Abstract
While recent ‘big data' analyses discovered structural brain networks that alter with age and relate to cognitive decline, identifying modifiable factors that prevent these changes remains a major challenge. We therefore aimed to determine the effects of common cardiovascular risk factors on vulnerable gray matter (GM) networks in a large and well-characterized population-based cohort. In 616 healthy elderly (258 women, 60–80 years) of the LIFE-Adult-Study, we assessed the effects of obesity, smoking, blood pressure, markers of glucose and lipid metabolism as well as physical activity on major GM-networks derived using linked independent component analysis. Age, sex, hypertension, diabetes, white matter hyperintensities, education and depression were considered as confounders. Results showed that smoking, higher blood pressure, and higher glycated hemoglobin (HbA1c) were independently associated with lower GM volume and thickness in GM-networks that covered most areas of the neocortex. Higher waist-to-hip ratio was independently associated with lower GM volume in a network of multimodal regions that correlated negatively with age and memory performance. In this large cross-sectional study, we found selective negative associations of smoking, higher blood pressure, higher glucose, and visceral obesity with structural covariance networks, suggesting that reducing these factors could help to delay late-life trajectories of GM aging.
Keywords: Alzheimer's disease, aging, brain structure, gray matter modifiers, Independent component analysis, structural covariance
Introduction
Recent ‘big data' analyses of structural co-variance between brain regions revealed large-scale gray matter (GM) networks that are linked to developmental changes and inter-individual behavioral differences.1–4 Douaud et al.3 described a network of transmodal cortical and limbic GM regions that showed correlated shrinkage in healthy aging and links to memory performance. That GM network also mirrored brain regions that exhibit accelerated atrophy in patients with Alzheimer's disease (AD).3
The observed network-based effects could hint towards a shared susceptibility of connected regions, indicative of unique morphological properties, to selective pathological processes.5,6 They also strengthen the hypothesis that fundamental mechanisms of aging may contribute to (or result from) neurodegenerative pathologies.7–11 A better understanding of possible modulators of GM networks that are vulnerable to aging would thus open a novel window towards targets for intervention of disease progression.
Using conventional analyses, global and regional decreases in GM volume and cortical thickness have been linked, though not unequivocally,12 to common cardiovascular risk factors comprising cigarette smoking, hypertension, obesity and metabolic changes.13–16 However, addressing potential impact of these factors at the network- and population-level remains a major challenge.17
We therefore aimed to systematically assess the effects of obesity, smoking, blood pressure, as well as markers of glucose and lipid metabolism and physical activity on major GM networks using linked independent component analysis of cortical volume, thickness, and surface area estimated from T1-weighted MRIs in a large cohort of community-dwelled healthy older individuals. We determined the unique contribution of each risk factor (selected according to the Framingham study18 and additionally physical activity19) to variations in these GM networks using multivariable statistics that were adjusted for confounders. Possible associations between GM networks and cognition were explored using a sumscore of verbal memory performance, known to be highly affected by age.20 We hypothesized negative effects of cardiovascular risk factors on major GM components that are prone to undergo age-associated changes and linked with cognition.
Materials and methods
Participants
Data were drawn from the baseline examination of the “Health Study of the Leipzig Research Centre for Civilization Diseases” (LIFE), a population-based cohort study of adult Leipzig inhabitants, randomly invited via the population registry. All subjects signed an informed consent form and received a small financial compensation. The study protocol was in accordance with the declaration of Helsinki and approved by the ethics committee of the University of Leipzig.
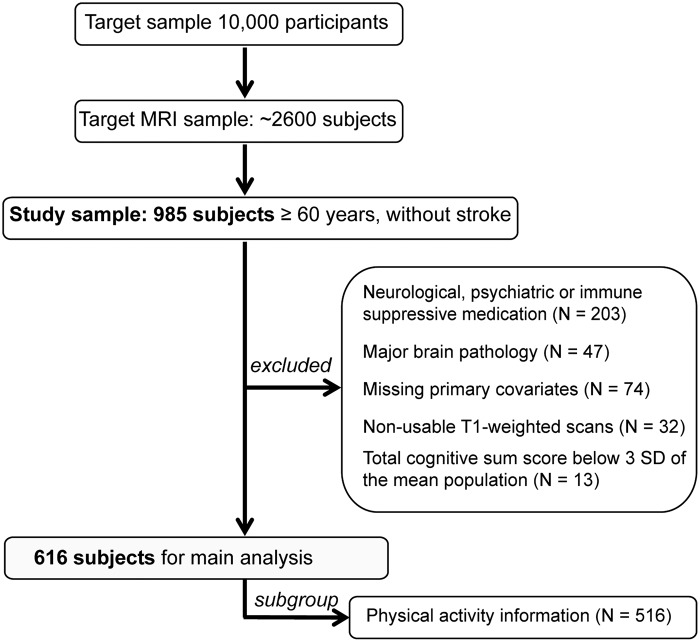
Participants underwent neuropsychological testing, medical examinations, and a randomly selected subset underwent magnetic resonance imaging (MRI) of the head at 3T (n ∼ 2600). For details on the study design, see Loeffler et al.21 Out of a sample of 985 older participants (≥ 60 years) available at the date of analyses, we excluded participants with dementia, neurological, psychiatric or immune suppressive medication (n = 203), as well as major brain pathology (e.g. tumors and stroke) (n = 47). Also, subjects with missing information on confounding factors (n = 74), severe movement artifacts on the MRI or other technical problems (n = 32), as well as non-intact cognitive performance (n = 13, defined as showing a total cognitive sumscore of < 3 SD of the mean population) were excluded, resulting in a sample of 616 subjects (Figure 1). Due to the nature of our exclusion criteria, participants excluded (n = 369) were on average slightly older (mean age: 69.5 ± 6 (SD) years, p = 0.021), more frequently women (p = 0.028), exhibited a higher BMI (mean BMI: 28.4 ± 4.25 (SD) kg/m2, p = 0.001) and were less educated compared to those included (p < 0.001).
