Abstract
Diabetes mellitus (DM) is a rapidly worsening global epidemic over the last thirty-five years. The increased prevalence of DM has changed the phenotypic expression of atherosclerotic limb threatening ischemia (LTI), resulting in an increase in lesions in the tibial vessels. These patients are also afflicted with peripheral neuropathy, foot deformities, and medial calcification of the vasculature. In response to the evolving phenotype of atherosclerosis, newer minimally invasive tools and techniques have been developed to improve the blood supply in LTI. Arterial access, traditionally obtained from the contralateral common femoral artery (CFA) in a retrograde fashion, is now also frequently being obtained in the ipsilateral limb in an antegrade fashion. Retrograde access of the tibial, pedal, tarsal, or calf collateral vessels is also being utilized to provide a route through which wires, catheters, balloons and stents may be placed. Wires have evolved to have a variety of diameters, materials and coatings providing interventionalists with a wide variety of choices when attempting to traverse blockages in the arteries. When catheters and wires fail to traverse the lesion, newer chronic total occlusion (CTO) devices have been developed to aid in the placement of a wire across the offending lesions. Due to medial calcification associated with DM, atherectomy devices have been developed to debulk the atherosclerotic plaque within the vessel. High pressure balloon angioplasty with or without stents remain the mainstay of intervention, with drug-coated balloons (DCBs) and drug-eluting stents (DESs) now being frequently used to prevent reocclusions of atherosclerotic lesions.
Keywords: endovascular, limb-threatening ischemia, diabetic complications, diabetic ulceration, percutaneous catheter based intervention
Diabetes mellitus (DM) is an exploding epidemic, with a current worldwide prevalence of 422 million, representing a four-fold increase over the last 35 years.1 The increased contribution of DM to the atherosclerotic phenotype has changed the presentation of limb-threatening ischemia (LTI).2-4 The risk of a major amputation is 10-30 times higher among individuals with DM.5 Worldwide, there are approximately one million major lower extremity amputations/year, which translates to a lower-extremity amputation every twenty seconds among individuals with DM.6
Hence, there is a pressing need to develop new tools to improve lower extremity perfusion. To meet this need, there have been multiple endovascular devices and techniques designed to augment perfusion in LTI. Understanding the strategies, advantages, disadvantages, and outcomes is daunting given the cornucopia of options. In this context, we aim to briefly describe patient selection and goals of endovascular revascularization in LTI among individuals with DM. We will then review some of the latest techniques and devices available, mechanisms of action, and outcomes.
Goals of Revascularization
There are two broad categories of revascularization: open and endovascular. The comparative efficacy of open versus endovascular revascularizations remains contentious, and is the subject of ongoing prospective randomized controlled trials.7,8 This article will focus on endovascular therapies.
Wound-healing and limb-salvage in LTI is multifactorial, but revascularization is critical.9-12 There is no absolute perfusion target threshold, making goals of revascularization difficult to define. A recent review found that skin perfusion pressure ≥ 25 mm Hg, toe pressure ≥ 30 mm Hg and a transcutaneous pressure of oxygen (TcPO2) ≥ 25 mm Hg was associated with ≥ 25% greater chance of healing.13 Ankle pressures often mislead in DM, due to medial calcification and incompressible tibial vessels.14 Palpable pulses have a greater than two-fold likelihood of healing. The interrater reliability in detecting pedal pulses is poor, however.15
Angiosome-Directed Revascularization (ADR)
ADR is the principle that guides revascularization strategies in LTI. Angiosomes are anatomical units of tissue (skin, subcutaneous tissue, fascia, muscles, nerves, and bone) that are supplied by a major vessel. There are three vessels that supply five angiosomes in the foot.16,17 The anterior tibial becomes the dorsalis pedis and supplies the dorsum of the foot. The peroneal artery provides a collateral vessel that supplies the lateral ankle and heel. The posterior tibial artery divides into the medial and lateral plantar arteries. These three vessels then supply the medial ankle, and the medial, and lateral plantar surface of the foot and digits.
ADR is not always feasible, and imprecisely defined between institutions.18-20 It appears that ADR results in approximately a 10-15% increase in wound healing rates relative to non-ADR.18-20 When possible, the author advocates for achieving direct flow to the pertinent angiosome.
Challenges in LTI Among Individuals With Diabetes
The changing distribution of obstructions mandates that techniques and devices be able to navigate and treat tortuous, and narrow tibial and pedal vessels. Among individuals with diabetes, LTI involves the tibial vasculature in over 70% of subjects.4,21 Occlusion of all of the crural vessels are found in 28% of subjects.4 The most common pattern of occlusive disease includes occlusion of the femoral-popliteal artery, with an occlusion of one or more of the tibial vessels. Isolated tibial disease is the next most frequent, where ≥ 1 tibial vessel(s) has an occlusion.4,21 Interestingly, the pedal vasculature is often spared, with almost 90% of limbs having ≥ 1 patent pedal artery.4
Medial calcification typifies LTI. It appears that exposure of advanced glycation end-products induce a change in vascular smooth muscle cells (VSMCs) to an osteogenic phenotype. These osteogenic VSMCs deposit hydroxyapetite in the media of medium-sized arteries.22 Calcified lesions are difficult to traverse with a wire, resistant to transluminal angioplasty and stenting, and often require other atherectomy to debulk the plaque. Calcification also results in incompressible vessels, making ankle-brachial indices less reliable measures of perfusion. Finally, calcium creates artifacts on computed tomographic and duplex ultrasound imaging, which obscures the ability to visualize the patency of tibial vessels. In many cases, arteriography is required simply to evaluate the infrapopliteal vasculature.
