Abstract
Background:
We investigated the long-term effects of continuous subcutaneous insulin infusion (CSII) on glucose control and microvascular complications in patients with type 1 diabetes (T1D).
Methods:
A total of 157 patients (59 M/98 W; age 39.1 ± 14.8 years) with T1D who switched from multiple daily injections to CSII and used CSII for at least one year were included. HbA1c levels and status of microvascular complications before and while under CSII were analyzed, retrospectively.
Results:
The follow-up period was 4.0 ± 1.5 years. HbA1c significantly decreased from 8.4 ± 1.3 to 7.7 ± 1.3% (68 ± 14 to 61 ± 14 mmol/mol) after 1-year CSII and remained lower than pre-CSII levels during four years. Patients with pre-CSII HbA1c >8.0% (64 mmol/mol) showed significant improvement of HbA1c for four years, while those with pre-CSII HbA1c <8.0% showed no significant change. The prevalence of retinopathy, albuminuria, and chronic kidney disease (CKD) were respectively 39%, 12%, and 9% at CSII initiation. During follow-up, the incidence of retinopathy, albuminuria, and CKD were 3.6, 2.5 and 1.4/100 patient-years. Onset or progression of retinopathy occurred in 16 (27.1%) patients with diabetes duration >15 years, and in three (4.3%) patients with diabetes duration <15 years (P < .01).
Conclusion:
CSII was effective in improving HbA1c for up to four years, specifically in patients with HbA1c >8% (64 mmol/mol) prior to CSII. Incidence and progression rates of retinopathy and albuminuria were low, particularly in patients with a diabetes duration <15 years at CSII initiation. These results argue for not delaying a proposal of CSII initiation in T1D with sustained HbA1c >8% (64 mmol/mol).
Keywords: continuous subcutaneous insulin infusion, insulin pump, type 1 diabetes, long-term effects, glucose control, microvascular complications
Continuous subcutaneous insulin infusion (CSII) has been used for more than 35 years, with an increasing adoption.1 CSII is expected to be proposed to patients with type 1 diabetes (T1D) with persistent HbA1c level above target and/or frequent or severe hypoglycemia or high glucose variability, despite a well-managed intensive insulin therapy with multiple daily injections (MDI), as recommended by several expert consensus statements.2,3 Indeed, meta-analyses have shown the efficacy of CSII compared to MDI on glucose control in patients with suboptimal control, with an average difference of −0.3% (–3 mmol/mol) HbA1c in favor of CSII therapy.4-7 Of note, most of the studies included in these meta-analyses had a short follow-up.
Few studies on the long-term efficacy of CSII on glucose control have been published so far. Some studies showed a persistent reduction of HbA1c for more than 5 years in children and adults.8,9 A recent single-center study confirmed sustained lower HbA1c levels over 1- to 10-year period compared with pre-CSII values in patients with T1D,10 while another recent single-center study in 151 patients with T1D treated by CSII for at least 5 years showed a decrease of severe hypoglycemia, but only transient decrease of HbA1c.11 Due to these divergent reports, there is a need for more evaluations of long-term CSII outcomes in patients with T1D. Moreover, data concerning the status of microvascular complications under CSII treatment were not available in these studies.
Our aim was to evaluate the long-term effects of CSII on glucose control and the evolution of microvascular complications in a large series of patients with T1D who switched from MDI to CSII in our clinic.
Patients and Methods
Patients
Among all patients with T1D who switched from MDI to CSII at our clinic between January 2008 and December 2011, a total of 157 patients who continued CSII for more than one year were included in this retrospective observational study. Data were collected until January 2015. The indications of insulin pump were coherent with consensual guidelines, that is, persistent HbA1c level above target, frequent or severe hypoglycemia, high glucose variability, dawn phenomenon, preconception and pregnancy planning, search for a more flexible treatment plan related to patient life conditions.