Figure 1.
Flow chart of the study. Out of 985 older adults free of stroke, 369 were excluded due to medication intake, brain pathology, missing covariates, non-usable MRI scans, or non-intact cognition, leaving 616 participants for main analyses. Out of this sample, 516 participants had physical activity information.
MRI acquisition
Anatomic T1-weighted images were acquired in a 3-Tesla Magnetom Verio scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head array coil, using a three-dimensional Magnetization-Prepared Rapid Gradient Echo (MPRAGE) sequence. GRAPPA parallel imaging technique22 was applied on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) standard protocol with the following parameters: TI 900 ms, TR 2300 ms, TE 2.98 ms, flip angle 9°, band width 240 Hz/pixel, image matrix 256 × 240, 176 partitions, FOV 256 × 240 × 176 mm3, sagittal orientation, voxel size 1 × 1 × 1 mm3, no interpolation.
Image processing
T1-weighted images were processed using FSL-VBM,23 an optimized voxel-based morphometry (VBM)24 protocol using FMRIB Software Library (FSL) tools (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM; FSL 4.1),25 in which a symmetric study-specific GM template was built from the images of a sub-group of 260 participants equally matched for males and females, which were not significantly different from the whole sample with regard to age and BMI range and frequency of hypertension and diabetes. Prior to the FSL-VBM processing, the volumes were masked by the full brain-segmented volume output from FreeSurfer (http://surfer.nmr.mgh.harvard.edu/; FreeSurfer 5.0.0),26 effectively excluding non-brain compartments. After nonlinearly registering all of the brain-extracted, GM-segmented images onto the symmetric study-specific GM template, the optimized FSL-VBM protocol involved a compensation (or “modulation”) for the local contraction/enlargement caused by the nonlinear component of the transformation. In addition, brain structural information was derived from vertex-wise cortical thickness and surface area calculated in FreeSurfer by means of an automated surface reconstruction scheme.26 All surface reconstructions were visually inspected in Freeview and manually edited in 31 cases. For computational reasons, we reduced the number of data points in each modality by lowering the resolution of the pre-processed images, while not losing any information about global patterns of structural covariance, due to the smoothness of the pre-processed images. The modulated registered GM-segmented images were first down-sampled to 4 mm isotropic and then were smoothed with an isotropic Gaussian kernel with a σ of 4 mm (≈9.4 mm full width at half maximum (FWHM)). Cortical thickness and surface area maps were sampled from subject space to the fsaverage5 template (10,242 vertices) and then smoothed with a surface FWHM of 10 mm.
Then linked-independent component analysis (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLICA) was applied to measures of GM volume, cortical thickness and surface area, decomposing the data into 70 components, see Douaud et al.3 and Groves et al.27,28 for further descriptions. Briefly, here the aim is to model the group data as a set of interpretable features (the independent components (ICs)), each one characterizing a single mode of variability. Each feature consists of a shared subject loading, which indicates which subjects have more or less of this feature, and the corresponding spatial pattern that is learned for each modality. We selected 12 global networks based on the elbow in the scree plot of the relative amount of total variance explained by each component (similar to Groves et al.27) and further denoted them as IC 1–12 (Table 1). Two components (IC1 and IC8) were considered of no further interest, as their respective variance was nearly fully explained by differences in head size (IC1) and image artifacts (IC8). See Supplementary Figure, for illustration of spatial maps of the remaining ten components.
Table 1.
Relative amount of explained variance by independent components (IC), according to linked-IC analysis of gray matter volume (GMV), cortical thickness, and surface area; and correlation with age and memory performance.
| # Component | Explained variance (%) |
Correlation (r,
p-value) |
|||
|---|---|---|---|---|---|
| GMV | Thickness | Area | Age | Memory | |
| IC1 | 1 | 2 | 47 | −0.136, 10−3 | −0.075, 0.062 |
| IC2 | 0 | 32 | 0 | −0.115, 0.004 | 0.036, 0.376 |
| IC3 | 9 | 4 | 14 | −0.581, 10−3 | 0.192, 10−3 |
| IC4 | 8 | 0 | 4 | 0.003, 0.95 | −0.078, 0.053 |
| IC5 | 0 | 12 | 0 | 0.177, 10−3 | −0.082, 0.042 |
| IC6 | 4 | 0 | 0 | −0.165, 10−3 | 0.023, 0.57 |
| IC7 | 3 | 1 | 4 | −0.150, 10−3 | 0.144, 10−3 |
| IC8 | 3 | 2 | 0 | −0.034, 0.4 | −0.061, 0.13 |
| IC9 | 2 | 1 | 1 | −0.086, 0.034 | 0.027, 0.5 |
| IC10 | 3 | 0 | 1 | 0.001, 0.97 | 0.184, 10−3 |
| IC11 | 3 | 0 | 0 | −0.178, 10−3 | −0.018, 0.65 |
| IC12 | 0 | 8 | 0 | −0.044, 0.28 | 0.047, 0.24 |
Note: r- and p-values are according to Spearman’s rank correlations. Significant associations are indicated by bolding the numbers (p < 0.005).
