (Active, Sham) were assessed with Fisher’s Exact test. Treatments were contrasted with regard to continuously distributed outcomes with a t-test. In an analysis of days to closure versus wound closure we used a repeated measures linear model with an autoregressive order one autocorrelation assumption. We used R and SAS for all analyses and graphics. All statistical tests were two-sided with a significance level of 5%. Corrections for multiple comparisons were not applied.
Results
As of October 31, 2016, 379 subjects had been screened. The first 50 to complete the study per protocol in each arm (n = 100) were included, 50 in the Active and 50 in the Sham arms (Figure 1). Of the 34 participating sites, 23 had subjects who completed the study with a mean of 4 subjects per site. There was no significant impact of any one given site on the results of the study. At baseline the two treatment arms were similar with regard to age, ethnicity, gender, wound size, HbA1c, ABI, and patients experiencing no pain in the wound (Table 1).
Table 1.
Baseline (Enrollment) Characteristics.
| Characteristics | Active (n = 50) | Sham (n = 50) | Total (n = 100) | P value |
|---|---|---|---|---|
| Age ± SD | 57.5 ± 10.9 | 59.1 ± 13.3 | 58.3 ± 12.1 | .5 |
| Female (%) | 11 (22) | 10 (20) | 21 (21) | 1 |
| Male (%) | 39 (78) | 40 (80) | 79 (79) | |
| Ethnicity (%) | ||||
| Black | 7 (14) | 9 (18) | 16 (16) | .83 |
| Hispanic | 20 (40) | 18 (36) | 38 (38) | |
| White | 23 (46) | 23 (46) | 46 (46) | |
| Wound area, cm2 ± SD | 3.4 ± 1.5 | 3.9 ± 2.0 | 3.6 ± 1.8 | .23 |
| HbA1c ± SD | 8.1 ± 1.7 | 8.3 ± 1.9 | 8.2 ± 1.8 | .56 |
| ABI ± SD | 17.4 ± 11.6 | 15.9 ± 11.9 | 16.7 ± 11.7 | .51 |
| No pain | 24 (48) | 22 (44) | 46 (46) | .84 |
| Pain | 26 (52) | 28 (56) | 54 (54) | |
The primary outcome of complete wound closure at 12 weeks (Table 2) was significantly associated with treatment per protocol, Active 23 (46.0%), Sham 11 (22.0%), RR 0.69 (95% CI 0.52, 0.93), P = .02.
Table 2.
Primary Outcome of Full Wound Closure.
| Primary outcome, n (%) | Treatment |
P value | RR (95% CI) | |
|---|---|---|---|---|
| Active | Sham | |||
| Closed | 23 (46) | 11 (22) | .02 | 0.69 (0.52, 0.93) |
| Open | 27 (54) | 39 (78) | ||
| Total | 50 | 50 | ||
Wound size (cm2) at baseline (Figure 2) did not vary significantly by treatment arm (P = .44) or wound closure (P = .11).
Figure 2.
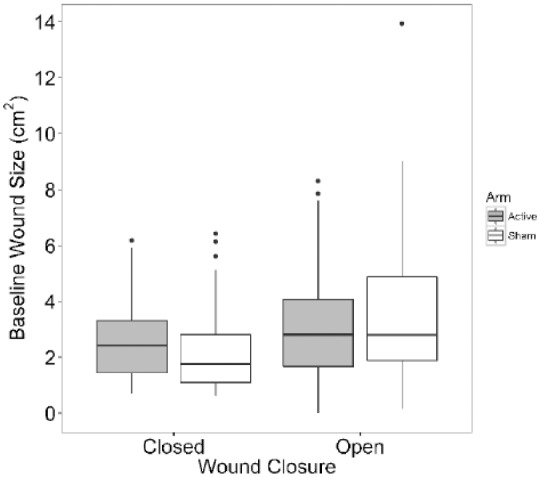
Wound size at baseline by treatment arm and wound closure in all subjects (Active n = 50, Sham n = 50). The interaction between wound closure and wound size at baseline was not significant (P = .61). After removing the interaction term, the wound size at baseline did not vary significantly with treatment (P = .44), or wound closure (P = .11).
Days to closure increased with wound closure in both arms (Figure 3) and the average days to closure was less among patients in the Active arm relative to those in the Sham arm. The treatment effect was significant (P = .026) and the wound closure effect was significant (P < .001). The relative reduction in time to reach 50%, 75% or 100% wound closure was higher initially and decreased as the wounds progressed to full closure (Figure 4).
Figure 3.