All patients were admitted for three to five days to initiate CSII. Basal rates were set on the first day according to usual basal insulin needs and individual glucose profiles under multiple daily injections, and further adjusted on each following day by the health care team based on requested daily eight capillary blood glucose measurements. At CSII initiation, daily meal carbohydrate (CHO) contents were kept at the same levels to facilitate bolus adjustments. Practical management of pump and consumables was taught on a daily basis. All patients attended an outpatient visit 2-3 weeks after CSII initiation. Training to CHO counting and bolus calculation was performed according to patient needs during outpatient visit. Due to the lack of reimbursement by National Health Insurance, continuous glucose monitoring was almost never combined with CSII in these patients. They were followed by their diabetologist on average quarterly for optimization of their insulin doses and evaluation of chronic complications. Risk factors such as hypertension or hyperlipidemia were also treated according to guidelines when applicable. The patients were instructed to visit our clinic at least once a year for an annual check-up.
Data Collection
Data on HbA1c and microvascular complications before and while under CSII were collected. HbA1c values were measured at the initiation of CSII and at yearly intervals thereafter. We defined HbA1c at 1-year CSII as HbA1c measured at 12 ± 6 months after CSII initiation, and when more than one HbA1c value was available, the HbA1c value obtained at the nearest date to year one was selected. Data for each subsequent year was extracted in the same manner. Only the HbA1c values measured by an High Pressure Liquid Chromatography (HPLC) method from venous samples were used for the analysis of HbA1c data.
Status of microangiopathy before CSII was extracted and any changes under CSII were recorded. Patients with microvascular data before CSII, and at least once under CSII, were included in the analysis. Retinopathy was evaluated in a variety of hospitals based on the patient’s ease of access, but according to the same classification chart. We classified retinopathy as absent (NDR), nonproliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR) including retinopathy after laser photocoagulation or vitrectomy. We defined progression of retinopathy as laser photocoagulation for patients with NPDR before CSII, or supplement laser photocoagulation or vitrectomy for patients with initial PDR. Albuminuria was defined as urinary albumin excretion >30 mg/gCreat or >30 mg/24 h. According to recommendations of French Health Authority, screening for microalbuminuria was performed every year based on one urine sample allowing measurement of microalbumin/creatinin ratio. If this ratio was equal or above 30 mg/g, microalbuminuria was measured on two to three 24-hour urine collections. Patients known to present previous pathological levels of microalbuminuria were assessed on two to three 24-hour urine collections on a yearly basis. Estimated glomerular filtration rate (eGFR) was calculated by the formula 175×(Scr)−1.154×(Age)−0.203 (×0.742 if female) and chronic kidney disease (CKD) was defined as eGFR <60 ml/min/1.73 m2 at two consecutive visits. Patients who received hemodialysis or renal transplantation were also defined as having CKD.
Statistical Analysis
The statistical analysis was carried out with SPSS software. Data was presented as mean ± SD. For analysis of categorical variables, the results were expressed as percentages. To compare frequencies, Pearson’s chi-square test was used, or in case of small frequencies, Fisher’s exact test was used. Incidence rates of complications were presented as the number of events per 100 patient-years based on the ratio of the observed number of events to the total number of patient-years of exposure. Repeated-measures analysis of variance was used to compare pre- with post-CSII HbA1c over time. All tests of significance were two-sided and P < .05 was considered significant.
Results
The follow-up period was 4.0 ± 1.5 years. Among 157 subjects, 11 stopped CSII and returned to MDI because of inadequate management of the insulin pump or patient’s wish, one patient switched to an implanted insulin pump for intraperitoneal insulin delivery, one patient underwent islet transplantation, two patients received renal-pancreas transplantation, and 28 patients had no recorded follow-up (Figure 1).
Figure 1.