For illustration purposes, we up-sampled the linked-ICA results to the high-resolution versions of the smoothed input data, similar to Groves et al.27 (GM volume images on 2 mm isotropic and surface measures sampled on fsaverage space, i.e. 163,842 cortical vertices per hemisphere).
Cardiovascular risk factors
All subjects underwent anthropometric assessments, donated blood after fasting overnight and were asked to fill in questionnaires about their lifestyle habits. Cardiovascular risk factors comprised obesity, assessed using “waist-to-hip ratio” (WHR, measured using an ergonomic circumference measuring tape (SECA 201) to the nearest 0.1 cm) and “BMI” (in kg/m2, measured) as continuous variables, “smoking status” (current, past or never smokers), systolic “blood pressure” (in mmHg, mean of three consecutive measurements in a seated position at rest), fasting serum concentrations of glucose and lipid metabolism, i.e. “glycated hemoglobin (HbA1c)” (in mmol/mol), “high-density lipoprotein (HDL)” (in mU/mL), and total “cholesterol” (in mmol/L). By using blood pressure and markers of glucose control (HbA1c) as continuous variables of interest related to hypertension and diabetes, we were able to increase statistical power and sensitivity in a dose–response relationship. Six subjects had missing blood values due to technical problems (HbA1c, n = 5, HDL, n = 1), these values were replaced by the sample’s medians. “Physical activity” (self-reported according to the German short version of the international physical activity questionnaire, IPAQ,29 in MET-minutes/week) was analyzed in a subgroup of 516 subjects due to unreturned questionnaires in 100 subjects.
Memory performance and assessment of confounders
“Memory performance” was assessed using the CERAD verbal learning task.30 Briefly, subjects were asked to remember and recall immediately and after a delay as many words as possible out of a list of 10 words. The memory performance sumscore was defined as the standardized mean performance in the sum of (1) immediate learning (no. of correct words across the three learning trials), (2) delayed recall (no. of correct words in the recall trial), and (3) recognition (no. of correctly recognized words in the recognition trial, minus false positives).31,32
“Depression” was measured using the Center for Epidemiologic Studies Depression Scale (CES-D) questionnaire,33 “arterial hypertension” was defined as a systolic blood pressure ≥ 160 mmHg, a diastolic blood pressure ≥ 95 mmHg or use of antihypertensive medication,14 “diabetes” was defined as none, type 1 medicated, type 2 medicated, type 2 unmedicated, and “other cardiovascular conditions” were defined as arrhythmia or tachycardia. “Education” was measured according to the International Standard Classification of Education (7 levels).34 In addition, “APOE4 carrier status” was defined as carrying one or two E4 alleles of the apolipopreotein E (APOE) gene (n = 32 missings due to lack of DNA samples). Genotyping was performed on a Roche Lightcylcer 480 using genomic DNA that was isolated from peripheral leukocytes using an automate protocol on the Qiagen Autopure LS (Qiagen, Hilden, Germany).
Statistical analysis
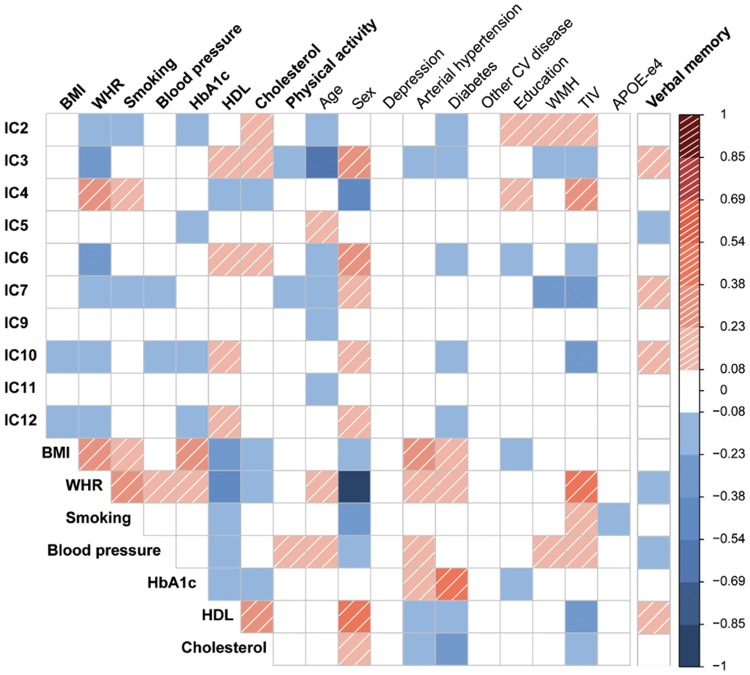
To determine unique associations between the GM networks and cardiovascular risk factors, we conducted partial correlations between subjects' loading on each of the 10 ICs and BMI, WHR, smoking, blood pressure, HbA1c, HDL and total cholesterol, respectively, in line with previous studies.35,36 When meeting the criteria of bivariate correlation with the respective IC (Figure 2), the remainder of the cardiovascular risk factors as well as the following confounder variables was considered in the partial correlation models: age, sex, depression, hypertension (except for blood pressure analyses), diabetes (except for HbA1c analyses), other cardiovascular conditions, white matter hyperintensities (WMH, assessed according to the Fazekas rating scale by means of 3D-FLAIR images37), education, total intracranial volume (TIV), and APOE4 carrier. We repeated analyses for physical activity in the physical activity subgroup (n = 516).
