Abstract
Background:
Jet injection has been shown to accelerate the absorption and action of rapid-acting insulin. In this study, we compared the variability of absorption characteristics between jet injection and conventional administration of the rapid-acting insulin analogue aspart.
Methods:
A total of 30 healthy volunteers were enrolled in this randomized controlled blinded parallel study. On two test days, they received insulin aspart (0.2 units/kg body weight), either by jet injection or conventional pen, followed by a 6-hour euglycemic glucose clamp. Plasma glucose and insulin levels and glucose infusion rates were measured every 5 to 10 minutes to calculate the variability in pharmacological endpoints.
Results:
Jet injection advanced the times until maximal insulin concentration (T-INSmax) and glucose infusion rate (T-GIRmax) by ~40% (both P < .01). The difference between the two test days for these endpoints did not differ between jet injection and conventional administration (T-INSmax: 7.3 ± 1.9 vs 22.3 ± 6.3 min, P = .074; T-GIRmax: 24.0 ± 3.5 vs 27.3 ± 6.6 min, P = .66). The corresponding intraindividual coefficients of variation for injection by jet or conventional pen were 15.3 ± 3.3 and 22.0 ± 4.6% (P = .25, Pvariance = .044) for T-INSmax and 34.5 ± 5.1 and 21.2 ± 4.6% for T-GIRmax (P = .064, Pvariance = .62). The variance in maximal insulin concentration was significantly less after conventional administration (P = .039). The variance in total glucose-lowering effect and total insulin exposure did not differ (P = .93 and P = .32)
Conclusion:
Using a jet injector for insulin administration was associated with slightly altered variability in pharmacokinetic endpoints, but with about similar variability in pharmacodynamic endpoints compared to conventional administration. Variability in these endpoints remains considerable, regardless of the method of insulin administration.
Keywords: diabetes mellitus, insulin aspart, jet injector, pharmacodynamics, pharmacokinetics, variability
Jet injection provides a needle-free alternative to conventional subcutaneous administration of insulin. The jet injector administers insulin at a high velocity (typically >100 m/s) directly across the skin into the subcutaneous tissue, where it is dispersed in a spray-like pattern which facilitates its uptake into the circulation.1 We have previously shown, both in healthy volunteers and in patients with diabetes, that insulin absorption from the subcutaneous tissue is considerably enhanced when insulin is administered by a jet injector instead of a conventional pen.2,3 This enhancement leads to a 40-50% advancement in the time to reach the maximal glucose-lowering effect,2 a shorter duration of insulin action,3 and a faster correction of incidental marked hyperglycemia.4
For such an improved insulin effect to become clinically relevant, it needs to be reproducible. Large day-to-day variability in insulin effect hampers the achievement of optimal glycemic control5,6 and is associated with a diminished health-related quality of life, at least in patients with type 2 diabetes.7 Variability in glucose-lowering effect can be explained by many parameters,6 but is in part determined by variability in the absorption of insulin from the subcutaneous area into the circulation. Prior studies have shown that the intraindividual variability of the metabolic effect of regular human insulin and of rapid-acting insulin analogues is still considerable, varying between 10-30%, when administered by needle and syringe.8
Studies published in the 1980s suggested about similar intraindividual variability for regular human insulin when injected by jet stream as compared to administration by needle and syringe.9,10 There are no data on the intraindividual variability of the pharmacology of rapid-acting insulin analogues, when administered by jet injection. Also, previous jet injectors were criticized because they were cumbersome to handle and had a high propensity for errors, including “wet injections.”1 The current jet injector (ie, Insujet™) has been technically improved to assure better performance and easier handling by patients. The aim of this study was to examine the variability of the pharmacologic effect of the rapid-acting insulin analogue, aspart, when administered by jet injection as compared to that by a conventional pen.
Materials and Methods
Participants
Written informed consent was obtained from 30 healthy volunteers, who were recruited by advertisements via websites. All patients were at least 18 years of age and had a body-mass index between 18-32 kg/m2. Patients were excluded if they had had a major vascular event (eg, myocardial infarction, stroke, symptomatic peripheral artery disease, coronary bypass surgery, percutaneous coronary or peripheral artery angioplasty) in the previous 6 months, used medication other than oral contraceptives or had a medical condition that interfered with the study protocol. Pregnancy was excluded where appropriate. The study was approved by the institutional review board of the Radboud university medical center (identification code 2014-1346) and conducted according to Good Clinical Practice and the Helsinki Declaration. The study was registered with Clinicaltrials.gov under NCT02272296.