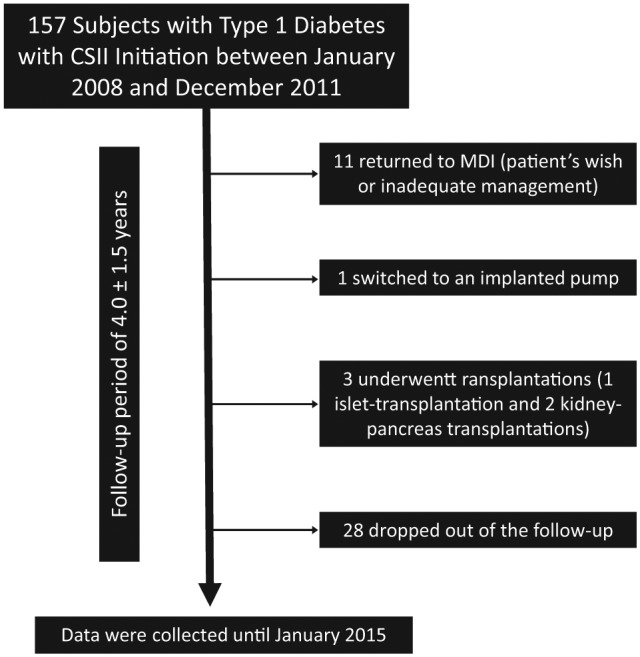
Patient flow chart: follow-up of study patients. All included subjects continued CSII for at least one year.
Characteristics of the patients at CSII initiation are shown in Table 1.
Table 1.
Baseline Characteristics and Prevalence of Microvascular Complications at CSII Initiation.
| Number of patients | 157 |
| Men/women | 59/98 |
| Age (years) | 39.1 ± 14.8 |
| Duration of diabetes (years) | 17.7 ± 10.6 |
| BMI (kg/m2) | 24.0 ± 3.8 |
| HbA1c in % (mmol/mol) | 8.4 ± 1.3 (68 ± 14) |
| Retinopathy (%) | 39.0 |
| Albuminuria (%) | 12.0 |
| CKD (%) | 9.0 |
Albuminuria is defined by a urinary albumin excretion >30 mg/gCreat or >30 mg/24 h. CKD, chronic kidney disease, defined by an estimated glomerular filtration rate <60 ml/min/1.73 m2 at two consecutive visits or subjects who received hemodialysis or renal transplantation. Values are presented as mean ± SD.
Before CSII, long-acting insulin analogues were used as basal insulin in 149 (94.9%) patients and rapid-acting insulin analogues were used as bolus insulin in 152 (96.8%) patients. After the initiation of CSII, all of the patients used rapid-acting insulin analogues (insulin aspart, lispro, or glulisine).
Evolution of HbA1c levels while under CSII is shown in Figure 2. HbA1c significantly decreased from 8.4 ± 1.3% (68 ± 14 mmol/mol) to 7.7 ± 1.3% (61 ± 14 mmol/mol) at 1 year after CSII initiation and remained significantly lower than pre-CSII HbA1c until 4 years after CSII initiation. When considering patients according to their pre-CSII HbA1c, patients with an HbA1c level >8.0% (64 mmol/mol) showed significant improvement of HbA1c up to 4 years after CSII, while those with an HbA1c level <8.0% (64 mmol/mol) showed no significant change (Figure 3). This cut-off set for pre-CSII Hba1c value of 8% was the most relevant to describe changes in HbA1c levels while under CSII. Of note, the same evolution of HbA1c levels was observed in the 80 patients who had a full 4-year follow-up compared to the whole included patient population.
Figure 2.
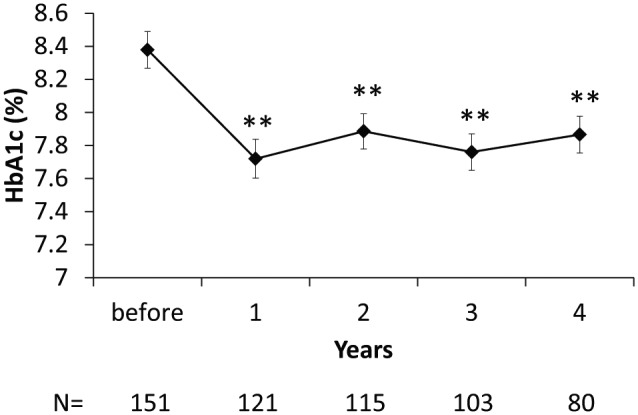
Evolution of HbA1c levels from CSII initiation. Mean ± SE. N, number of patients with available data.