Figure 2.
Bivariate correlations among independent components (IC), cardiovascular risk factors, confounders, and verbal memory score. Significant associations (Spearman's correlations, p < 0.05) are color-coded in red-shaded (positive) and blue (negative). CV: cardiovascular; WMH: white matter hyperintensities; TIV: total intracranial volume; APOE-e4: apolipoprotein E epsilon-4 carrier status; BMI: body mass index; WHR: waist-to-hip ratio; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein.
To assess if the relation between blood pressure (or HbA1c) and GM networks would change with medication, we repeated the analysis in those with anti-hypertensive medication (or anti-diabetic medication, respectively), and in those without. In addition, given the link between estrogen replacement therapy and brain aging,38,39 we excluded women with current estrogen medication in confirmatory analyses. All variables were normally distributed (unimodal, |skewness| ≤ 1), except IC7, HbA1c, HDL, and physical activity, therefore non-parametric statistics were used. For partial correlations, missing APOE4 values (n = 32) were substituted by the sample mean. The significance threshold of partial correlations was set to p < 0.05 (two-sided) and corrected for the number of ICs tested (n = 10), resulting in pα < 0.005. All statistical analyses were performed in SPSS 20 (PASW, SPSS, IBM).
Results
In total, 616 older participants (258 women) were included in the analyses, see Table 2 for demographic characteristics. Subjects without physical activity questionnaires (n = 100) were slightly older (mean age: 70 ± 5 (SD) years; p < 0.001) and less educated (p = 0.005) in comparison to those with complete information (n = 516).
Table 2.
Sample characteristics.
| Participants n = 616 (258 women) | |
|---|---|
| Age [y] | 69 ± 5 (60–79) |
| Waist-to-hip ratio | 0.96 ± 0.085 (0.73–1.14) |
| BMI [kg/m2] | 27.5 ± 4 (17–41) |
| Smoking [%] (current/previous/never) | 7.5/33.1/59.4 |
| Mean Systolic BP [mmHg] | 136 ± 17 (89–197) |
| HbA1c [mmol/mol] (n = 611) | 5.4 [5.16–5.68] (3.84–12.38) |
| HDL [mU/mL] (n = 615) | 1.6 [1.32–1.92] (0.45–4.17) |
| Total cholesterol [mmol/L] | 5.9 ± 1.1 (2.3–10.8) |
| Physical activity [MET-minutes/week] (n = 516) | 4159 [2374.5–6919.5] (33.0−16398.0) |
| APOE status [% e4-carrier] (n = 584) | 20.9 |
| Depression scale (CES-D) [score] | 9.4 ± 5.1 (0–34) |
| Arterial hypertension [%] (yes) | 55.7 |
| Diabetes status [%] (none / type 1-medicated, type 2-medicated, type 2-non-medicated) | 84.4/0.5/12.3/2.8 |
| Current estrogen supplement [% females] (yes) | 7.3 |
| Cardiovascular diseases [%] (any) | 19.2 |
| Education [%] (without SS-LD/SS-LD/advanced SS-LD / advanced technical SS-LD / technical college ED / university ED) | 0/10.7/6.3/42.7/5.2/35.1 |
| White matter hyperintensities [%] (Fazekas score 0/1/2/3) | 23.5/59.8/16.2/0.5 |
Note: Data are mean ± SD (minimum-maximum) or median [Interquartile range] (minimum-maximum), unless indicated otherwise.
BMI: body mass index; BP: Blood pressure; MET: multiples of the resting metabolic rate; HbA1c: glycated hemoglobin A1c; HDL: high-density lipoproteins; APOE: Apolipoprotein E; CES-D: center for epidemiologic studies depression scale; SS-LD: secondary school-leaving degree; ED: entrance degree.
Structural networks and cardiovascular risk factors
Out of 10 independent GM components, global networks IC2 and IC7 showed an overall decrease in cortical thickness and volume, respectively, with age (IC2: r = −0.115, p = 0.004; IC7: r = −0.150, p < 10−3; Table 1). IC7 was also associated with memory performance (r = 0.144, p < 10−3).
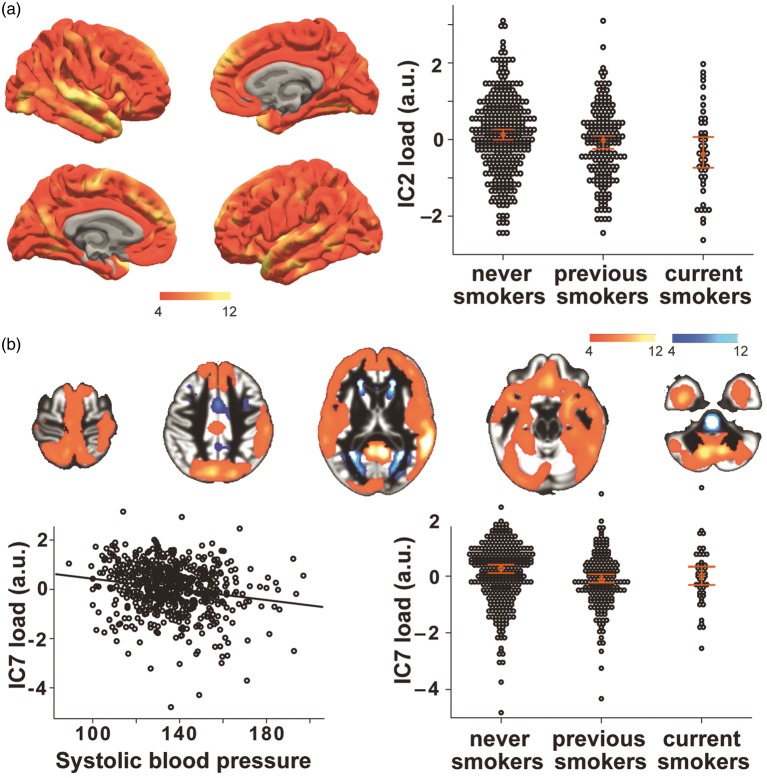
Independent of further associated risk factors and confounders, cigarette smoking was significantly linked to lower thickness and volume throughout the neocortex in these two global networks (Figure 3(a) and (b), IC2: partial-r = 0.120, p = 0.003; IC7: partial-r = −0.143, p < 0.001).