Experimental Study Design
Fifteen participants were randomized to the jet injector study arm and the other 15 participants to the conventional pen study arm, in blinded fashion and using a double-dummy design.2,3 All participants were examined on two study days, separated by a minimum of 1 day and a maximum of 7 days, during which they underwent a 6-hour euglycemic glucose clamp, as described previously.2 Briefly, all experiments started at 7:30 am and were conducted in a temperature-controlled room (22-24°C), with the subjects in fasting condition and having abstained from smoking, alcohol use and caffeine use for at least 24 hours. A heated box at 55°C was used to arterialize venous blood.11 After obtaining baseline variables, insulin aspart (Novo Nordisk, Bagsvaerd, Denmark) was administered in a dose of 0.2 U per kg of body weight by either jet injection (Insujet, European Pharma Group bv, Schiphol-Rijk, the Netherlands) or by conventional pen with a 31 G, 6 mm needle (NovoPen IV, Novo Nordisk, Bagsvaerd, Denmark). The alternate device was used as placebo to administer an “empty” injection, that is, the device looked and felt like it delivered insulin but it contained no solution. All injections were given in the lower half of the abdomen by an independent research nurse. Both investigators and patients were blinded to which device contained the insulin. Randomization was done by a computer program with the use of blocks of two subjects.
After insulin administration, glucose infusion rate (GIR) was adjusted to maintain euglycemia, based on plasma glucose levels, measured at the bedside at 5-min intervals during the first three hours of the study and at 10-min intervals for the following three hours. Blood for plasma insulin levels was sampled every 5 minutes during the first hour, every 15 minutes for the second hour, and every 30 minutes thereafter.
Analytical Procedures
Plasma glucose was measured by the glucose enzymatic-amperometric method (Biosen C-line GP+, EKF-diagnostic GmbH, Barleben, Germany). Blood sampled for determination of plasma insulin levels was collected in lithium-heparin tubes. After centrifugation, the supernatant was stored at -80°C. Plasma insulin was measured by radioimmunoassay (RIA).11
Calculations and Statistical Analyses
The pharmacokinetic parameters were derived from plasma insulin levels and consisted of the time to maximal insulin concentration (T-INSmax), the maximal insulin concentration (C-INSmax), the area under the insulin concentration curve (AUCINS) and the time until 50% of insulin absorption (T-INSAUC50%). For these parameters, we calculated the variance (ie, standard deviation squared), inter- and intraindividual coefficient of variation (CV) and mean day-to-day difference. The pharmacodynamic parameters were derived from the body-weight standardized GIR-curves and included variance, inter- and intraindividual CV and mean difference in the time until maximal GIR (T-GIRmax), reflecting the maximal glucose lowering effect, the maximal GIR (GIRmax), the area under the GIR curve (AUCGIR), and the time until 50% of glucose disposal (T-GIRAUC50%), as described previously.2 The variance in T-GIRmax was the primary endpoint. Mean outcomes measuring variance endpoints were log-transformed and tested using a one-way ANOVA with the device as between-subject factor. Other endpoints were tested for normal distribution and compared with an independent samples t-test or Mann-Whitney U test, as appropriate. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 20.0, IBM Corp, Armonk, NY). All data are expressed as means ± SEM, unless otherwise indicated, and a P value of <.05 was considered statistically significant.
Results
Table 1 summarizes the baseline characteristics of the participants. We screened and included 33 subjects. Replacements were required for two subjects who dropped out due to lack of time before the first experimental day, and for one participant for whom rescheduling the second test day (because of a technical problem with the Biosen glucose analyzer) proved impossible. The subjects were well matched for sex, age, BMI, and fasting glucose and insulin levels.
Table 1.
Baseline Characteristics.
| Jet injection | Conventional administration | |
|---|---|---|
| Number of patients | 15 | 15 |
| Male : female | 4 : 11 | 4 : 11 |
| Age, years | 21.3 ± 2.1 | 21.5 ± 2.2 |
| BMI, kg/m2 | 21.6 ± 2.2 | 22.0 ± 2.2 |
| Insulin dose, units | 13.0 (10-17) | 12.0 (11-19) |
| Fasting plasma glucose, mmol/l | 5.4 ± 0.38 | 5.3 ± 0.23 |
| Fasting plasma insulin, mU/l | 10.0 ± 2.5 | 10.3 ± 2.8 |
Data are presented as mean ± SD, median and range, or number.