Revascularization in DM
Due to the preponderance of calcific, tibial occlusive disease in DM, interventionalists have adapted their tools. Newer techniques involving the pedal vasculature have become a larger fraction of the armamentarium of the modern interventionalist. Newer devices also improve the treatment of calcific lesions.
Obtaining Vascular Access
Interventions begin with vascular access. There are multiple options, without a significant difference in safety or technical success associated with a particular access vessel or combination of vessels.23 The two most common options are retrograde puncture of the common femoral artery (CFA) contralateral to the affected foot, and antegrade ipsilateral access of the CFA. Physical exam and duplex ultrasound are instrumental in surveying the patient prior to intervention. These will reveal whether there is disease in the CFA, or proximally in the iliacs or aorta. The author recommends ultrasound-guided access to ensure accurate localization of the CFA. The author will choose the contralateral CFA retrograde access if there is a flush occlusion of the superficial femoral artery (SFA) at the bifurcation, or if the extent of disease to the ipsilateral leg is unclear from the preoperative pulse and duplex examination.
If examinations confirm there is no significant disease in the aorta, iliacs, CFA, and proximal SFA, the author favors antegrade CFA access, ipsilateral to the affected limb. Antegrade access shortens the distance between the sheath and the targets, which eases treatment. The size of the sheath ranges between 5-8 Fr. The larger sheath sizes accommodate dual wires, or larger devices, as needed. The sheath is advanced as far distally as there is healthy artery, minimizing the distance between the end of the sheath and the target lesion. This facilitates passage of devices, performance of lower extremity arteriograms and infusion of vasodilatory medications.
Retrograde pedal vessel (dorsalis pedis, medial plantar, lateral plantar, distal posterior tibial, peroneal) access is frequently utilized, with a 7-15 MHz “hockey-stick” shaped ultrasound probe, and a pedal-access micropuncture kit (Cook, Bloomington, IN).23 Other aids in pedal access include intraarterial vasodilators, such as Verapamil (1.0-2.5 mg), nitroglycerin (100-200 mcg), and papaverine (5-10 mg) to prevent vasospasm. Brachial and radial access are infrequently used for tibial interventions, as the length of endovascular instruments are inadequate. The author typically avoids placing a sheath in the distal tibial or pedal vessels. Instead, the dilator of the micropuncture kit, or 0.018-inch catheters are used to secure access. Once the wire is advanced, catheters or balloons are advanced without a sheath, thereby minimizing trauma to distal tibial or pedal vessels.
Dual tibial/pedal access with femoral access is frequently used, which provides the advantage of exteriorizing the wire and obtaining “through-and-through” access, or “flossing.” This gives the operator firm control of wire position, increasing the support necessary to cross severely stenotic, fibrocalcific lesions.23 When wires are passed into the subintimal space, this is referred to as the SAFARI technique (Subintimal Arterial Flossing with Anterograde Retrograde Intervention).24,25 Collateral vessels and the pedal loop can also be treated, and used to cross lesions in a retrograde fashion.26-29 Caution is required at bifurcations of vessels, as the wire itself can cut through the artery at angulated bifurcation points. Upon completion of the procedure, hemostasis at the retrograde puncture can be achieved with manual compression alone, or with brief balloon inflation.26-29
Crossing the Lesion
Ideally, the lesion is traversed intraluminally. The most difficult areas are the fibrocalcific “caps” (proximal and distal ends of the occlusion). Newer catheters and wires have made this easier, with the technology and techniques largely borrowed from those developed to cross coronary chronic total occlusions (CTOs).27 Catheters now have improved support with wire braiding within the catheters, as well as hydrophilic coatings which permit the catheter to slide past lesions more easily. Coaxial combinations of sheaths and/or catheters can be used in a “telescoping” fashion to provide improved wire passage. The author has found the CXI™ family of catheters (Cook, Bloomington, IN) to be especially useful for crossing CTOs.
Wires are supplied in 0.014-, 0.018-, and 0.035-inch diameters. The diameter is critical, as balloons, devices, or stents have specific wire requirements. Wires of 0.035 inches offer excellent support for devices, though are too large for tibial vessels. The coating varies, with many new polymers developed to create hydrophilic, durable coatings that are well suited to crossing CTOs. Some wires have a “core-to-tip” design, where the tip of the wire is an extension of the core of the wire that has simply been tapered. In contrast, others have a coil that extends from the core which is radio-opaque and shapeable, allowing the operator to select smaller branches and channels. The stiffness and tip-load of the wire varies also, depending on the material and design. Stiffer wires aid in passing subsequent balloons across the lesion, and may provide improved ability to push the wire across an occlusion, but are less able to navigate tortuous vessels. In the tibial vessels, 0.014-inch wires become the workhorse diameter.27-29
Subintimal angioplasty was first described in the late 1980’s as a technique to cross femoropopliteal CTOs.30 Typically, a wire and catheter are used to enter the subintimal space at or just proximal to where the occlusion begins. Within the subintimal space, a small wire loop is created, which, in combination with a braided catheter, is used to dissect subintimally until the lesion is traversed. The catheter and wire then reenter the true lumen of the vessel distal to the CTO. Multiple combinations of wires and catheters have been used, with the precise combination varying significantly by the individual practitioner. Popular combinations include a Glidewire™ (Terumo, Somerset, NJ) or a Treasure (Asahi Intecc, Santa Ana, CA ) wire, with a Navicross™ (Terumo, Somerset, NJ) or a Quickcross™ (Spectranetics, Colorado Springs, CO).
