Abstract
Background:
In 2 previous clinical trials, fingertip capillary blood samples were evaluated using prespecified blood glucose monitoring systems (BGMSs) and a reference YSI glucose analyzer. In post hoc analyses, hypothetical insulin doses were calculated using these blood glucose measurements; dosing errors were compared for each trial.
Method:
For each blood glucose measurement, premeal bolus insulin dosing was determined for a hypothetical person, assuming a 60-g carbohydrate meal and 100-mg/dL target blood glucose level (adjusting 1/25 insulin sensitivity and 1/15 insulin:carbohydrate ratio inputs to account for BGMS measurement error). Dosing error was the difference between doses calculated using the BGMS and YSI results.
Results:
In Clinical Trial 1, 95% dose error ranges (in units of insulin) were: CONTOUR®NEXT EZ BGMS (EZ), –0.9 to 0.5; Accu-Chek® Aviva BGMS (ACA), –0.5 to 1.8; FreeStyle Freedom Lite® BGMS (FFL), –3.2 to −0.3; OneTouch® Ultra®2 BGMS (OTU2), –4.1 to 0.3; and Truetrack® BGMS (TT), –3.9 to 2.2. In Clinical Trial 2, these ranges were: CONTOUR®NEXT BGMS (CN), –0.7 to 1.7; Accu-Chek® Aviva Nano BGMS (ACAN), –1.3 to 1.8; FreeStyle Lite® BGMS (FSL), –5.1 to 0.2; OTU2, –1.9 to 1.2; OneTouch® Verio® Pro BGMS (OTVP), –1.0 to 1.9; and TT, –5.1 to 1.7. Within each trial, EZ and CN had statistically significantly smaller insulin dose error ranges than other BGMSs (P <0.0001).
Conclusions:
The ranges of insulin dose errors were statistically significantly smaller with EZ and CN than with all other BGMSs in this post hoc analysis. Differences in BGMS accuracy could result in clinically important differences in insulin dosing.
Keywords: blood glucose monitoring, diabetes management, dosing errors, insulin, self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) can serve to motivate individuals with diabetes to improve their self-care practices as they see the effects of lifestyle choices (eg, physical activity and meal planning) on their blood glucose levels.1 SMBG results can also be used to determine appropriate insulin doses for individuals who use insulin. Knowledge of their measured blood glucose levels, as opposed to their self-estimated blood glucose levels (ie, testing versus guessing), can give people with diabetes more confidence in adjusting their insulin doses.1 For example, in a study in which subjects were asked to estimate their current blood glucose before learning their blood glucose value using a blood glucose monitoring system (BGMS), 98% agreed or responded neutrally to the statement “Knowing my blood sugar by checking gives me more confidence in adjusting my daily insulin dose.”1 In addition, SMBG results can be used by people who take oral diabetes medications to guide decisions regarding medication adjustments.2
Modeling analyses have demonstrated that blood glucose measurement error can lead to insulin dosing inaccuracy.3-5 The effect of measurement error on insulin dosing may be further influenced by inaccurate carbohydrate estimation.6 A simulation model showed that while both BGMS error and carbohydrate estimation contribute to insulin dosing accuracy, BGMSs with the best performance are associated with the greatest likelihood of on-target insulin doses.5 These potential insulin dosing errors could result in adverse blood glucose outcomes, such as clinically significant hypoglycemia or hyperglycemia, as shown in other modeling analyses.7 The accuracy of BGMS results is important; the International Organization for Standardization (ISO) 15197:2013 criteria8 provide a standard for acceptable BGMS accuracy.
Another previous modeling analysis demonstrated that differences in performance can exist between BGMSs that meet accuracy standards.9 Yet another modeling analysis examined the relationship between BGMS error and the risk of hypoglycemia.10 In this article, we present a model that adds to the previous analyses and further demonstrates that blood glucose measurement error can lead to insulin dosing inaccuracies.