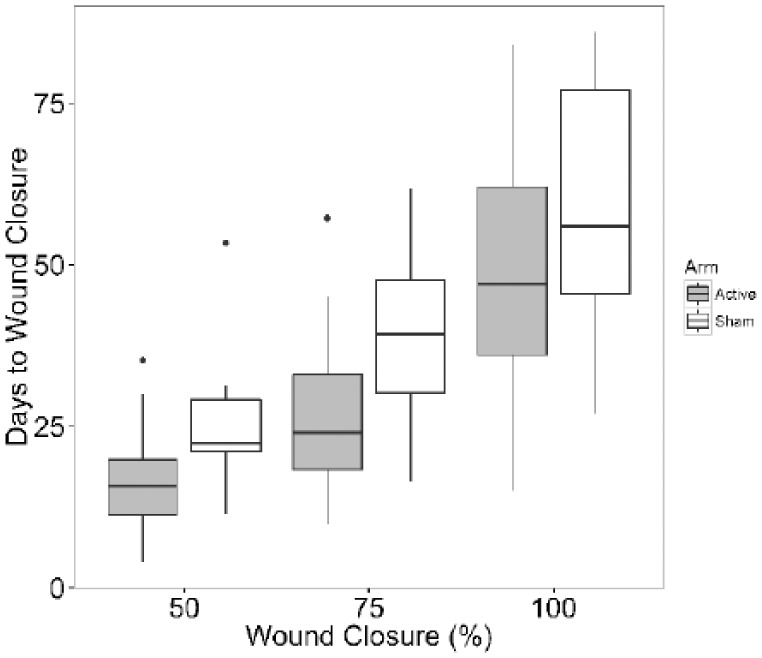
Days to wound closure by treatment arm and wound closure (%) among subjects who experienced full closure (Active n = 23, Sham n = 11). The interaction of wound closure (%) and treatment was not significant (P = .75). After removing the interaction term, the treatment effect was significant (P = .026) and the wound closure (%) effect was significant (P < .001).
Figure 4.

Relative reduction in time to 50%, 75%, and full wound closure by treatment arm and wound closure among subjects who fully healed (Active n = 23, Sham n = 11).
The effect of run-in wound closure rate on the primary outcome shows a linear decrease in the Sham arm with essentially no change in the Active arm as the run-in wound closure rate decreased (Table 3). There were significant beneficial effects of the Active arm at 25%/40% PWAR (P = .01) and 20%/30% PWAR (P = .006). Table 3 shows that the Active arm was relatively insensitive to reducing the run-in wound closure rate (values ranged from 46.0% to 42.5% full wound closure), whereas the Sham arm experienced a significant drop from 22.0% full wound closure to 13.5% full wound closure, corresponding to a 39% reduction in efficacy as the wounds become more chronic. This resulted in an overall increase in relative performance of the Active versus Sham from 209% to 315% as the wounds become more difficult to heal.
Table 3.
Wound Closure (%) by Treatment Arm and Run-In Wound Closure Rate; Sample Sizes Are Indicated in Parentheses.
| Run-in closure rate (PWAR) | Wound closure, % (n) |
P value | Relative risk | Confidence interval | Relative performance | |
|---|---|---|---|---|---|---|
| Active | Sham | |||||
| 30%/50% | 46.0% (50) | 22% (50) | .02 | 0.69 | (0.52, 0.93) | 209% |
| 25%/40% | 43.5% (46) | 17.1% (41) | .01 | 0.68 | (0.51, 0.91) | 255% |
| 20%/30% | 42.5% (40) | 13.5% (37) | .006 | 0.67 | (0.50, 0.89) | 315% |
The effect of baseline minimum wound size on full wound closure showed a decrease in both arms (Figure 5) as the wound size increased. The relative performance showed an increasing trend as the minimum wound size increased (Figure 6).
Figure 5.
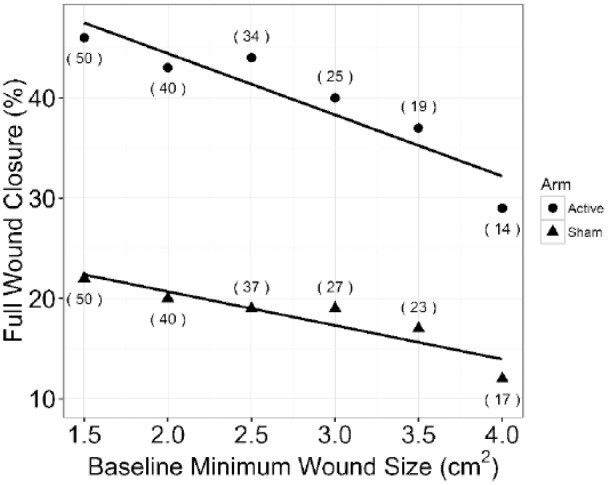
Full wound closure (%) by minimum baseline wound size and treatment arm. The effect of increasing the minimum wound size at randomization is shown. For example, 2.5 cm2 indicates all wounds between 2.5 and 10.0 cm2 at randomization were included in the sample. Samples sizes are indicated in parentheses.