**P < .01 vs before CSII.
Figure 3.
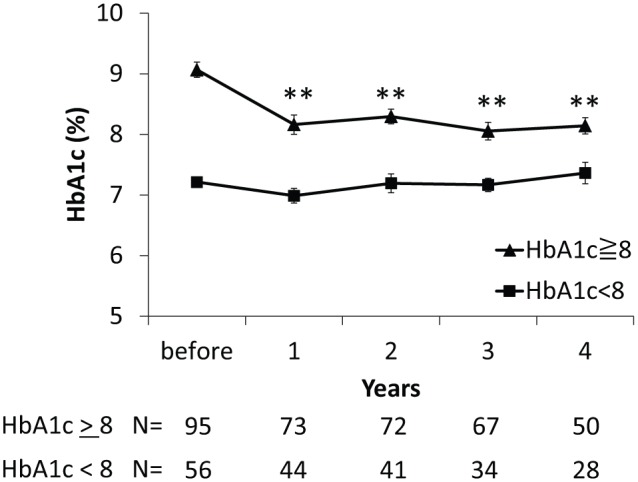
Evolution of HbA1c levels from CSII initiation according to pre-CSII HbA1c level below or above 8% (64 mmol/mol). Mean ± SE. N, number of patients with available data.
**P < .01 vs before CSII.
Table 2 shows the occurrence and incidence rates of microvascular complications during the cumulated follow-up from CSII initiation. Diabetic retinopathy was newly diagnosed in 9 patients (11.5%) and progression of diabetic retinopathy occurred in 10 patients (20.0%). The incidence rate and the progression rate of retinopathy were 3.6 and 6.9 per 100 patient-years, respectively. Albuminuria and CKD developed in 8 (8.5%) and 5 (5.1%) patients, respectively, and the incidence rates were 2.5 and 1.4 per 100 patient-years, respectively.
Table 2.
Occurrence and Incidence Rate of Microvascular Complications From CSII Initiation.
| Number of events (% of patients) | Incidence rate (/100 patient-years) (95% CI) | |
|---|---|---|
| Onset of retinopathy | 9 (11.5%) | 3.6 (1.9-6.9) |
| Progression of retinopathy | 10 (20.0%) | 6.9 (3.8-12.5) |
| Onset of albuminuria | 8 (8.5%) | 2.5 (1.3-5.0) |
| Onset of CKD | 5 (5.1%) | 2.5 (1.3-5.0) |
Albuminuria is defined by a urinary albumin excretion >30 mg/gCreat or >30 mg/24 h. CKD, chronic kidney disease, defined by an estimated glomerular filtration rate <60 ml/min/1.73 m2 at two consecutive visits or subjects who received hemodialysis or renal transplantation.
Table 3 shows the occurrence of onset or progression of retinopathy under CSII according to baseline status of retinopathy, duration of diabetes, HbA1c level before and under CSII, or the degree of reduction in HbA1c at one year after CSII initiation. Progression of retinopathy occurred in seven (29.2%) patients with PDR, which was significantly more frequent (P = .04) than the onset of retinopathy in patients with NDR (9 patients, 11.5%). There was no significant difference between number of cases with progression from NPDR (n = 10) and those with onset of NPDR from NDR (n = 9). Sixteen (27.1%) patients with a duration of diabetes >15 years developed or impaired retinopathy, while only three (4.3%) patients with duration of diabetes <15 years developed a retinopathy (P < .01). Hence a longer duration of diabetes at CSII initiation was significantly more prone to emergence or progression of retinopathy. Since retinopathy was newly diagnosed in 6 (35.3%) patients with a duration of diabetes >15 years, the severity of baseline retinopathy was not the only cause of impaired retinal status. Besides, onset or progression of retinopathy occurred in 5 (10.4%) patients with pre-CSII HbA1c <8% (64 mmol/mol), and in 14 (17.5%) patients with pre-CSII HbA1c >8% (64 mmol/mol), which showed no significant difference. Furthermore, the average HbA1c level under CSII and the degree of reduction in HbA1c at one year after CSII initiation showed no significant association with onset or progression of retinopathy under CSII.