In each of 2 previous clinical trials, the differences in accuracy between the CONTOUR®NEXT EZ BGMS (EZ; Clinical Trial 1) and the CONTOUR®NEXT BGMS (CN; Clinical Trial 2) and 4 and 5 other BGMSs, respectively, were evaluated.11,12 For both trials, mean absolute relative difference (MARD) from the reference value was used to assess accuracy; EZ and CN each had a statistically significantly lower MARD compared with the other BGMSs in their respective studies. In the post hoc analyses presented herein, blood glucose measurements generated in the previous trials were used to calculate bolus insulin dosing for a hypothetical person with diabetes. The resulting errors in bolus insulin dosing for EZ and CN were compared with those of the other BGMSs in the corresponding trial. The objective of these analyses was to determine and compare the distribution of bolus insulin dosing errors that may result from inaccuracies in blood glucose measurements.
Methods
Clinical Trial Design
Clinical Trial 1
In a previous study,11 fingertip capillary blood samples from 146 subjects were tested using 5 BGMSs. The meter systems evaluated were the following: EZ (Ascensia Diabetes Care, Parsippany, NJ); Accu-Chek® Aviva BGMS (ACA; Roche Diagnostics, Indianapolis, IN); FreeStyle Freedom Lite® BGMS (FFL; Abbott Diabetes Care, Inc, Alameda, CA); OneTouch® Ultra®2 BGMS (OTU2; Life-Scan, Inc., Milpitas, CA); and Truetrack® BGMS (TT; Nipro Diagnostics Inc, Fort Lauderdale, FL). Using the same sample source, all BGMS results were compared with results from a YSI glucose analyzer (YSI; YSI Life Sciences, Inc, Yellow Springs, OH).
Clinical Trial 2
In another previous study,12 fingertip capillary blood samples from 146 subjects were tested using 6 BGMSs. The meter systems evaluated were the following: CN (Ascensia Diabetes Care, Parsippany, NJ); Accu-Chek® Aviva Nano BGMS (ACAN; Roche Diagnostics, Indianapolis, IN); FreeStyle Lite® BGMS (FSL; Abbott Diabetes Care, Inc, Alameda, CA); OTU2; OneTouch® Verio® Pro BGMS (OTVP; LifeScan, Inc., Milpitas, CA); and TT. All BGMS results were compared with results from the same sample source measured on a YSI glucose analyzer.
Assessments and Analyses
Blood Glucose Measurement Accuracy
In both clinical trials, unmodified and modified blood samples were tested. Subjects did not test their own blood using the BGMSs; all blood sample testing was performed by trained study staff. Recognizing the potential impact of sample modification on glucose oxidase–based meters, the post hoc analyses shown here are based on unmodified samples only. Unmodified blood samples best represent the hypothetical scenario in these analyses (a person using BGMS results to determine an appropriate bolus insulin dose). The calibration method used by each manufacturer for their BGMS can be found in Appendix 1 of the Supplementary Materials available online. Blood glucose measurements were randomly sampled, and these analyses did not attempt to identify “target ranges” for each subject.
Insulin Dose Error
The specific scenario under consideration in these post hoc analyses involved a hypothetical person with diabetes calculating an appropriate premeal bolus insulin dose, assuming a meal containing 60 g of carbohydrates and a target blood glucose level of 100 mg/dL. For each clinical trial, blood glucose measurement pairs (BGMS and YSI) from the trial were randomly sampled, and each was used in the dosing scenario, generating 2 insulin doses (1 based on the BGMS blood glucose measurement and 1 based on the YSI blood glucose measurement). When calculating the bolus insulin dose based on the YSI measurement, an insulin sensitivity input of 1/25 and an insulin:carbohydrate ratio input of 1/15 were used. When calculating the insulin dose based on the BGMS measurement, these initial inputs were adjusted using the proportion of error between the BGMS measurement and the YSI measurement. These adjustments were made to account for the fact that a personi with diabetes will compute these values using blood glucose measurements from a BGMS rather than from a laboratory reference method. The adjustment formula used was as follows: adjusted factor = original factor/(1 + p), where p = (BGMS – YSI)/YSI. The original factor was what the subject believed to be the truth; namely, the sensitivity factor = 1/25 and the insulin:carbohydrate factor = 1/15. The formula used in the computer model was as follows, where BG is blood glucose level: bolus insulin dose = [(current BG – target BG) × (adjusted insulin sensitivity factor)] + [(carbohydrate intake) × (adjusted insulin:carbohydrate ratio)].