Figure 6.
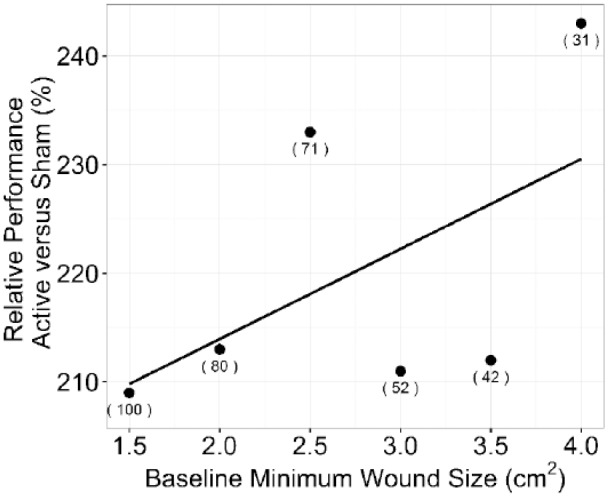
Relative performance versus minimum baseline wound size. The effect of increasing the minimum wound at randomization size is shown. For example, 2.5 cm2 indicates all wounds between 2.5 and 10.0 cm2 at randomization were included in the sample. Samples sizes are indicated in parentheses.
Discussion
In this per protocol analysis we found significant and beneficial treatment effect in the Active arm for full wound closure as well as time to wound closure. More than twice as many wounds closed in the subjects receiving CDO therapy versus MWT delivered through a sham device (Table 2). When stratified by the primary outcome (Figure 3), the Active arm experienced significantly faster rates of closure relative to the Sham arm (P < .001). The Active arm experienced significantly shorter times to reach 50%, 75%, and 100% closure (P = .026). This appears to support the findings of others that earlier, aggressive methods of intervention such as CDO are not only beneficial, yet also cost-effective by bringing wounds to closure more quickly. In doing so, the increased costs and burden of ongoing care, infection, and potential hospitalization might be reduced or avoided.34
We found increasingly significant and beneficial treatment effect in the Active arm for wounds which were more chronic at the beginning of treatment: subjects who experienced slower run-in wound closure rates (measured as PWAR) during the run-in period experienced significant and beneficial effects, and this benefit was greater as the run-in wound closure rates were reduced (a measure of more chronic wounds). The sample set for the lowest run-in wound closure rate (≤30% PWAR during the two-week period) eliminated subjects with easier to close wounds: the wounds that were eliminated experienced relatively high rates of closure substantially similar to those found to have significantly higher probability of healing in published literature as described above (≥50% in 4 weeks34 or ≥60% in 4 weeks35). These other studies found that diabetic foot wounds which exhibit a relatively high rate of closure are significantly more likely to close without intervention of advanced therapies.33 At the lowest run-in wound closure rate of ≤30% PWAR in two weeks, which contains the subset of the most chronic wounds, there was a significant and beneficial treatment effect in the Active CDO arm of 42.5% full wound closure versus 13.5% for the Sham MWT arm (n = 77, P = .006). These most chronic wounds were more than three times (315%) as likely to close with the treatment of CDO versus MWT alone.
The effect of wound size on primary outcome shows that the Active CDO arm has a sustained beneficial improvement as the wound size increases (Figure 5). The slopes of the regression lines are similar and the relative effect is equal or greater (Figure 6), indicating that as the wound size increases, the Active CDO arm has a relative beneficial effect that does not diminish and could potentially be of more benefit.
The Active CDO arm versus the Sham arm demonstrates improved healing with more than twice as many wounds reaching full closure in the 12-week timeframe. Table 4 shows a comparison of CDO to other advanced treatments in wound closure, all of which assess wound closure within similar time frames. The absolute performance of 46% in the Active CDO arm compares very favorably to published results from other advanced wound therapies (30-52%). This comparison becomes even stronger when one considers that the other studies allow for closure by secondary means other than the therapy being studied, including but not limited to surgical closure and amputation. Furthermore, the CDO study is the only study that has a double blind with a sham/placebo design. In all of the other studies, the subjects and physicians were aware of the treatment arm. Only the CDO study eliminates this variable, known commonly as the placebo effect.