Technical success rates of crossing CTOs (transluminally or via subintimal angioplasty) range between 80-90%.30,31 Hence, several devices have been developed to facilitate wire passage for the minority of cases where wire-and-catheter techniques fail. The data are heterogeneous for each of these devices, with wide variation in the rates of technical success, and complications (Table 1). The devices are dubbed “CTO devices,” and fall into two categories: those that maintain transluminal wire position, and those that improve “reentry” from the subintimal plane. Each device has unique mechanisms of action that may prove advantageous in specific cases.
Table 1.
Summary of CTO Devices.
| Device | Mechanism | Technical successa | Complications |
|---|---|---|---|
| Reentry devices | |||
| Outback (Cordis) 6 Fr |
Reentry from subintimal plane | 88-93% | 1.6-4.2% vessel perforation |
| Pioneer (Medtronic) 7 Fr |
Intravascular-ultrasound guided reentry from subintimal plane | 95-100% | 0% vessel perforation |
| Enteer (Covidien) 5 Fr |
Flat balloon with sideport for wire reentry | 70-86% | 3.3% embolization, 0.6% perforation |
| Intraluminal devices | |||
| Crosser (Bard) 5-7 Fr |
21 Hz mechanical vibrations, 4 µm amplitude | 65-83.5% | 1.6-3.0% vessel perforation, 16% dissection |
| Frontrunner (Cordis) 6 Fr |
Blunt actuating tip microdissects through plaque | 65-95% | 3.8% dissection, 3.8% vessel perforation |
| Ocelot (Avinger) 6 Fr |
Optical coherence tomography with directional atherectomy | 72% | 2% vessel perforation |
| TruePath (Boston Scientific) (0.018-inch compatible) | Microdissection with diamond coated-tip wire at 13,000 rpm | 77% | 0% reported |
| Viance (Covidien) 5 Fr (similar to 0.035-inch wire) |
Metal microcatheter with rounded tip, rotated rapidly | 70% | 1.5% perforation |
After failure to pass a wire in either the transluminal or subintimal plane.
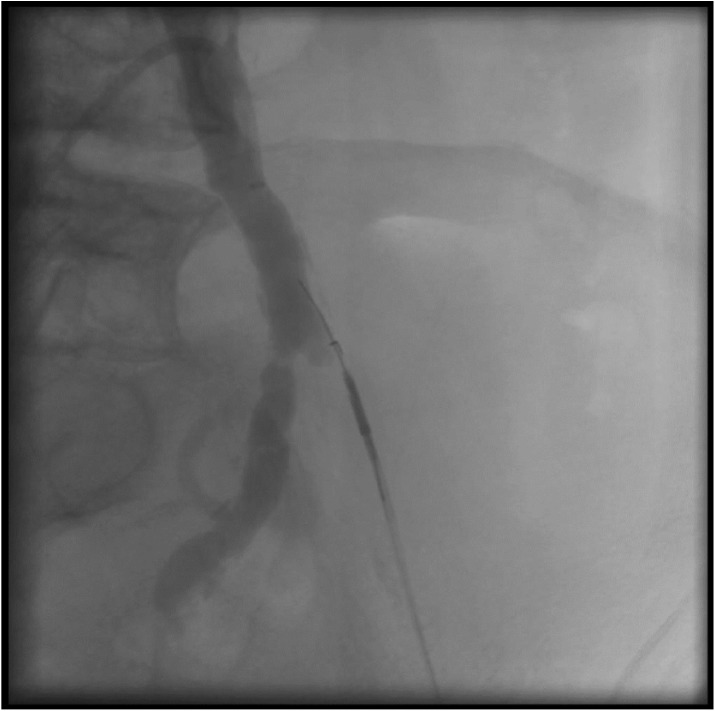
One of the earliest reentry devices available is the Outback™ catheter (Cordis, Warren, NJ), which has two separate wire lumens: one for the device, and one for the reentry needle. The device is deployed in the subintimal plane past the area of stenosis under fluoroscopic guidance, with markers on the catheter indicating the direction the needle will be deployed (Figure 1). After orthogonal views confirm the appropriate position of the catheter, the 22-gauge needle is deployed to gain entry into the true lumen distal to the occlusion. A separate wire is then advanced into the true lumen through the needle. The needle and catheter are then withdrawn, and 0.014-inch-compatible balloons and stents are advanced over the wire.32
Figure 1.
Example of the Outback Cather (Cordis) utilized to reenter the common iliac artery after crossing an external iliac artery chronic total occlusion (CTO) in the subintimal plane. Note the L shaped marker at the tip, with the wire extending from the subintimal plane into the true lumen of the common iliac artery.