Bolus insulin dose error was calculated as the difference between the doses calculated using the BGMS and YSI results. For each BGMS, bolus insulin dose errors were calculated for 20,000 randomly sampled measurement pairs (with replacement) to generate an empirical dose error distribution. With a large number of data points, this bootstrapping procedure was employed to provide a more robust dose error distribution than would have been generated using each of the BGMS measurements from the clinical trials once. Significant differences in dose error distributions were observed without the use of bootstrapping. The bootstrapping was performed to make sample sizes uniform across all meter systems; however, differences in those sample sizes were relatively small (differing by 1 or 2 subjects).
For both clinical trials, bolus insulin dose error distributions were generated for each BGMS in the trial. The median dose error and the 95% dose error range were used to describe the distribution of bolus insulin dose errors for each BGMS. The bolus insulin dose error distributions for EZ and CN were then compared with the dose error distributions for each of the other BGMSs in Clinical Trials 1 and 2, respectively, by using the Kolmogorov-Smirnov test to assess the differences for statistical significance.
Results
Clinical Trial 1
Blood Samples
One unmodified capillary fingertip blood sample from each of 146 subjects was included in this analysis. The blood glucose concentration of the 146 unmodified samples ranged from 50 mg/dL to 386 mg/dL, as measured on the YSI.
Insulin Dose Error
Using the blood glucose values measured in the clinical trial with the BGMSs and the blood glucose values measured with the YSI, appropriate premeal bolus insulin doses were calculated for a hypothetical person with diabetes. The median bolus insulin dose error and 95% dose error range for each BGMS are plotted in Figure 1. Insulin underdosing is reflected by negative dose errors, and insulin overdosing is reflected by positive dose errors. In the plot, a central tendency (median bolus insulin dose error) nearest 0 units of insulin and a relatively small dispersion (shorter bar representing the 95% dose error range) would indicate more accurate bolus insulin dosing for that BGMS. The median dose errors, represented by solid white circles in Figure 1, were as follows (in units of insulin): EZ, –0.1; ACA, 0.2; FFL, –1.0; OTU2, –1.5; and TT, –0.7. For each BGMS, the lower and upper limits of the 95% dose error range are represented by the termini of the horizontal bars in Figure 1; these values are shown in Table 1. EZ was associated with a smaller bolus insulin dose error range than those of the other 4 BGMSs. Based on the bolus insulin dose error distributions, EZ was associated with a statistically significantly smaller range of dose errors than those of ACA, FFL, OTU2 and TT (Kolmogorov-Smirnov test; P <0.0001 for all 4 BGMSs).
Figure 1.
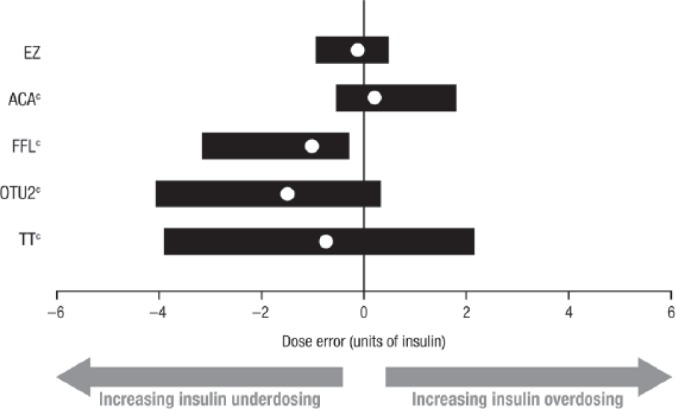
Middle (median) 95% distribution of bolus insulin dose errors (Clinical Trial 1).a,b
BGMS, blood glucose monitoring system; YSI, YSI glucose analyzer.
aThe insulin sensitivity input (1/25) and the insulin:carbohydrate ratio (1/15) were adjusted based on the observed error of the BGMS result versus the YSI measurement.
bSolid white circles represent the median dose error; horizontal bars represent the 95% dose error range.
cP <0.0001 versus EZ (Kolmogorov-Smirnov test).