Table 3.
Occurrence of Onset or Progression of Retinopathy From CSII Initiation According to Baseline Retinopathy, Duration of Diabetes, HbA1c at CSII Initiation, Average HbA1c Level Under CSII, and the Degree of Reduction in HbA1c at Year 1.
| Number of events (% of patients) | P value | |
|---|---|---|
| Baseline retinopathy | ||
| NDR | 9 (11.5%) | |
| NPDR | 3 (11.5%) | NS (vs NDR) |
| PDR | 7 (29.2%) | .04 (vs NDR) |
| Duration of diabetes | ||
| <15 years | 3 (4.3%) | |
| >15 years | 16 (27.1%) | <.01 |
| Baseline HbA1c | ||
| <8% (64 mmol/mol) | 5 (10.4%) | |
| ⩾8% (64 mmol/mol) | 14 (17.5%) | NS |
| Average HbA1c under CSII | ||
| <8% (64 mmol/mol) | 12 (15.2%) | |
| ⩾8% (64 mmol/mol) | 7 (14.3%) | NS |
| Reduction of HbA1c after one year | ||
| <0 | 5 (19.2%) | |
| 0-1% (0-11 mmol/mol) | 8 (13.6%) | NS |
| >1% (>11 mmol/mol) | 6 (15.8%) | NS |
Progression of retinopathy defined on laser photocoagulation in patients with NPDR or supplement laser photocoagulation or vitrectomy in patients with PDR at CSII initiation.
We also analyzed the relationship between onset of albuminuria and duration of diabetes or HbA1c. Onset of albuminuria under CSII occurred in 14.6% of patients with a duration of diabetes >15 years and in 3.8% of those with a duration <15 years. Hence patients with a longer duration of diabetes showed a higher trend to develop albuminuria after CSII initiation, although not reaching statistical significance (P = .07). According to HbA1c level at CSII initiation, average HbA1c level under CSII, or degree of reduction in HbA1c at one year after CSII initiation, no significant difference was found in the onset of albuminuria.
Discussion
Our study demonstrates the sustained efficacy of CSII on glucose control but a persistent impairment of retinal and kidney status when applied to patients with a long duration of T1D in a large series of patients during an average follow-up of 4 years after CSII initiation. A noticeable strength of this study is the number of 157 patients who initiated CSII in a period shorter than 3 years. Thanks to this short period of time, the education staff and methods for the management of T1D, the used devices and consumables for CSII and the data collection were very similar for all the patients, both at CSII initiation and during follow-up. Moreover, since the patients were not involved in any clinical trial, the reported responses to CSII can be considered as reflecting an everyday clinical practice.
Our investigations found that the average HbA1c level of patients who switched from MDI to CSII decreased by 0.7% (7 mmol/mol) during the first year following CSII initiation, and this improvement was sustained for up to four years. Among the previous recent studies which assessed long-term efficacy of CSII, a study in children and adolescents with T1D also showed this sustained reduction of HbA1c,8 while another study found only a transient improvement of HbA1c in this population.12 In adults with T1D, Carlsson et al reported a 0.2% (2 mmol/mol) reduction in HbA1c level after 5 years in 331 patients treated with CSII compared with a matched control group of patients treated with MDI.9 Orr et al found that adult patients on CSII therapy maintained lower HbA1c values over a 1- to 10-year period compared with their pre-CSII values, and that the predictors for failure to achieve an HbA1c target of <7.0% (53 mmol/mol) on CSII were high pre-CSII HbA1c levels, missed appointments, mental illness, and active smoking.10 A single-center Spanish study of 151 patients with T1D treated by insulin pump for at least 5 years showed a persistent decrease of severe hypoglycemia, but an only transient decrease in HbA1c levels. Nevertheless, in the subgroup with a suboptimal metabolic control, HbA1c decreased from 8.4% (68 mmol/mol) to 8% (64 mmol/mol) after 5 years.11 In our study, most of the overall benefit in HbA1c reduction was due to the subgroup that started with an HbA1c level of >8% (64 mmol/mol), which is consistent with previous studies. Of note, the vast majority of our patients used long- and rapid-acting insulin analogues before switching to CSII which indicates that CSII treatment was more effective on glucose control than current optimal MDI regimen in adult T1D patients whereas most studies included in published meta-analyses compared CSII to MDI regimen with no insulin analogues. .