Glucose Clamp
Baseline glucose values did not differ between the 2 groups (table 1). Plasma glucose values during the glucose clamps were comparable on all test days (5.12 ± 0.03 and 5.12 ± 0.03 mmol/l for jet injection versus 5.11 ± 0.03 and 5.11 ± 0.02 mmol/l for conventional pen, P = .99). The CVs for glucose levels also showed no difference between the two groups (7.15 ± 0.54% and 7.67 ± 0.61% for jet injection versus 6.54 ± 0.40% and 6.49 ± 0.45% for the conventional pen, P = .31).
Pharmacokinetic Endpoints
After jet injection, insulin levels rose more rapidly and to higher levels as compared to conventional administration (P < .001 for both endpoints; Table 2, Figure 1a). The average time difference for T-INSmax between the two test days tended to be smaller for the jet injector (7.3 ± 1.9 versus 22.3 ± 6.3 min, P = .074) and the variance in T-INSmax was less after jet injection (P = .044). However, conventional pen administration was associated with smaller differences (P = .021) and variance (P = .039) in peak insulin concentrations between the two test days ( table 2). The intraindividual differences and variances for the other pharmacokinetic parameters did not differ between the two devices (table 2 and 3). The inter- and intraindividual CVs were largely comparable for both devices, except for the interindividual CV of T-INSmax, which seemed less after jet injection (Table 3).
Table 2.
Pharmacodynamic and Pharmacokinetic Outcome Measurements.
| Mean values |
Mean differences |
Standard deviations* |
P value for variance | ||||
|---|---|---|---|---|---|---|---|
| Jet injection | Conventional pen | Jet injection | Conventional pen | Jet injection | Conventional pen | ||
| Pharmacokinetics | |||||||
| T-INSmax50%, min | 11.8 ± 0.7 | 25.2 ± 1.2§ | 3.7 ± 1.0 | 5.7 ± 1.1 | 2.6 ± 0.7 | 4.0 ± 0.8 | .21 |
| T-INSmax, min | 31.0 ± 1.4 | 64.2 ± 4.9§ | 7.3 ± 1.9 | 22.3 ± 6.3 | 5.2 ± 1.3 | 15.8 ± 4.4 | .044 |
| T-INSAUC50%, min | 87.5 ± 3.2 | 122.0 ± 4.3§ | 13.0 ± 3.5 | 14.0 ± 4.0 | 9.2 ± 2.5 | 9.9 ± 2.8 | .91 |
| C-INSmax, mU/l | 147.6 ± 7.1 | 94.6 ± 3.3§ | 32.5 ± 6.8 | 14.9 ± 3.1# | 23.0 ± 4.8 | 10.5 ± 2.2 | .039 |
| AUCINS, mU·min·l-1 | 16403.9 ± 634.3 | 14781.5 ± 457.9# | 1922.9 ± 410.8 | 1140.4 ± 238.8 | 1359.7 ± 290.5 | 806.4 ± 168.8 | .32 |
| Pharmacodynamics | |||||||
| T-GIRmax, min | 52.4 ± 4.3 | 91.2 ± 5.4§ | 24.0 ± 3.5 | 27.3 ± 6.6 | 17.0 ± 2.4 | 19.3 ± 4.6 | .59 |
| T-GIRAUC50%, min | 115.5 ± 3.6 | 144.7 ± 3.9§ | 15.7 ± 3.1 | 11.3 ± 3.4 | 11.1 ± 2.2 | 8.0 ± 2.4 | .22 |
| GIRmax, mg/kg/min | 10.3 ± 0.6 | 9.2 ± 0.5 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 | .35 |
| Gluctotal, g | 101.4 ± 6.0 | 108.0 ± 6.4 | 20.5 ± 5.0 | 13.9 ± 2.1 | 14.5 ± 3.5 | 9.9 ± 1.5 | .93 |
Data are represented as mean ± SEM.
Standard deviation of the individual mean value of the two test days.
P < .05. §P < .001.
Figure 1.