Figure 3.
In two global networks, lower gray matter thickness (IC2, a) and volume (IC7, b) were associated with smoking (a) and higher blood pressure (b). Scatter plots show the individual’s loading (black dots) and the group’s median with 95% SE or linear fit. Colors indicate positive (red/yellow) or negative (blue/light-blue) co-variations within the network (z > 4), maps are drawn on a standard brain.
Also, blood pressure was independently associated with IC7, showing an overall cortical volume decrease in association with increased blood pressure (Figure 3(b), partial-r = −0.122, p = 0.003).
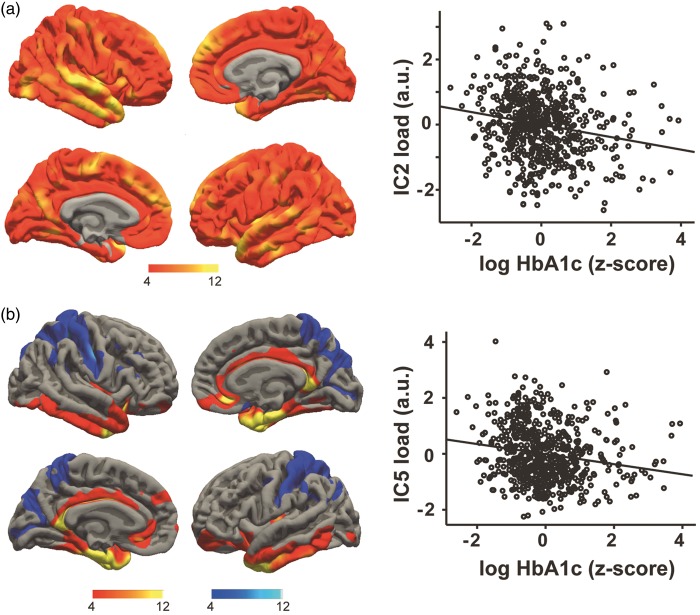
Considering glucose metabolism, we observed that higher serum concentrations of HbA1c were associated with decreased cortical thickness in IC2 after controlling for other risk factors and confounders (partial-r = −0.158, p < 10−3) (Figure 4(a)). A more regionally specific effect was noted for HbA1c in network IC5, showing lower thickness in medial frontal, insular, cingulate and inferior temporal areas and higher thickness in the postcentral gyrus and in the intraparietal sulcus (partial-r = −0.206, p < 10−3) (Figure 4(b)). This network exhibited a positive correlation with age (r = 0.177, p < 10−3).
Figure 4.
Higher fasting serum levels of HbA1c were associated with lower cortical thickness of IC2 (a) and IC5 (b). Scatter plots show the individual’s loading on each network (black dots) and linear fit. Colors indicate positive (red/yellow) or negative (blue/light-blue) co-variations within the network (z > 4), maps are drawn on a standard brain.
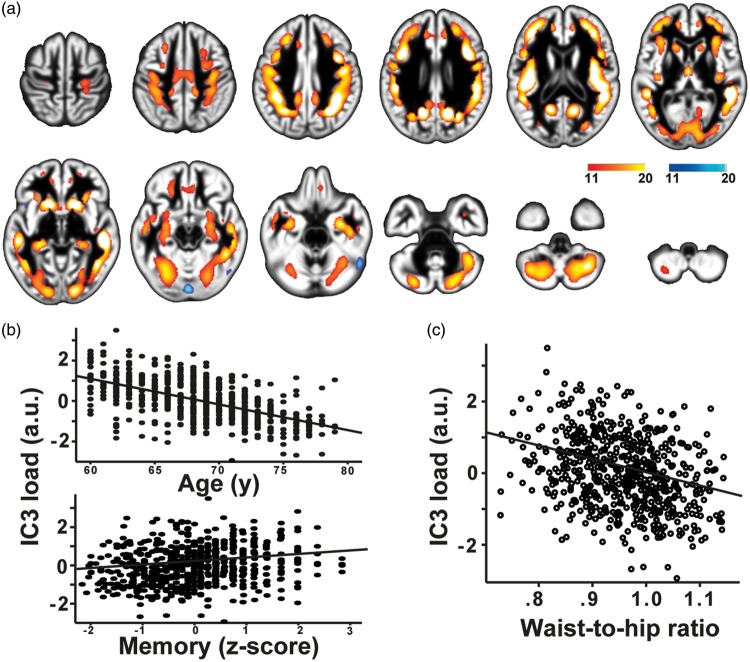
A strong negative association with age was present in IC3 (r = −0.58, p < 10−3), which was characterized by changes in GM volume predominantly within the fundus of the sulci in prefrontal, temporal and parietal regions, as well as in limbic and paralimbic areas and in the cerebellum (Figure 5(a) and (b)). Notably, this network had a good spatial agreement with the GM component in Douaud et al.3 (http://www.fmrib.ox.ac.uk/analysis/LIFO+AD+AOS/), showing late development and accelerated decline in aging, and with GM atrophy seen in AD (spatial correlation of Z-maps: r = 0.82, p < 10−3; r = 0.67 p < 10−3, respectively). Lower GM volume in this network correlated with worse memory performance in our sample (r = 0.192, p < 10−3 Figure 5(b)). In addition, lower GM was independently associated with higher WHR in this component (partial-r = −0.149, p < 10−3) (Figure 5(c)).