In this study, we also evaluated the incidence rates of microvascular complications after switching from MDI to CSII. While incidence of retinopathy was 3.6/100 patient-years, incidence of albuminuria was 2.5/100 patient-years. Hence, the incidence rate of retinopathy in this study was higher than that reported in the intensively treated group of the Diabetes Control and Complications Trial (DCCT).13 However, our study included a large subset (close to 50%) of patients with a diabetes duration >15 years, who were not eligible for recruitment in the DCCT. When we analyzed the patients with a pre-CSII duration of diabetes <15 years, the incidence rate of retinopathy was only 1.5/100 patient-years, that is, similar to that in the intensively treated group of the DCCT. Incidence rate of albuminuria in this study was similar to that in the intensive therapy group of the DCCT. Among the patients with a diabetes duration <15 years in our study, incidence rate of albuminuria was 1.1/100 patient-years, that is, lower than that in the DCCT. A decreasing incidence of albuminuria or further advanced nephropathy has been suggested over the recent decades, mainly due to the better management of blood glucose and blood pressure.14 Although we have no detailed data on blood pressure and antihypertensive treatment in our study population, guideline-based treatment of hypertension and frequent use of renin-angiotensin system blocking drugs may be reasons for the lower incidence of albuminuria beside an overall lower HbA1c level under CSII.
Duration of diabetes, HbA1c levels, blood pressure, and baseline severity of retinopathy are known as the risk factors of onset or progression of diabetic retinopathy.15 Our study also showed higher occurrence of onset or progression of diabetic retinopathy in patients with longer duration of diabetes and those with severe retinopathy at the initiation of CSII. On the other hand, HbA1c before and while under CSII, and the change in HbA1c one year after CSII initiation had no significant relationship with the occurrence of onset and progression of retinopathy. These results might suggest that the effect of improved glucose control under CSII for an average period of 4 years on reducing impairment of retinopathy status was small compared to the influence of long-standing hyperglycemia under previous treatment. There was no significant increase in the incidence rate of retinopathy progression for subjects with a high reduction of HbA1c at one year (>1% [11 mmol/mol]), probably because of a systematic screening of retinal status (and treatment if needed) before CSII initiation.
Few studies have compared the effects of CSII compared to MDI on the development of diabetic retinopathy. Downie et al found a reduced risk of retinopathy in adolescents with T1D treated with CSII versus those treated with MDI, although the difference did not reach statistical significance.16 Another recent study showed that in adolescents, CSII use is associated with lower rate of retinopathy (OR 0.66, 95% CI 0.45-0.95, P = .029), lower rate of peripheral neuropathy (OR 0.63, 95% CI 0.42-0.95, P = .026) but no significant difference on albuminuria, versus MDI.17 However, another report suggested the superiority of CSII over MDI in adults to obtain a lower albumin excretion rate.18
The size of our series and the duration of our follow-up period did not allow a reliable evaluation of the development of CKD and macroangiopathy. A recent Swedish study showed that T1D adults treated by CSII had a lower cardiovascular mortality, versus MDI:19 with a mean follow-up of 6.8 years of 18 168 subjects (2441 with insulin pump), the adjusted HR was 0.58 (0.40 to 0.85) for fatal cardiovascular disease (coronary heart disease or stroke) in favor of CSII.
Limitations of our study are mainly related to the retrospective nature of our analyses. Hence available data for assessing glucose control were limited to HbA1c levels. The role of individual management of CSII, for example, frequency of daily glucose measurements and adjustments of insulin delivery, could not be assessed as potentially involved in glucose control under CSII. Besides, no control population was available to investigate whether MDI therapy associated with a similar assistance in the management of insulin treatment would result in similar outcomes albeit all our patients benefitted from reinforced education and intensified follow-up in the three to six months before switching to CSII.