Since then, the Pioneer™ (Medtronic, Minneapolis, MN) catheter has been developed which is a reentry device also with two wire lumens and a needle. The Pioneer utilizes intravascular ultrasound to orient the needle, and can deploy the needle to variable depths, providing the operator with an additional level of control. One of the limitations is that it is bulkier than the Outback.33 One of the newer reentry devices utilized in peripheral arterial disease is the Enteer™ (Medtronic, Minneapolis, MN) catheter, which borrows the similar platform as the Stingray™ (Boston Scientific, Marlborough, MA) device, which is utilized in the coronary vasculature. This device consists of a flat balloon with two wire ports. The device inflated in the subintimal plane. The flat shape of the balloon orients the two ports such that one faces the luminal side, while the other faces the adventitia. The balloon functions as a fulcrum against which an angled 0.014-inch wire can be pushed more forcefully against a plaque, and into the true lumen. The Enteer is smaller, and able to be used in the tibials (Table 1).34
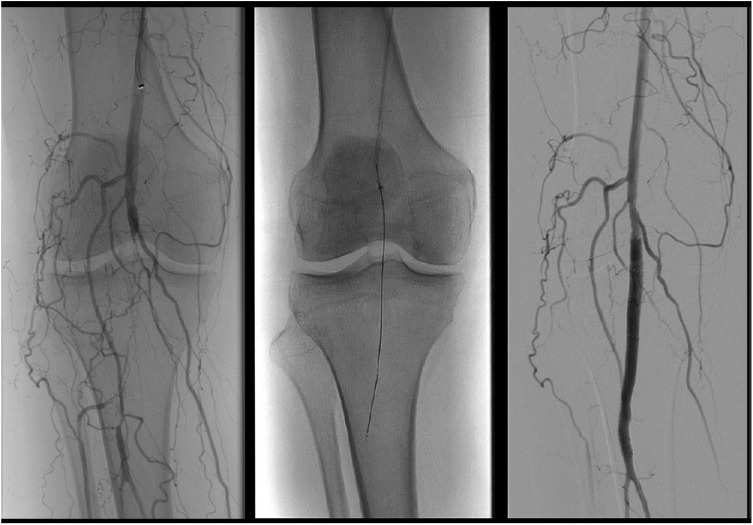
The other strategy of CTO devices is to optimize intraluminal wire placement. The oldest such device is the Frontrunner™ (Cordis, Warren, NJ), which utilizes an actuating tip that is opened and closed, allowing the operator to micro-dissect through the occlusion, and utilize the difference between the more rigid fibrocalcific plaque, and more compliant adventitia to prevent perforation.35 The Crosser™ (Bard, Murray Hill, NJ) utilizes a tip that functions similarly to a jackhammer by vibrating at 21,000 Hz, to slowly dissect through a lesion. Theoretically, the vibrations loosen the plaque, while guiding the catheter through microchannels within the plaque. After the catheter crosses the lesion, a catheter is advanced over the device, into the true lumen, and replaced with a wire (Figure 2).36,37 The devices by Avinger™ (Redwood City, CA) utilize optical coherence tomography to confirm intraluminal wire guidance through the CTO, and avoid inadvertent perforation. The devices also has directional atherectomy capability to enlarge the lumen.38
Figure 2.
The Crosser (Bard, Murray Hill, NJ) utilized to cross a CTO of the below-the-knee popliteal artery.
TruePath™ (Boston Scientific, Marlborough, MA) uses an 0.018-inch wire, attached to a motor housing. The last 130 cm of the wire is hydrophilic-coated, with the last 9 cm tapering down to 0.009 inches, with a diamond coated tip that rotates at 13,000 rpm. This is utilized to drill through a CTO, ideally finding microchannels, as it traverses the CTO.39 Finally, the Viance™ catheter (Covidien, Minneapolis, MN) is analogous to the CrossBoss™ catheter (Boston Scientific, Marlborough, MA), and is composed of a stainless steel catheter with a rounded-tip. This is utilized with a Fast-spin™ device to rotate the catheter as it advances through CTOs. This can be performed over or in front of the wire. Ideally, the device passes intraluminally, though its actual path cannot be controlled.40
The lesions treated, as well as operator experience are heterogeneous in the literature, making the comparative efficacy of the devices unclear. Banerjee et al suggest that perhaps a CTO device-first technique may be most effective at crossing lesions, compared to a wire-and-catheter-first technique.41 However, their success rate for the wire-and-catheter strategy for traversing the CTO was 52%, which is considerably lower than the approximately 80% rates of success in other series.24-31
Atherectomy to Debulk the Lesion
Balloon angioplasty alone is imperfect to restore luminal diameter.42 Much of the plaque is ruptured leaving significant amounts of residual lumen compromise. Hence, atherectomy devices evolved to decrease the plaque burden. Atherectomy appears adjunctively important to debulk plaque within vessels, especially heavily calcified plaques.43 The role of atherectomy is evolving, with the devices, techniques and experience constantly growing. While successfully debulking lesions,43,44 atherectomy is insufficient alone to revascularize lesions.43 Further data with suitable control arms will be required to clarify its comparative efficacy as an adjunct to modify the lesion in preparation for balloon angioplasty and/or stenting.
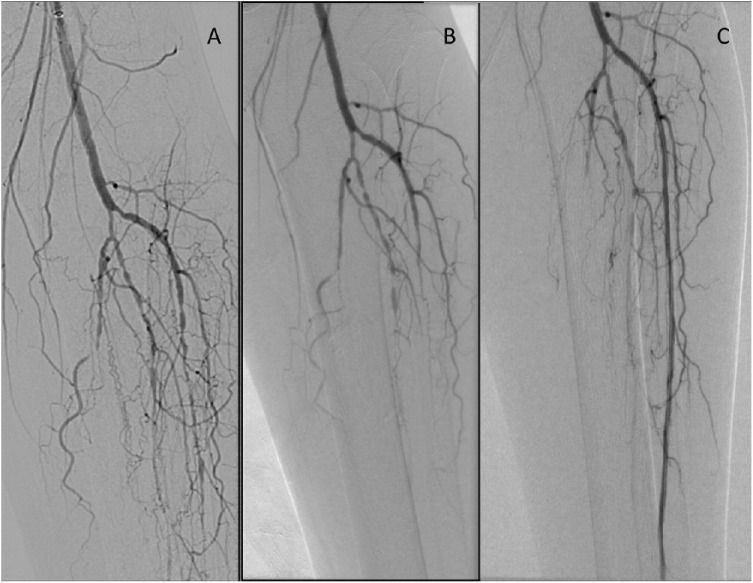
There are four categories of atherectomy devices (Table 2). Patency rates vary widely with their use, ranging between 54-93%.43-50 Directional atherectomy devices utilize a cutting device along the longitudinal plane of the vessel. These devices vary in the size and number of blades, and are limited by relatively high rates of distal embolization,47 leading many experts to recommend distal embolization protection devices, such as the Spyder FX™ (Ev3, Irvine, CA) with their use. Rotational atherectomy relies on blades or abrasive materials on the front of the device that rotates at high speeds to cut a lumen through the plaque.44 Orbital atherectomy utilizes rotational devices coated with an abrasive surface that spin eccentrically within the vessel, thereby resulting in luminal gain (Figure 3).48 Excimer lasers rely on exciplex phenomena to excite the tissues that react to specific laser wavelengths to differentially destroy plaque while preserving the vessel.50
Table 2.