Figure 5.
Higher waist-to-hip ratio was associated with lower gray matter volume in a network of multimodal regions (IC3, a, c) that also correlated negatively with age and memory performance (b). Scatter plot shows the individual’s loading on the network and linear fit. Colors indicate positive (red/yellow) or negative (blue/light-blue) co-variations within the network (z > 11), maps are drawn on a standard brain.
Considering components covering parts of the cerebellum, GM volume in IC10 correlated with memory performance (r = 0.184, p < 10−3). In addition, IC10 was independently associated with lower blood pressure (partial-r = −0.129, p = 0.001), showing decreased GMV in the lateral cerebellum, including bilateral Crus I, part of Crus II, area VI, VII-b, and VIII-a.
Subsample analysis
Considering physical activity in the subgroup of 516 subjects, no independent significant associations with the GM networks were found. Additionally, controlling for physical activity did not change the pattern of above-described effects of cardiovascular risk factors and GM networks, as well as when excluding women on estrogen replacement therapy (n = 19) (data not shown).
In participants with anti-hypertensive medication (n = 325), higher blood pressure was associated with lower GM volume in both IC7 (partial-r = −0.176, p = 0.002) and IC10 (partial-r = −0.139, p = 0.013). In those without anti-hypertensive medication (n = 291), however, associations did not reach significance. In participants without anti-diabetic medication (n = 534), but not in those with (n = 82), higher HbA1c, similar to the whole sample analysis, was negatively associated with IC2 (partial-r = −0.159, p < 10−3) and IC5 (partial-r = −0.230, p < 10−3)
Discussion
In this large cross-sectional study, we identified unique associations of cardiovascular risk factors, independent from confounders, on major structural covariance brain networks in a well-characterized cohort of 616 healthy older adults. In two age-associated networks that covered most cortical areas, we detected lower GM volume and thickness in smokers, in participants with higher blood pressure, and in those with higher long-term glucose. Also, WHR was associated with lower GM volume in a multimodal, age- and memory-sensitive network, known to be affected in both normal aging and AD.3,40
Smoking
We observed a significant negative association between smoking and global networks IC2 and IC7, pointing to a negative impact of smoking throughout all areas of the neocortex. Our findings are in line with recent results of Karama,15 which linked smoking to widespread cortical thinning in a similarly large sample of older individuals (n = 504). Considering the pattern of GM covariance, these networks could be indicative of ubiquitous neuronal properties that are affected by smoking.41 This could be due to direct and indirect toxic effects of cigarette smoking, for example as shown in rodents after prenatal exposure to nicotine,42 or in humans with regard to chronic effects of cigarette smoking on cerebral perfusion.43
Blood pressure
Our results indicate that higher blood pressure exerts negative effects on GM volume in networks that covered most parts of the neocortex and cerebellum, with stronger associations in subjects taking anti-hypertensive medication. There is consistent evidence that higher blood pressure in mid-life is a risk factor for cognitive decline and AD,44 and medication intake could indicate a prolonged period of elevated blood pressure, leading to stronger effects of high blood pressure in this group. A meta-analysis of neuroimaging findings concluded that high blood pressure leads to lower GM volume, particularly in frontal and temporal lobes.13 Possible underlying mechanisms include cerebrovascular lesions due to chronic high blood pressure and GM loss.45
Glucose metabolism
Higher concentrations of the long-term marker of glucose metabolism, HbA1c, were associated with two networks of covariance mainly in cortical thickness. Considering the additive nature of components in our analysis, this indicates a negative impact of higher glucose on most parts of the neocortex with stronger effects in medial frontal, cingulate and temporal areas, in line with previous reports in young.46 Due to neurotoxic effects of glucose and accumulation of advanced glycation end products (AGEs),47 persistent episodes of hyperglycemia might have led to GM damage in subjects with higher long-term glucose. It could be speculated that regions of higher metabolism in young and higher Aß accumulation in older cognitively normal subjects48 would show stronger correlations; however, future studies that for example implement AGE-receptor-PET49 are needed to expand on these speculations.