In conclusion, our study showed that CSII is effective in improving HbA1c for up to four years, specifically in patients with HbA1c levels above 8% (64 mmol/mol) prior to CSII. The recorded data did not allow a further assessment of glucose control while under CSII, for example, rate of hypoglycemic events or glucose variability. Emergence and progression rates of retinopathy and albuminuria were low in patients with a diabetes duration <15 years at CSII initiation whereas impairment of retinal and kidney status was significantly higher in patients with a diabetes duration >15 years. Hence long-term failure to keep glucose control close to normoglycemia appears as a limitation for improvement of microvascular lesions with CSII. These results argue for not delaying the proposal of CSII initiation in subjects with T1D with sustained HbA1c > 8% (64 mmol/mol).
Acknowledgments
We acknowledge the assistance of the staff of Association d’Aide aux Malades Traités par Infusion Médicamenteuse (AMTIM) in the collection of study data.
Footnotes
Abbreviations: BMI, body mass index; CHO, carbohydrate; CI, confidence interval; CKD, chronic kidney disease; CSII, continuous subcutaneous insulin infusion; DCCT, Diabetes Control and Complications Trial; eGFR, estimated glomerular filtration rate; HPLC, High Pressure Liquid Chromatography; HR, Hazard Ratio; MDI, multiple daily injections; NDR, no diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; NS, not significant; OR, odds ratio; PDR, proliferative diabetic retinopathy; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev. 2009;25(2):99-111. [DOI] [PubMed] [Google Scholar]
- 2. Lassmann-Vague V, Clavel S, Guerci B, et al. When to treat a diabetic patient using an external insulin pump. Expert consensus. Societe francophone du diabete (ex ALFEDIAM) 2009. Diabetes Metab. 2010;36(1):79-85. [DOI] [PubMed] [Google Scholar]
- 3. Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology insulin pump management task force. Endocr Pract. 2014;20(5):463-489. [DOI] [PubMed] [Google Scholar]
- 4. Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26(4):1079-1087. [DOI] [PubMed] [Google Scholar]
- 5. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941-951. [DOI] [PubMed] [Google Scholar]
- 6. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765-774. [DOI] [PubMed] [Google Scholar]
- 7. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010(1):CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56(11):2392-2400. [DOI] [PubMed] [Google Scholar]
- 9. Carlsson BM, Attvall S, Clements M, et al. Insulin pump-long-term effects on glycemic control: an observational study at 10 diabetes clinics in Sweden. Diabetes Technol Ther. 2013;15(4):302-307. [DOI] [PubMed] [Google Scholar]
- 10. Orr CJ, Hopman W, Yen JL, Houlden RL. Long-term efficacy of insulin pump therapy on glycemic control in adults with type 1 diabetes mellitus. Diabetes Technol Ther. 2015;17(1):49-54. [DOI] [PubMed] [Google Scholar]
- 11. Quiros C, Gimenez M, Rios P, et al. Long-term outcome of insulin pump therapy: reduction of hypoglycaemia and impact on glycaemic control. Diabet Med. 2016;33(10):1422-1426. [DOI] [PubMed] [Google Scholar]
- 12. Batajoo RJ, Messina CR, Wilson TA. Long-term efficacy of insulin pump therapy in children with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2012;4(3):127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 14. Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26(4):1258-1264. [DOI] [PubMed] [Google Scholar]
- 15. Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care. 2012;35(3):592-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Downie E, Craig ME, Hing S, Cusumano J, Chan AK, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care. 2011;34(11):2368-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zabeen B, Craig ME, Virk SA, et al. Insulin pump therapy is associated with lower rates of retinopathy and peripheral nerve abnormality. PLOS ONE. 2016;11(4):e0153033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lepore G, Bruttomesso D, Bonomo M, et al. Continuous subcutaneous insulin infusion is more effective than multiple daily insulin injections in preventing albumin excretion rate increase in Type 1 diabetic patients. Diabet Med. 2009;26(6):602-608. [DOI] [PubMed] [Google Scholar]
- 19. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]