Summary of Atherectomy Devices.
| Device | Mechanism | Technical success with PTA ± stent | Complications |
|---|---|---|---|
| Directional atherectomy | |||
| Silverhawk (Medtronic) 6-8 Fr |
Single-contoured blade, cuts longitudinally on one side | 2.5% embolization 5.7% perforation |
|
| Turbohawk (Medtronic) 6-8 Fr |
Four contoured blades, cuts longitudinally on one side | 77% 1-year 1° patency | 2.5% dissection |
| Rockhawk (Medtronic) 6-8 Fr |
Additional burr to the solitary blade to cut calcified lesions | SpiderFx for embolization protection recommended | |
| Orbital atherectomy | |||
| Diamondback (CSIa) 4-6 Fr |
1.25-2 mm crown that spins 360° eccentrically abrading the lesions | 93% 1 year 1° patency | 3.4% dissection 0% perforation or embolization |
| Rotational atherectomy | |||
| Jetstream (Boston Scientific) 7 Fr |
Two sets of rotating stainless steel blades at 70 krpm with an aspiration port aspirating 51 mL/min | 62% 1-year 1° patency | 1% occlusion, 9% dissection, 10% minor embolization, 2% perforation |
| Phoenix (Volcano) 5-6 Fr |
Rotating blades 1.2 krpm and an Archimedes screw to pull the plaque debris away | 94% 30-day 1° patency | 1.9% dissection, 0.95% embolization |
| Excimer laser atherectomy | |||
| Turbo-Elite (Spectranetics) | Exciplex phenomena, resulting in photoablation of the plaque | 54% 1-year 1° patency | 0% occlusion, dissection, embolization, or perforation |
Cardiovascular Systems Incorporated.
Figure 3.
Results of orbital atherectomy. Panel (A) shows the initial angiogram. The proximal anterior tibial artery lesions were resistant to balloon angioplasty alone, as shown in panel (B). After orbital atherectomy with the Diamondback (CSI, St. Paul, MN), and repeat balloon angioplasty, we were able to restore flow to the anterior tibial artery (C).
Ballooning the Lesion
High-pressure percutaneous transluminal angioplasty (PTA) remains the mainstay of endovascular therapy.42 Materials have evolved, enabling miniaturization of balloons while still maintaining the ability to be inflated to high pressures without rupture. These balloons are designed to inflate to a specific diameter with increasing insufflation. The acronym POBA (plain old balloon angioplasty) is used to describe high-pressure PTA performed without specialized balloons.51
Specialized balloons include cutting-balloons and drug-coated balloons (DCBs), and are designed to minimize recurrent stenoses.51,52 Cutting balloons have wires covering the balloon, with sharp edges on the side facing the lesion. Upon insufflation, these wires then cut into the surface of the atherosclerotic plaque. Theoretically, this should create a more controlled rupture of the atherosclerotic plaque than occurs with POBA.42 This appears helpful in SFA lesions; however, a number of these lesions require stenting as well, and meta-analyses suggest that cutting-balloon angioplasty remains inferior to other alternatives with respect to primary patency.53 The author favors these in more fibrotic lesions, which tend to exhibit greater recoil and are more resistant to POBA.
DCBs were initially conceived for the coronary vasculature, and are coated with medications that are applied to the surface of the lesion upon PTA. Over time, these medications diffuse into the underlying smooth muscle cells and inhibit neointimal hyperplasia.54 There are two DCBs available in the United States currently: the Lutonix™ (Bard, Murray Hill, NJ) and the In.Pact Admiral™ (Medtronic, Minneapolis, MN). Both utilize paclitaxel, though the In.Pact applies a higher dose of paclitaxel (3.0 micrograms/mm2) compared to the Lutonix (2.0 micrograms/mm2).54-57 It appears that the higher dose may achieve higher concentrations of paclitaxel in the VSMCs, though with comparable levels of inhibition of neointimal hyperplasia relative to the lower dose formulation.55
Early results in the SFA appear encouraging for DCBs,56 though utility may be limited in the tibial vasculature.57 Moreover, regardless of the modifications that have been made for balloons, complications with PTA persist, including vessel recoil and dissection. Some have proposed that DCBs be used to prepare lesions for eventual stent placement to maximize patency. Whether this should be performed adjunctively with atherectomy also remains contested, with mixed results.53,56,57
Stenting the Lesion
Stents are metallic tubular scaffolds that are designed to prevent recoil, treat focal dissections, and maintain the patency of the treated segment. They are most commonly composed of nitinol, which is a nickel-titanium alloy with shape-memory and superelastic properties.58 Stainless steel is also utilized for stents, as it is a relatively inert material with excellent radial force and durability. It fails to exhibit the shape memory traits found with nitinol, and is relatively bulky, and is hence less frequently utilized.59 Each material corrodes differently, which also influences the durability.59
There are several characteristics used to categorize stents. Stents can be either self-expanding or balloon-expandable. Self-expanding stents are typically made of nitinol, and expand to the desired diameter and shape upon deployment. PTA is often utilized to dilate the stent and the lesion afterward to ensure the stent is fully opened. In contrast, balloon-expandable stents are mounted on a balloon, and exert greater outward radial force. When the stent is at the desired location, the balloon is dilated, thereby expanding the stent precisely. Stents may also be covered or uncovered, which describes whether there is a fabric that covers the metallic struts. In theory, the fabric may help to prevent further ingrowth of intimal hyperplasia between the struts. However, they also occlude the collateral branches in the area that it is deployed, which may result in profound ischemia when they do fail.