Visceral obesity
We found an independent association of higher WHR and lower GM volume in IC3, covering multimodal cortices at the gray-to-white matter border as well as limbic regions. This finding is in line with previous studies that showed negative effects of obesity on regional and total GM volume in older cohorts,31,50 and extends previous reports that observed more severe changes when looking at visceral obesity measures in comparison to “crude” BMI.51 This might be due to the more severe negative effects of visceral adipose tissue compared to (gluetal-) subcutaneous fat, including a higher expression of proinflammatory cytokines.52 Chronic low-grade inflammation has been speculated to particularly harm myelination, thus affecting white matter tracts and intra-cortical axon collaterals.53 The network depicted by IC3 in our cohort has been previously linked to AD and described to display a “last-in-first-out” trajectory.3,40 These effects had been further ascribed to the myelination process of intracortical fibers depicted by the network.3 Therefore, our data-driven analysis supports the hypothesis that higher visceral fat, possibly through higher inflammatory activity, exerts detrimental effects on the late-myelinated GM. However, future studies combining imaging techniques capable of quantifying myelin content and AD pathology as well as more detailed measures of visceral fat-related inflammation are needed to test this hypothesis.
Lipid markers and physical activity
We did not observe robust association between markers of lipid metabolism or physical activity and GM networks. In our sample of healthy elderly, we observed higher HDL levels and far less individuals on anti-hyperlipidemic medication compared to others,16,54,55 rendering a low sensitivity to detect HDL-effects on GM structure in our cohort likely. Considering physical activity, longitudinal studies including older adults observed protective effects on GM volume and thickness,55–57 raising the possibility that our cross-sectional questionnaire (IPAQ short version) might not have been sensitive enough to capture these effects.58 Furthermore, accumulated evidence suggests a positive impact of leisure activities on cognitive function and lowered risk of AD.59,60 Specifically, in an elderly population, such as ours, use of standardized leisure activity questionnaire might better depict factors with possible beneficial effect on brain aging.
Limitations
We are unable to infer causal relationships due to the cross-sectional nature of our study, thus we cannot exclude that changes in GM structure were prior to the differences in cardiovascular risk factors. However, it has been suggested that modifiable risk factors at middle-age are a better predictor of structural decline and cognitive outcomes in later life,61 which potentially imply even stronger associations of risk factors on GM networks than seen here. The effects of smoking in our cohort could have been underestimated as result of cortical recovery after quitting smoking15 that might have led to a higher variance within our “previous smoker” group. Despite possible effects of further factors linked with higher cardiovascular risk, such as low “cognitive reserve” or depressive illness, on GM measures,62–65 we did not evaluate these conditions in detail. Strengths of the study include the large population-based sample and the data-driven multi-modal analysis of GM networks, instead of focusing on traditional voxel-wise associations in one modality. This systems-view could increase the interpretability of the effects in older populations on the brain, especially with regard to underlying mechanisms and potential preventive options.1,7,8
Conclusions
Using a large cohort of healthy older adults and a data-driven approach, we were able to replicate and further characterize large-scale, age-sensitive GM networks that inversely correlated with major cardiovascular risk factors, i.e. smoking, blood pressure, long-term glucose, and visceral obesity. The spatial extent and composition of covarying GM measures within the different networks indicated that smoking and, to a lesser degree, higher blood pressure affected GM throughout the brain, which might be attributed to direct and indirect damage of neuronal tissue. Higher HbA1c was found to predominantly affect areas that are known to have high glucose metabolism and early Abeta deposition. In addition, we detected negative effects of visceral obesity on a structural network covering areas rich in intracortical myelinated fibres, possibly pointing to detrimental effects of visceral fat-induced low-grade inflammation on myelin. This proposed mechanism might also help to better understand how a cardiovascular risk factor, in this case WHR, could be a trigger or booster of cognitive decline and regional AD pathology, as this network has specifically been linked to accelerated aging and vulnerability to AD in previous studies.3 Our additional observation of a negative correlation of both age and memory performance with IC3 further underlines the congruency and the functional relevance of this specific network. Future longitudinal studies including the LIFE follow-up data (starting in August 2017) or those that incorporate more detailed microstructural assessments are now needed to prove our hypotheses and to test if improving cardiovascular risk, specifically visceral obesity, would help to maintain the integrity of GM networks sensitive to aging throughout old age.
Supplementary Material
Acknowledgements
We thank all members of the LIFE study center for conducting the LIFE-Adult-Study as well as all participants for their valuable collaboration. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative, and LIFE–Leipzig Research Center for Civilization Diseases, University of Leipzig (project numbers 713-241202, 14505/2470, 14575/2470), and by the German Research Foundation (CRC1052 Obesity mechanisms Project A01 to AV and MS).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
SKM conducted data analysis, statistical analysis, and was involved in the interpretation of results and writing of the manuscript. ML, MLS, SGR-H, MS and TL were involved in study design, data collection and revision of the manuscript. FB and LL contributed to data analysis and manuscript revision. AV was involved in study design and revision of the manuscript. AVW contributed to analysis and interpretation of the data and writing of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci 2013; 14: 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brickman AM, Habeck C, Ramos MA, et al. A forward application of age associated gray and white matter networks. Hum Brain Mapp 2008; 29: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douaud G, Groves AR, Tamnes CK, et al. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A 2014; 111: 201410378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans AC. Networks of anatomical covariance. Neuroimage 2013; 80: 489–504. [DOI] [PubMed] [Google Scholar]
- 5.Raji CA, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging 2012; 33: 834.e7–834.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Gennatas ED, Kramer JH, et al. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73: 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 2011; 32: 1341–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron 2013; 77: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz N. Ageing and the Brain. Encycl Life Sci 2005, pp. 1–6. DOI: 10.1038/npg.els.0004063. [Google Scholar]