Table 1.
95% Dose Error Ranges (Clinical Trial 1).
| Meter system | Lower limit | Upper limit |
|---|---|---|
| EZ | –0.9 | 0.5 |
| ACA | –0.5 | 1.8 |
| FFL | –3.2 | –0.3 |
| OTU2 | –4.1 | 0.3 |
| TT | –3.9 | 2.2 |
Clinical Trial 2
Blood Samples
Three unmodified capillary fingertip blood samples from each of 146 subjects were included in this analysis. The YSI blood glucose concentration range of the 438 unmodified samples was 36 mg/dL to 408 mg/dL.
Insulin Dose Error
The blood glucose values measured in the clinical trial with the BGMSs and those measured with the YSI were used to calculate the appropriate premeal bolus insulin doses for a hypothetical person with diabetes. The median bolus insulin dose error and 95% dose error range for each BGMS are plotted in Figure 2. As in Figure 1, negative dose errors indicate insulin underdosing, and positive dose errors indicate insulin overdosing; moreover, more accurate bolus insulin dosing is represented by a central tendency nearest 0 units of insulin and a relatively small dispersion. The median dose errors, represented by solid white circles in Figure 2, were as follows (in units of insulin): CN, 0.2; ACAN, 0.1; FSL, –1.0; OTU2, 0.1; OTVP, 0.0; and TT, –0.7. The lower and upper limits of the 95% dose error range for each BGMS, represented by the termini of the horizontal bars in Figure 2, are shown in Table 2. CN was associated with a smaller bolus insulin dose error range than those of the other 5 BGMSs. Based on the bolus insulin dose error distributions, CN was associated with a statistically significantly smaller range of dose errors than those of ACAN, FSL, OTU2, OTVP, and TT (Kolmogorov-Smirnov test; P <0.0001 for all 5 BGMSs).
Figure 2.
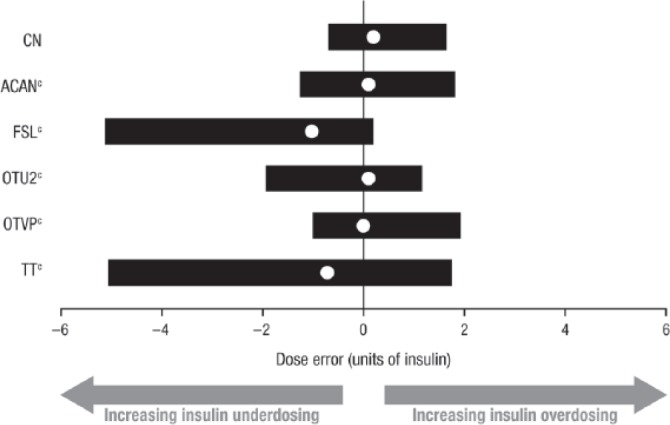
Middle (median) 95% distribution of bolus insulin dose errors (Clinical Trial 2).a,b
BGMS, blood glucose monitoring system; YSI, YSI glucose analyzer.
aThe insulin sensitivity input (1/25) and the insulin:carbohydrate ratio (1/15) were adjusted based on the observed error of the BGMS result versus the YSI measurement.
bSolid white circles represent the median dose error; horizontal bars represent the 95% dose error range.
cP <0.0001 versus CN (Kolmogorov-Smirnov test).
Table 2.