Recently, drug-eluting stents (DESs) have been approved for peripheral use. These stents couple the ability to deploy medications to inhibit intimal hyperplasia, with the mechanical stent properties. Moreover, DESs elute drug over longer periods of time than is possible with the single application of DCB. The most notable example of DES utilized in LTI is the Zilver™ stent (Cook Medical, Bloomington, IN), which also elutes paclitaxel. Zilver stents, however, are too large to be deployed in the tibial vasculature where they would be most useful for LTI among individuals with DM.
In general, longer lesions (>70 mm) are better treated with stents in the femoro-popliteal and external iliac segments.60,61 The use of stents in the tibial vasculature is germane for individuals with DM, and has traditionally been avoided, as outcomes are similar with PTA alone.62-66 Provisional stenting is required in some settings, however, due to excessive recoil, calcification, or dissection. For these situations, there have been comparisons with the off-label use of coronary DESs with bare metal stents in the tibial vasculature.62-66 With respect to tibial DCB, DES also appears to provide lower reintervention rates.66 While early results favor DES with respect to reintervention rates, these studies are small, and heterogeneous. Further work is required to determine the precise role of DES in the tibials. The author currently relies on a combination of atherectomy and PTA for infrapopliteal interventions, reserving the off-label use of coronary DES to treat lesions with excessive recoil, calcium, or dissection.
Conclusions
The latest iterations of the balloons, stents and devices have evolved to treat LTI individuals with DM. Alternative access using retrograde techniques, collateral vessels, and SAFARI have enhanced the ability to reperfuse in LTI significantly. Medial calcinosis remains the main barrier to successful revascularization, as calcium is frequently recalcitrant to any intervention. Future research will be required to improve the materials, devices, durability and cost-effectiveness of endovascular procedures for LTI. Future trials7,8 will also clarify the most appropriate roles for open and endovascular interventions in LTI.
Footnotes
Abbreviations: ADR, angiosome-directed revascularization; CE Mark, Conformité Européene Mark; CFA, common femoral artery; cm, centimeter; CTOs, chronic total occlusions; DCB, drug-coated balloon; DES, drug-eluting stents; DM, diabetes mellitus; Fr, French (unit used to size catheters and sheaths); Hg, mercury; LTI, limb-threatening ischemia; mcg, microgram; mg, milligram; mm, millimeter; PTA, percutaneous balloon angioplasty; POBA, plain old balloon angioplasty; PTA, percutaneous transluminal angioplasty; RPM, revolutions per minute; SAFARI, subintimal arterial flossing with anterograde retrograde intervention; SFA, superficial femoral artery; TcPO2, transcutaneous pressure of oxygen.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980:a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell PR, Charlesworth RG, DePalma HH, et al. The definition of critical ischaemia of the limb. Br J Surg. 1982;69(suppl):S2. [PubMed] [Google Scholar]
- 3. Mills JL, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia and foot infection (WIfI). J Vasc Surg. 2014;59:220-234. [DOI] [PubMed] [Google Scholar]
- 4. Graziani L, Silvestro A, Vertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453-460. [DOI] [PubMed] [Google Scholar]
- 5. 2014 National Diabetes Statistics Report. Available at: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Accessed June 3, 2016.