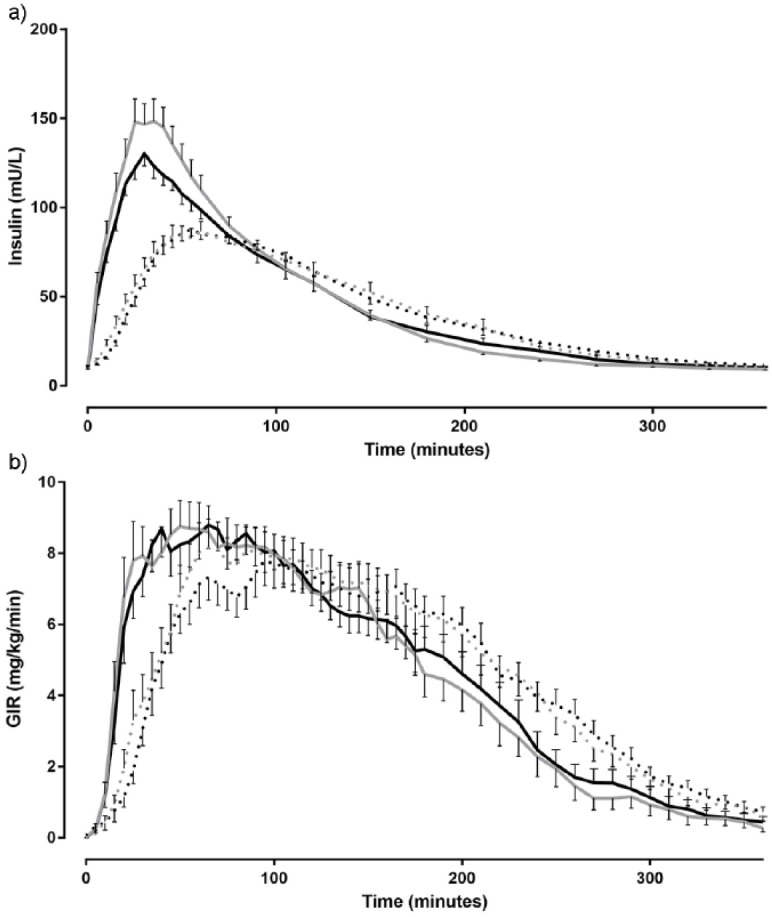
Mean plasma insulin levels (a) and glucose infusion rates (b) after insulin injection by the jet injector (solid line) and conventional pen (dashed line) on day 1 (black line) and day 2 (gray line) during the euglycemic glucose clamp.
Table 3.
Inter- and Intraindividual Coefficients of Variation for Jet Injection and Conventional Pen.
| Interindividual CV (%) |
Intraindividual CV (%) |
P value | |||
|---|---|---|---|---|---|
| Jet injection | Conventional pen | Jet injection | Conventional pen | ||
| Pharmacokinetics | |||||
| T-INSmax50% | 30.4 | 26.9 | 20.1 ± 5.4 | 17.5 ± 4.0 | 1.000 |
| T-INSmax | 24.8 | 41.6 | 15.3 ± 3.3 | 22.0 ± 4.6 | .25 |
| T-INSAUC50% | 20.2 | 19.3 | 9.7 ± 2.4 | 8.4 ± 2.2 | .540 |
| C-INSmax | 26.4 | 18.9 | 15.0 ± 2.5 | 11.0 ± 2.2 | .24 |
| AUCINS | 21.2 | 17.0 | 8.7 ± 1.9 | 6.0 ± 1.6 | .35 |
| Pharmacodynamics | |||||
| T-GIRmax | 37.1 | 25.2 | 34.5 ± 5.1 | 21.2 ± 4.6 | .06 |
| T-GIRAUC50% | 14.8 | 13.8 | 9.6 ± 2.0 | 5.8 ± 2.0 | .08 |
| GIRmax | 31.3 | 28.8 | 5.6 ± 1.3 | 8.7 ± 1.8 | .17 |
| Gluctotal | 29.6 | 32.0 | 13.6 ± 2.9 | 10.0 ± 1.7 | .30 |
Data are represented as percentage ± SEM.
Pharmacodynamic Endpoints
On both test days, the T-GIRmax was reached approximately 40% faster when insulin was administered by jet injection than when this was done by conventional pen (P≤ .001 for both test days; Table 2, Figure 1b). The mean intraindividual time differences in T-GIRmax between the two test days were 24.0 ± 3.5 and 27.3 ± 6.6 min for jet injection and conventional administration, respectively (P = .66). The variance of this endpoint was about similar (P = .59) and the intraindividual CV tended to be slightly higher for jet injection than for conventional administration (P = .064, table 3). The average variance and intraindividual differences for the other pharmacodynamic endpoints did not differ between jet injection and conventional pen administration (Table 3), although there was a trend toward greater intraindividual CVs for jet injection with respect to T-GIRAUC50% (Table 3).