95% Dose Error Ranges (Clinical Trial 2).
| Meter system | Lower limit | Upper limit |
|---|---|---|
| CN | –0.7 | 1.7 |
| ACAN | –1.3 | 1.8 |
| FSL | –5.1 | 0.2 |
| OTU2 | –1.9 | 1.2 |
| OTVP | –1.0 | 1.9 |
| TT | –5.1 | 1.7 |
Discussion
Differences in BGMS accuracy were observed in the 2 prior clinical trials comparing both EZ and CN with several other BGMSs11,12; blood glucose measurement data from these trials were then used to determine bolus insulin dose errors for the post hoc analyses described here. Results from the first post hoc analysis from Clinical Trial 1 demonstrated that the range of bolus insulin dose errors was statistically significantly smaller with EZ than with ACA, FFL, OTU2, and TT. In the post hoc analysis from Clinical Trial 2, CN was associated with a range of bolus insulin dose errors that was statistically significantly smaller than those of ACAN, FSL, OTU2, OTVP, and TT. The 2 studies were conducted independently of each other and unequally sampled; however, there was no attempt to compare data between the 2 studies, so differences in sample size were not a concern.
According to the American Diabetes Association (ADA) Standards of Care in Diabetes guidelines,13 SMBG results can be integrated into a diabetes management plan to help guide decisions related to medication adjustment, meal planning, and physical activity. These ADA guidelines note the particular importance of SMBG for people with diabetes who use multiple-dose insulin or insulin pump therapy,13 as they use SMBG results to adjust their insulin dose.14
In the past, a number of analyses have demonstrated that inaccurate BGMS results can lead to errors in insulin dosing.3,4,7 As more recently published, errors in blood glucose readings may result in contraindicated treatment decisions.9,15 While large errors may result in clinically relevant differences in insulin dosing, even small measurement errors may combine to a substantial total effect due to factors that include human error, meter accuracy, test strip error, environmental and physiological conditions, and concomitant medication.9,15
Potential limitations of the post hoc analyses reported here should be considered when evaluating the results. There are other sources of error in calculating bolus insulin doses that were not considered in these analyses. Some possible sources of error relate to the many decisions that a person with diabetes who uses insulin must make every day, such as estimating carbohydrate intake, determining an appropriate insulin:carbohydrate ratio, or considering whether other circumstances may affect dosing (eg, dosing while sick).6,16 In addition, technological challenges, such as bolus calculators that may be complicated and a general lack of compatibility among devices, also have the potential to affect decision making for people with diabetes who use insulin therapy.16
While outside the scope of this article, considerations for future research might include the potential impact on dosing error of people with diabetes who use multiple BGMSs. As we chose a deliberately simplistic insulin dose calculation model and selected 1 set of parameters to illustrate the point that BGMS measurement error can have an effect on insulin dosing error, we recognize that other factors could have an impact, including different patient types and different types of insulin. Herein, we discuss a model in which we examined the entire blood glucose range, although the potential consequences for insulin dosing decisions at low blood glucose values would be relevant and of interest and, in fact, previous models have focused on hypoglycemia, including severe hypoglycemia.9 The results from our model demonstrate that BGMSs may read higher or lower than the YSI value, and it is possible that such differences could alter insulin dose. A broad assessment of the potential clinical relevance and implications of the findings from this model would be of interest, but further assessment is necessary to explore and validate findings of model-based approaches.
Conclusions
EZ and CN were each associated with a range of bolus insulin dose errors that was statistically significantly smaller than those of all other BGMSs in their respective trials in these post hoc analyses for a hypothetical person with diabetes. The model presented is designed to provide a sense of the potential impact of BGMS error on the distribution of errors that one might encounter in an array of clinical circumstances. As such, in the real clinical world, the assumptions made may vary, as would the results. Nonetheless, the results of such a model are useful to develop a sense of the issues that result from BGMS error. When considered together with the results of other previous modeling analyses,3-5,7,10 the results of the analyses presented here suggest that differences in BGMS accuracy may result in bolus insulin dosing errors that have the potential to result in adverse blood glucose outcomes, including clinically significant hypoglycemia or hyperglycemia.