Table 4.
A Summary of Results Compared to Four Other Published Studies Using Advanced Therapies to Treat Diabetic Foot Ulcers.
| Study | Wound type | Test device (therapy) | Level of evidence | Comparator | N | Length of study (weeks) | Wound closure (%) |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| Test | Control | ||||||||
| Present study | DFU | TransCu O2 (CDO) | 1A | Sham device MWT with specific foam and thin film, optional alginate | 100 | 12 | 46 | 22 | .02 |
| Blume et al 200737 | DFU | VAC (NPWT) | 1B | MWT with alginates, foams, hydrocolloids, or hydrogels | 335 | 16 | 43 | 29 | .007 |
| Armstrong et al 201238 | DFU and VLU | Snap (NPWT) | 1B | VAC | 83 | 12 | 50 | 52 | Not available |
| Marston et al 200339 | DFU | Dermagraft (skin subst.) | 1B | Saline-moistened gauze | 245 | 12 | 30 | 18 | .03 |
| Edmonds 200940 | DFU | Apilgraf (skin subst.) | 1B | Nonadherent dressing | 72 | 12 | 52 | 26 | .03 |
The CDO study has level 1A evidence (double-blind, prospective RCT with a sham). All other studies have level 1B evidence (unblinded, prospective RCT).
It is important to note that, while the results here are shown to result in significant and beneficial treatment of DFUs, the oxygen flow rate from the CDO device was set to the minimum flow rate of 3 ml/hr. Higher flow settings, up to 15 ml/hr, could plausibly result in improvements in larger or more ischemic wounds through the availability of additional oxygen and higher potential oxygen concentrations. Higher local oxygen tensions may result in proportional increases in the rates of cell proliferation and reepithelialization,11,12 collagen synthesis and tensile strength,13-15 angiogenesis,16 and antibacterial activity through respiratory burst.17,18 Similar results have been demonstrated in other studies of CDO in various wound types.11,22-24 Further experience with the device may ultimately reveal that the device benefits patients who need it the most.
Conclusions
We report a significantly greater percentage of and rate of healing in patients receiving CDO therapy compared to a sham device providing standard wound therapy. The study also revealed that CDO therapy appears to have a similar or greater effect as the wound size increases. Furthermore, the relative effect of CDO therapy on more chronic wounds appears to be more pronounced as the wounds become more chronic. These results appear to indicate that the more a wound needs CDO therapy, the greater the apparent effect. We look forward to further works that may confirm or build on these data.
Footnotes
Abbreviations: CDO, continuous diffusion of oxygen; CI, confidence interval; DFU, diabetic foot ulcer; MWT, moist wound therapy; PWAR, percentage wound area reduction; RR, relative risk.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MQN is a full-time employee of EO2 Concepts. DGA is a member of the Scientific Advisory Board of EO2 concepts.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by EO2 Concepts Inc.
References
- 1. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29(6):1288-1293. [DOI] [PubMed] [Google Scholar]
- 2. Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract. 2009;83:347-352. [DOI] [PubMed] [Google Scholar]
- 3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217-228. [DOI] [PubMed] [Google Scholar]
- 4. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286-287. [DOI] [PubMed] [Google Scholar]
- 6. Morbach S, Furchert H, Groblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35:2021-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle. 2013;4. doi: 10.3402/dfa.v4i0.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tandara AA, Mustoe TA. Oxygen in wound healing—more than a nutrient. World J Surg. 2004;28:294-300. [DOI] [PubMed] [Google Scholar]
- 9. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Bilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257-268. [DOI] [PubMed] [Google Scholar]
- 10. Sen CK. Wound healing essentials: let there be oxygen. Wound Rep Reg. 2009;17:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asmis R, Qiao M, Zhao Q. Low-flow oxygenation of full-excisional skin wounds on diabetic mice improves wound healing by accelerating wound closure and reepithelialization. Int Wound J. 2010;7:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandit AS, Faldman DS. Effect of oxygen treatment and dressing oxygen permeability on wound healing. Wound Repair Regen. 1994;2:130-137. [DOI] [PubMed] [Google Scholar]