Discussion
This study confirmed that using a jet injector for administration of rapid-acting insulin advances the onset of insulin’s glucose lowering effect in comparison to insulin administered by a conventional insulin pen. The pharmacological variability of insulin administered by the two devices was largely, but not entirely, similar. In particular, the time until maximal insulin concentration was less variable after jet injection than after conventional administration, whereas the maximal insulin concentration was more variable. There were no differences in the variability of other pharmacokinetic endpoints or in that of the pharmacodynamic endpoints between the two devices.
The disparities with regard to the variability of pharmacokinetic parameters between insulin administered by jet injection or by conventional means were not mirrored by such differences in the more clinically relevant pharmacodynamic endpoints. Therefore, the play of chance cannot be excluded and the implications for clinical practice are probably limited. The intraindividual CV for the time to maximal glucose-lowering effect (T-GIRmax) tended to be somewhat greater after jet than after conventional injection. Notably, this greater variability of jet injection was due to the ~40% advancement of insulin’s time-action profile (the denominator of the equation) rather than to an actual extension in intraindividual time differences (the numerator). The “predictability” of the insulin effect will thus be of similar magnitude for the two devices, when used in daily clinical practice.
Our results extend those of two previous studies that compared the variability of regular human insulin administered by jet injection with that by needle and syringe.9,10 One study investigated the variability in insulin levels after injection of 10 units of regular insulin in 8 patients with type 1 diabetes. The authors found comparable intraindividual variation in peak insulin levels and time to peak levels for the two injection methods,9 albeit slightly higher than what we found with aspart insulin. The second study reported comparable intraindividual variability of both insulin effect and insulin action for jet injection and injection by syringes in healthy volunteers, based on two glucose clamps, although one of the clamps had a duration of only 4 hours.10 Our data are also in line with a study investigating the variability of insulin aspart. When injected by syringes in healthy volunteers, intraindividual CVs for pharmacodynamic parameters ranged from 11 to 21% and those for pharmacokinetic parameters from 14 to 16%.8 Finally, in a study among patients with type 1 diabetes, the variability in T-INSmax for insulin aspart administered by subcutaneous pump was 27%.12
The present observations should be seen in the context of the more rapid glucose-lowering effect of insulin after jet injection. We have previously shown that this reduces the postprandial hyperglycemic burden and advances the correction of marked hyperglycemia in patients with diabetes.3 Our findings suggest that these benefits can be sustained with repeated injections. This is important, since high variability in the absorption and action of insulin impacts on glucose variability, which not only hampers efforts to achieve optimal glucose control, but also affects patient’s wellbeing and trust in insulin therapy.6
Limitations of our study include the parallel study design, which was chosen over a cross-over design for practical reasons, and the small sample size, so that we cannot prove with statistical certainty that the pharmacological variability of the two devices are similar. When using the data from this study, power calculations show that with a power of 80% and a significance level of .05, 63 participants in each study arm would be necessary to demonstrate superiority. However, since the confidence intervals for the main outcome parameters largely overlapped (data not shown, but available upon request), such statistical prove may have limited relevance for daily clinical practice. A second limitation is that the healthy, young volunteers enrolled in this study do not necessarily represent the majority of patients, certainly not those with type 2 diabetes. However, although variability of the insulin effect may be greater in patients with diabetes,6,13 the impact of clinical parameters on the pharmacokinetic variability of rapid-acting insulin has been reported small.12 Third, we used “empty injections” as placebo, since prefilled insulin pens with placebo solution (Testmedium®) are not registered for human use. We do not think this caused a major bias since the same injection was used twice in each participant, an independent research nurse carried out the injections to assure blinding of researcher and participant, and the injections still looked and felt like a real insulin injection. Fourth, the laboratory assay we used was not specific for insulin aspart, which meant that it did not discriminate between insulin aspart and endogenous insulin. However, since we clamped glucose values below each individuals’ fasting glucose levels, we expect their endogenous insulin production to be suppressed and therefore negligible.14 Finally, this study was not designed to assess the impact of factors relating to the site of injection, handling of the injection pen and the technique of injecting insulin on the variability of insulin.15 Notably, the jet injector differs from conventional pens in that specific training is required to fill the device with the correct amount of insulin, to remove air bubbles from the nozzle, and to apply the right amount of pressure when putting the device to the skin.
Conclusions
In conclusion, the variability in glucose-lowering effect is considerable, but broadly comparable for jet injection and conventional pen administration. When coupled with the approximately 40% advancement in insulin action, switching to jet injection may provide important clinical benefits for patients with diabetes on basal-bolus insulin regimens. Clinical studies of sufficient duration are necessary to more precisely assess these benefits in daily clinical practice.