Supplementary Material
Footnotes
Abbreviations: ACA, Accu-Chek® Aviva blood glucose monitoring system; ACAN, Accu-Chek® Aviva Nano blood glucose monitoring system; ADA, American Diabetes Association; BGMS, blood glucose monitoring system; CN, CONTOUR®NEXT blood glucose monitoring system; EZ, CONTOUR®NEXT EZ blood glucose monitoring system; FFL, FreeStyle Freedom Lite® blood glucose monitoring system; FSL, FreeStyle Lite® blood glucose monitoring system; ISO, International Organization for Standardization; MARD, mean absolute relative difference; OTU2, OneTouch® Ultra®2 blood glucose monitoring system; OTVP, OneTouch® Verio® Pro blood glucose monitoring system; SMBG, self-monitoring of blood glucose; TT, Truetrack® blood glucose monitoring system; YSI, YSI glucose analyzer.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SP and ND are full-time employees of Ascensia Diabetes Care. DAS was an employee of Ascensia Diabetes Care at the time of the study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study and the analyses presented here were supported by Bayer HealthCare, the predecessor-in-interest of Ascensia Diabetes Care, Parsippany, NJ. Medical writing assistance was provided by Allison Michaelis, PhD, of MedErgy, and was funded in part by Ascensia Diabetes Care, and in part by Bayer HealthCare as Ascensia’s predecessor-in-interest.
Supplemental Material: The online supplementary material is available at http://journals.sagepub.com/doi/suppl/10.1177/1932296817713025.
References
- 1. Pettus J, Stenger P, Schachner HC, et al. Testing versus guessing blood glucose values: impact on self-care behaviors in type 2 diabetes. Curr Med Res Opin. 2014;30(9):1795-1802. [DOI] [PubMed] [Google Scholar]
- 2. Kirk JK, Stegner J. Self-monitoring of blood glucose: practical aspects. J Diabetes Sci Technol. 2010;4(2):435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47(2):209-214. [PubMed] [Google Scholar]
- 4. Raine CH, III, Schrock LE, Edelman SV, et al. Significant insulin dose errors may occur if blood glucose results are obtained from miscoded meters. J Diabetes Sci Technol. 2007;1(2):205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Virdi NS, Mahoney JJ. Importance of blood glucose meter and carbohydrate estimation accuracy. J Diabetes Sci Technol. 2012;6(4):921-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kildegaard J, Randlov J, Poulsen JU, Hejlesen OK. The impact of non-model-related variability on blood glucose prediction. Diabetes Technol Ther. 2007;9(4):363-371. [DOI] [PubMed] [Google Scholar]
- 7. Raine CH, III, Pardo S, Parkes JL. Predicted blood glucose from insulin administration based on values from miscoded glucose meters. J Diabetes Sci Technol. 2008;2(4):557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Organization for Standardization. ISO 15197:2013(E): In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva, Switzerland: International Organization for Standardization; 2013. [Google Scholar]
- 9. Budiman ES, Samant N, Resch A. Clinical implications and economic impact of accuracy differences among commercially available blood glucose monitoring systems. J Diabetes Sci Technol. 2013;7(2):365-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4(3):562-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halldorsdottir S, Warchal-Windham ME, Wallace JF, Pardo S, Parkes JL, Simmons DA. Accuracy evaluation of five blood glucose monitoring systems: the North American comparator trial. J Diabetes Sci Technol. 2013;7(5):1294-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klaff LJ, Brazg R, Hughes K, et al. Accuracy evaluation of Contour Next compared with five blood glucose monitoring systems across a wide range of blood glucose concentrations occurring in a clinical research setting. Diabetes Technol Ther. 2015;17(1):8-15. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S108. [DOI] [PubMed] [Google Scholar]
- 14. Choleau C, Albisser AM, Bar-Hen A, et al. A novel method for assessing insulin dose adjustments by patients with diabetes. J Diabetes Sci Technol. 2007;1(1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erbach M, Freckmann G, Hinzmann R, et al. Interferences and limitations in blood glucose self-testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. Public workshop—regulatory science considerations for software used in diabetes management, November 13, 2014. Available at: https://www.fda.gov/downloads/medicaldevices/newsevents/workshopsconferences/ucm430215.pdf. Accessed November 16, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