- 6. Boulton AJ, Vilekyte I, Ragnarson-Tenvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [DOI] [PubMed] [Google Scholar]
- 7. Popplewell MA, Davies H, Jarrett H, et al. Bypass versus angioplasty in severe ischaemia of the leg-2. (BASIL-2) trial: study protocol for a randomised controlled trial. Trials. 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menard MT, Farber A. The BEST-CLI trial: a multidisciplinary effort to assess whether surgical or endovascular therapy is better for patients with critical limb ischemia. Semin Vasc Surg. 2014;27:82-84. [DOI] [PubMed] [Google Scholar]
- 9. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:132-173. [DOI] [PubMed] [Google Scholar]
- 10. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(suppl):3S-21S. [DOI] [PubMed] [Google Scholar]
- 11. Ward R, Dunn J, Clavijo L, et al. Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores. Ann Vasc Surg. 2016;38:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung J, Timaran DA, Modrall JG, et al. Optimal medical therapy predicts amputation-free survival in chronic critical limb ischemia. J Vasc Surg. 2013;58:972-980. [DOI] [PubMed] [Google Scholar]
- 13. Brownrigg JRW, Hinchliffe RJ, Apelqvist J, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):128-135. [DOI] [PubMed] [Google Scholar]
- 14. Brechow A, Slesaczeck T, Munch D, et al. Improving major amputation rates in the multicomplex diabetic foot patient: focus on the severity of peripheral arterial disease. Ther Adv Endocrinol Metabol. 2013;4:83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bunt TJ, Holloway A. TcPO2 as an accurate predictor of therapy in limb salvage. Ann Vasc Surg. 1996;10:224-227. [DOI] [PubMed] [Google Scholar]
- 16. Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40:113-141. [DOI] [PubMed] [Google Scholar]
- 17. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102:599-616. [PubMed] [Google Scholar]
- 18. Neville RF, Attinger CE, Bulan EJ, et al. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg. 2009;23:367-373. [DOI] [PubMed] [Google Scholar]
- 19. Alexandrescu V, Vincent G, Azdad K, et al. A reliable approach to diabetic neuroischemic foot wounds: below-the-knee angiosome-oriented angioplasty. J Endovasc Ther. 2011;18:376-387. [DOI] [PubMed] [Google Scholar]
- 20. Lida O, Nanto S, Uematsu M, et al. Importance of the angiosome concept for endovascular therapy in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2010;75:830-836. [DOI] [PubMed] [Google Scholar]
- 21. Chung J, Modrall JG, Knowles M, et al. Arteriographic patterns of atherosclerosis and associations between diabetes mellitus and ethnicity in chronic critical limb ischemia [published online ahead of print November 28, 2016]. Ann Vasc Surg. [DOI] [PubMed] [Google Scholar]
- 22. Suga T, Iso T, Shimizu T, et al. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011;18:670:683. [DOI] [PubMed] [Google Scholar]
- 23. Mustapha JA, Diaz-Sandoval LJ, Jaff MR, et al. Ultrasound-guided arterial access: outcomes among patients with peripheral artery disease and critical limb ischemia undergoing peripheral interventions. J Invasive Cardiol. 2016;28:259-264. [PubMed] [Google Scholar]
- 24. Spinosa DJ, Leung DA, Harthun NL, et al. Simultaneous antegrade and retrograde access for subintimal recanalization of peripheral arterial occlusion. J Vasc Interv Radiol. 2003;14:1449-1454. [DOI] [PubMed] [Google Scholar]
- 25. Spinosa DJ, Harthun NL, Bissonette EA, et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J Vasc Interv Radiol. 2005;16:37-44. [DOI] [PubMed] [Google Scholar]
- 26. Gandini R, Uccioli L, Spinelli A, et al. Alternative techniques for treatment of complex below-the-knee arterial occlusions in diabetic patients with critical limb ischemia. Cardiovasc Intervent Radiol. 2013;36:75-83. [DOI] [PubMed] [Google Scholar]
- 27. Godino C, Sharp AS, Carlino M, Colombo A. Crossing CTO-the tips, tricks and specialist kit that can mean the difference between success and failure. Catheter Cardiovasc Interv. 2009;74:1019-1046. [DOI] [PubMed] [Google Scholar]
- 28. Montero-Baker M, Schmidt A, Braunlich, et al. Retrograde approach for complex popliteal and tibioperoneal occlusions. J Endovasc Ther. 2008;15:594-604. [DOI] [PubMed] [Google Scholar]
- 29. Nakatsuji T, Kawareda O, Noguchi T, et al. Potential of using occult collateral vessels for trans-collateral intervention in infrapopliteal chronic occlusion. Ann Vasc Surg. 2017;38:320.e9-320.e12. [DOI] [PubMed] [Google Scholar]
- 30. Bolia A, Brennan J, Bell PR. Recanalization of femoropopliteal occlusions: improving success rate by subintimal recanalization. Clin Radiol. 1989;40:325. [DOI] [PubMed] [Google Scholar]
- 31. Met R, Van Lienden KP, Loelemay MJ, et al. Subintimal angioplasty for peripheral arterial occlusive disease: a systematic review. Cardiovasc Intervent Radiol. 2008;31:687-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitrou P, Parthipun A, Dimantopoulos A, et al. Targeted true lumen re-entry with the outback catheter: accuracy, success and complications in 100 peripheral chronic total occlusions and systematic review of the literature. J Endovasc Surg. 2015;22:538-545. [DOI] [PubMed] [Google Scholar]
- 33. Jacobs DL, Motaganahalli RL, Cox DE, et al. True lumen re-entry devices facilitate subintimal angioplasty and stenting of total chronic occlusions: initial report. J Vasc Surg. 2006;43:1291-1296. [DOI] [PubMed] [Google Scholar]
- 34. Wosik J, Shorrock D, Christopoulos G, et al. Systematic review of the BridgePoint system for crossing coronary and peripheral chronic total occlusions. J Invasive Cardiol. 2015;27:269-276. [PubMed] [Google Scholar]
- 35. Shetty R, Vivek G, Thakkar A, et al. Safety and efficacy of the frontrunner XP catheter for recanalization of chronic total occlusion of the femoropopliteal arteries. J Invasive Cardiol. 2013;25:344-347. [PubMed] [Google Scholar]