Acknowledgments
First of all, we would like to thank all participants who volunteered in this study. We are indebted to Karin Saini, Anja Rasing, Veroniek Harbers, and Evertine Abbink for preparing the insulin pens and administering the insulin and placebo injections, and Simone Hins, Adrianne Hofboer, Sanne Houba, and Inge ter Horst for their assistance during the clamps. Clinicaltrials.gov number: NCT02272296.
Footnotes
Abbreviations: AUCINS, area under the insulin concentration curve; C-INSmax, maximal insulin concentration during the 6-hour study; CV, coefficient of variation; GIR, glucose infusion rate; GIRAUC50%, time until 50% of glucose disposal; GIRmax, maximal GIR during the 6-hour study; Gluctotal, total amount of glucose administered during the experiment; HbA1c, glycosylated hemoglobin; RIA, radioimmunoassay; SD, standard deviation; SEM, standard error of the mean; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; T-GIRAUC50%, time of 50% of glucose disposal; T-GIRmax, variability in time until maximal glucose lowering effect; T-INSAUC50%, time until 50% of insulin absorption; T-INSmax, time to maximal insulin concentration; T-INSmax50%, time until 50% of maximal plasma insulin value is reached.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: European Pharma Group funded the study, but was not involved in the design or execution of the study or in the writing of the manuscript. BEdG served as a consultant for, or gave lectures organized by Novo Nordisk, Merck, and Sanofi. CJT (or his employer) has received research grants, served as a consultant for, or gave lectures organized by Merck, Janssen, AstraZeneca, and Novo Nordisk. The remaining author declares that she has no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: European Pharma Group funded the study, but was not involved in the design or execution of the study or in the writing of the manuscript.
References
- 1. Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543-548. [DOI] [PubMed] [Google Scholar]
- 2. Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care. 2011;34(8):1804-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engwerda EE, Tack CJ, de Galan BE. Needle-free jet injection of rapid-acting insulin improves early postprandial glucose control in patients with diabetes. Diabetes Care. 2013;36(11):3436-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Wit HM, Engwerda EE, Tack CJ, de Galan BE. Insulin administered by needle-free jet injection corrects marked hyperglycaemia faster in overweight or obese patients with diabetes. Diabetes Obes Metab. 2015;17(11):1093-1099. [DOI] [PubMed] [Google Scholar]
- 5. Garg SK, Voelmle MK, Beatson CR, et al. Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study. Diabetes Care. 2011;34(3):574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673-682. [DOI] [PubMed] [Google Scholar]
- 7. Penckofer S, Quinn L, Byrn M, Ferrans C, Miller M, Strange P. Does glycemic variability impact mood and quality of life? Diabetes Technol Ther. 2012;14(4):303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910-1914. [DOI] [PubMed] [Google Scholar]
- 9. Pehling GB, Gerich JE. Comparison of plasma insulin profiles after subcutaneous administration of insulin by jet spray and conventional needle injection in patients with insulin-dependent diabetes mellitus. Mayo Clin Proc. 1984;59(11):751-754. [DOI] [PubMed] [Google Scholar]
- 10. Houtzagers CM, Berntzen PA, van der Stap H, Heine RJ, van der Veen EA. Absorption kinetics of short- and intermediate-acting insulins after jet injection with Medi-Jector II. Diabetes Care. 1988;11(9):739-742. [DOI] [PubMed] [Google Scholar]
- 11. Abbink EJ, Walker AJ, van der Sluijs HA, Tack CJ, Smits P. No role of calcium- and ATP-dependent potassium channels in insulin-induced vasodilation in humans in vivo. Diabetes Metab Res Rev. 2002;18(2):143-148. [DOI] [PubMed] [Google Scholar]
- 12. Haidar A, Elleri D, Kumareswaran K, et al. Pharmacokinetics of insulin aspart in pump-treated subjects with type 1 diabetes: reproducibility and effect of age, weight, and duration of diabetes. Diabetes Care. 2013;36(10):e173-e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab. 2005;31(4 Pt 2):4S7-4S24. [DOI] [PubMed] [Google Scholar]
- 14. Heinemann L, Anderson JH., Jr. Measurement of insulin absorption and insulin action. Diabetes Technol Ther. 2004;6(5):698-718. [DOI] [PubMed] [Google Scholar]
- 15. Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(suppl 2):S3-S18. [DOI] [PubMed] [Google Scholar]