- 13. Niinikoski J. Effect of oxygen supply on wound healing and formation of experimental granulation tissue. Acta Physiol Scand. 1970;78:1-72. [PubMed] [Google Scholar]
- 14. Hunt T, Pai M. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135:561-567. [PubMed] [Google Scholar]
- 15. Stephens F, Hunt T. Effect of changes in inspired oxygen and carbon dioxide tensions on wound tensile strength. Ann Surg. 1971;173:515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262-270. [PubMed] [Google Scholar]
- 17. Knighton D, Halliday B, Hunt T. Oxygen as an antibiotic: the effect of inspired oxygen on infection. Arch Surg. 1984;119:199-204. [DOI] [PubMed] [Google Scholar]
- 18. Knighton D, Halliday B, Hunt T. Oxygen as an antibiotic. Arch Surg. 1986;121:191-195. [DOI] [PubMed] [Google Scholar]
- 19. Sen CK. The general case for redox control of wound repair. Wound Repair Regen. 2003;11:431-438. [DOI] [PubMed] [Google Scholar]
- 20. Roy S, Khanna S, Sen CK. Redox regulation of the VEGF signaling path and tissue vascularization: hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic Biol Med. 2008;44:180-192. [DOI] [PubMed] [Google Scholar]
- 21. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers. Arch Dermatol. 2000;136:1531-1535. [DOI] [PubMed] [Google Scholar]
- 22. Urrea-Botero G. Can continuous diffusion of oxygen heal chronic toe ulcers? Podiatry Today. 2015;28(10). [Google Scholar]
- 23. Brannick B, Engelthaler M, Jadzak J, Wu S. A closer look at continuous diffusion of oxygen therapy for a chronic, painful venous leg ulcer. Podiatry Today. 2014;27(11). [Google Scholar]
- 24. Couture M. Does continuous diffusion of oxygen have potential in chronic diabetic foot ulcers? Podiatry Today. 2015;28(12). [Google Scholar]
- 25. Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19-26. [PubMed] [Google Scholar]
- 26. Yu J, Lu S, McLaren A, Perry JA, Cross KM. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Rep Regen. 2016;24(6):1066-1072. [DOI] [PubMed] [Google Scholar]
- 27. Said HK, Jijjawi J, Roy N, Mogford J, Mustoe T. Transdermal sustained-delivery oxygen improves epithelial healing in a rabbit ear wound model. Arch Surg. 2005;140:998-1004. [DOI] [PubMed] [Google Scholar]
- 28. Brannick B, Engelthaler M, Jadzak J, Wu S. A closer look at continuous diffusion of oxygen therapy for a chronic, painful venous leg ulcer. Podiatry Today. 2014;27(11). [Google Scholar]
- 29. Mani R. Topical oxygen therapy for chronic wounds: a report on the potential of Inotec, a new device for delivering oxygen to chronic wounds. J Wound Technol. 2010;9:1-4. [Google Scholar]
- 30. Lowell DL, Nicklas B, Weeily W, Johnson F, Lyons MC. Transdermal continuous oxygen therapy as an adjunct for treatment of recalcitrant and painful wounds. Foot Ankle Online J. 2009;2(9):4. [Google Scholar]
- 31. Hirsh F, Berlin SJ, Holtz A. Transdermal oxygen delivery to wounds: a report of 6 cases. Adv Skin Wound Care. 2008;22:20-24. [DOI] [PubMed] [Google Scholar]
- 32. Niederauer MQ, Michalek JE, Armstrong DG. Interim results for a prospective, randomized, double-blind multicenter study comparing continuous diffusion of oxygen therapy to standard moist wound therapy in the treatment of diabetic foot ulcers. Wound Med. 2015;8:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armstrong DG, Boulton AJM, Andros G, et al. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J. 2009;6:211-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006;117(suppl):239S-244S. [DOI] [PubMed] [Google Scholar]
- 35. Lavery LA, Barnes SA, Keith MS, Seaman JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care. 2008;31:26-29. [DOI] [PubMed] [Google Scholar]
- 36. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855-859. [DOI] [PubMed] [Google Scholar]
- 37. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy utilizing vacuum-assisted closure to advanced moist wound therapy in the treatment of diabetic foot ulcers. Diabetes Care. 2008;31(4):631-636. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong DG, Marston WA, Reyzelman AM, Kirstner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Rep Regen. 2012;20(3):332-341. [DOI] [PubMed] [Google Scholar]
- 39. Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers. Diabetes Care. 2003;26:1701-1705. [DOI] [PubMed] [Google Scholar]
- 40. Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8(1):11-18. [DOI] [PubMed] [Google Scholar]