- 36. Staniloae CS, Mody KP, Yadav SS, et al. Endoluminal treatment of peripheral chronic total occlusions using the crosser recanalization catheter. J Invasive Cardiol. 2011;23:359-362. [PubMed] [Google Scholar]
- 37. Laird J, Joye J, Sachdev N, et al. Recanalization of infrainguinal chronic total occlusions with the Crosser System: results of the PATRIOT Trial. J Invasive Cardiol. 2014;26:497-504. [PubMed] [Google Scholar]
- 38. Selmon MR, Schwindt AG, Cawich IM, et al. Final results of the Chronic Total Occlusion Crossing With the Ocelot System II (CONNECT II) study. J Endovasc Ther. 2013;20:770-781. [DOI] [PubMed] [Google Scholar]
- 39. Banerjee S, Sarode K, Das T, et al. Endovascular treatment of infrainguinal chronic total occlusions using the truepath device: features, handling, and 6-month outcomes. J Endovasc Ther. 2014;21:281-288. [DOI] [PubMed] [Google Scholar]
- 40. Sethi S, Mohammad A, Ahmed SH, et al. Recanalization of popliteal and infrapopliteal chronic total occlusions using Viance and CrossBoss crossing catheters: a multicenter experience from the XLPAD Registry. J Invasive Cardiol. 2015;27:2-7. [PubMed] [Google Scholar]
- 41. Banerjee S, Sarode K, Patel A, et al. Comparative assessment of guidewire and microcatheter vs a crossing device-based strategy to traverse infrainguinal peripheral artery chronic total occlusions. J Endovasc Ther. 2015;22:525-534. [DOI] [PubMed] [Google Scholar]
- 42. Block PC, Myler RK, Stertzer S, et al. Morphology after transluminal angioplasty in human beings. N Engl J Med. 1981;305:1652. [DOI] [PubMed] [Google Scholar]
- 43. Panaich SS, Arora S, Patel N, et al. In-hospital outcomes of atherectomy during endovascular lower extremity revascularization. Am J Cardiol. 2016;117:676-684. [DOI] [PubMed] [Google Scholar]
- 44. Singh T, Koul D, Szpunar S, et al. Tissue removal by ultrasound evaluation (the TRUE study): the Jetstream G2 system post-market peripheral vascular IVUS study. J Invasive Cardiol. 2011;23:269-273. [PubMed] [Google Scholar]
- 45. McKinsey JF, Zeller T, Rocha-Singh KJ, et al. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv. 2014;7:923-933. [DOI] [PubMed] [Google Scholar]
- 46. McKinsey JF, Goldstein L, Khan HU, et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Ann Surg. 2008;248:519-528. [DOI] [PubMed] [Google Scholar]
- 47. Garcia LA, Jaff MR, Rocha-Singh KJ, et al. A comparison of clinical outcomes for diabetic and nondiabetic patients following directional atherectomy in the DEFINITIVE LE Claudicant Cohort. J Endovasc Ther. 2015;22:701-711. [DOI] [PubMed] [Google Scholar]
- 48. Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19:480-488. [DOI] [PubMed] [Google Scholar]
- 49. Zeller T, Krankenberg H, Steinkamp H, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the Multicenter Pathway PVD Trial. J Endovasc Ther. 2009;16:653-662. [DOI] [PubMed] [Google Scholar]
- 50. Dave RM, Patlola R, Kollmeyer K, et al. Excimer laser recanalization of femoropopliteal lesions and 1-year patency: results of the CELLO registry. J Endovasc Ther. 2009;16:665-675. [DOI] [PubMed] [Google Scholar]
- 51. Liistro F, Porto I, Angioli P, et al. Drug-Eluting Balloon in Peripheral Intervention for Below the Knee Angioplasty Evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615-621. [DOI] [PubMed] [Google Scholar]
- 52. Poncyljusz W, Falkowski A, Safranow K, et al. Cutting-balloon angioplasty versus balloon angioplasty as treatment for short atherosclerotic lesions in the superficial femoral artery: randomized controlled trial. Cardiovasc Intervent Radiol. 2013;36:1500-1507. [DOI] [PubMed] [Google Scholar]
- 53. Antonopoulos CN, Mylonas SN, Moulakakis KG, et al. A network meta-analysis of randomized controlled trials comparing treatment modalities for de novo superficial femoral artery occlusive lesions. J Vasc Surg. 2017;65:234-245. [DOI] [PubMed] [Google Scholar]
- 54. Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:89-699. [DOI] [PubMed] [Google Scholar]
- 55. Gongora CA, Shibuya M, Wessler JD, et al. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug-coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1115-1123. [DOI] [PubMed] [Google Scholar]
- 56. Rosenfield K, Jaff MR, White CJ, et al. Trial of paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145-153. [DOI] [PubMed] [Google Scholar]
- 57. Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568-1576. [DOI] [PubMed] [Google Scholar]
- 58. Elahinia MH, Hashemi M, Tabesh M, Bhaduri SB. Manufacturing and processing of NiTi implants. Prog Mater Sci. 2012;57:911-946. [Google Scholar]
- 59. Siddiqui DA, Sivan S, Weaver JD, Di Prima M. Effect of wire fretting on the corrosion resistance of common medical alloys [published online ahead of print September 23, 2016]. J Biomed Mater Res B Appl Biomater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879-1888. [DOI] [PubMed] [Google Scholar]
- 61. Dick P, Wallner H, Sabeti S, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74:1090-1095. [DOI] [PubMed] [Google Scholar]
- 62. Spreen MI, Marten JM, Knippenberg B, et al. Percutaneous transluminal angioplasty and drug-eluting stents for infrapopliteal lesions in critical limb ischemia (PADI) trial. Circ Cardiovasc Interv. 2016;9:e002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rastan A, Brechtel K, Krankenberg H, et al. Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: long-term results from a randomized trial. J Am Coll Cardiol. 2012;60:587-591. [DOI] [PubMed] [Google Scholar]
- 64. Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs.bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274-2281. [DOI] [PubMed] [Google Scholar]
- 65. Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluging stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290-2295. [DOI] [PubMed] [Google Scholar]
- 66. Siablis D, Kitrou PM, Spiliopoulos S, et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1048-1056. [DOI] [PubMed] [Google Scholar]