- 10.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016; 539: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman JI, Tang CY, de Haas HJ, et al. Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC Cardiovasc Imaging 2014; 7: 1039–1053. [DOI] [PubMed] [Google Scholar]
- 13.Beauchet O, Celle S, Roche F, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens 2013; 31: 1502–1516. [DOI] [PubMed] [Google Scholar]
- 14.Biessels GJ, De Leeuw F-E, Lindeboom J, et al. Increased cortical atrophy in patients with Alzheimer’s disease and type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2006; 77: 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karama S, Ducharme S, Corley J, et al. Cigarette smoking and thinning of the brain’s cortex. Mol Psychiatry 2015; 20: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villeneuve S, Reed BR, Madison CM, et al. Vascular risk and Aβ interact to reduce cortical thickness in AD vulnerable brain regions. Neurology 2014; 83: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 2016; 19: 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation 2009; 119: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walhovd KB, Storsve AB, Westlye LT, et al. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging 2014; 35: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 20.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 2013; 29: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffler M, Engel C, Ahnert P, et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015; 15: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 23.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007; 130: 2375–2386. [DOI] [PubMed] [Google Scholar]
- 24.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14: 21–36. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 27.Groves AR, Smith SM, Fjell AM, et al. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage 2012; 63: 365–380. [DOI] [PubMed] [Google Scholar]
- 28.Groves AR, Beckmann CF, Smith SM, et al. Linked independent component analysis for multimodal data fusion. Neuroimage 2011; 54: 2198–2217. [DOI] [PubMed] [Google Scholar]
- 29.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 2007; 9: 755–762. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 31.Kharabian Masouleh S, Arélin K, Horstmann A, et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging 2016; 40: 1–10. [DOI] [PubMed] [Google Scholar]
- 32.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008; 71: 430–438. [DOI] [PubMed] [Google Scholar]
- 33.Lewinsohn PM, Seeley JR, Roberts RE, et al. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997; 12: 277–87. [DOI] [PubMed] [Google Scholar]
- 34.UNESCO. International standard classification of education ISCED 1997, Geneva,Switzerland: Author, 1997. [Google Scholar]
- 35.Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci 2011; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang Y-F, Eldreth D, Erickson KI, et al. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging 2014; 35: 1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 38.Erickson KI, Colcombe SJ, Elavsky S, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging 2007; 28: 179–185. [DOI] [PubMed] [Google Scholar]
- 39.Henderson VW, Paganini-Hill A, Emanuel CK, et al. Estrogen replacement therapy in older women. Arch Neurol 1994; 51: 896. [DOI] [PubMed] [Google Scholar]
- 40.Fjell AM, Westlye LT, Grydeland H, et al. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex 2014; 24: 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durazzo TC, Meyerhoff DJ, Mon A, et al. Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-acetylaspartate and glutamate levels. Biol Psychiatry 2016; 79: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mychasiuk R, Muhammad A, Gibb R, et al. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res 2013; 1499: 53–60. [DOI] [PubMed] [Google Scholar]
- 43.Durazzo T, Meyerhoff D, Murray D. Comparison of regional brain perfusion levels in chronically smoking and non-smoking adults. Int J Environ Res Public Health 2015; 12: 8198–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13: 788–794. [DOI] [PubMed] [Google Scholar]
- 45.Veglio F, Paglieri C, Rabbia F, et al. Hypertension and cerebrovascular damage. Atherosclerosis 2009; 205: 331–341. [DOI] [PubMed] [Google Scholar]
- 46.Weinstein G, Maillard P, Himali JJ, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 2015; 84: 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta 2017; 1863: 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh H, Madison C, Baker S, et al. Dynamic relationships between age, amyloid-β deposition, and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain 2016; 139: 2275–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cary BP, Brooks AF, Fawaz MV, et al. Synthesis and evaluation of [18F]RAGER: a first generation small-molecule PET radioligand targeting the receptor for advanced glycation endproducts. ACS Chem Neurosci 2016; 7: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing Res Rev 2015; 20: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol 2010; 68: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swarbrick MM. A lifetime on the hips: programming lower-body fat to protect against metabolic disease. Diabetes 2014; 63: 3575–3577. [DOI] [PubMed] [Google Scholar]
- 53.Cardoso FL, Herz J, Fernandes A, et al. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation 2015; 12: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leritz EC, Salat DH, Williams VJ, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 2011; 54: 2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walhovd KB, Storsve AB, Westlye LT, et al. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging 2014; 35: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 56.Benedict C, Brooks SJ, Kullberg J, et al. Association between physical activity and brain health in older adults. Neurobiol Aging 2013; 34: 83–90. [DOI] [PubMed] [Google Scholar]
- 57.Flöel A, Ruscheweyh R, Krüger K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage 2010; 49: 2756–2763. [DOI] [PubMed] [Google Scholar]
- 58.Heesch KC, van Uffelen JG, Hill RL, et al. What do IPAQ questions mean to older adults? Lessons from cognitive interviews. Int J Behav Nutr Phys Act 2010; 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HX, Xu W, Pei JJ. Leisure activities, cognition and dementia. Biochim Biophys Acta 2012; 1822: 482–491. [DOI] [PubMed] [Google Scholar]
- 60.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348: 2508–2516. [DOI] [PubMed] [Google Scholar]
- 61.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011; 77: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012; 11: 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grieve SM, Korgaonkar MS, Koslow SH, et al. Widespread reductions in gray matter volume in depression. Neuroimage Clin 2013; 3: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benedict C, Byberg L, Cedernaes J, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement 2015; 11: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 65.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 2013; 16: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.