Abstract
Agents that augment cerebral blood flow (CBF) could be potential treatments for vascular cognitive impairment. Phosphodiesterase-5 inhibitors are vasodilating drugs established in the treatment of erectile dysfunction (ED) and pulmonary hypertension. We reviewed published data on the effects of phosphodiesterase-5 inhibitors on CBF in adult humans. A systematic review according to PRISMA guidelines was performed. Embase, Medline and Cochrane Library Trials databases were searched. Sixteen studies with 353 participants in total were retrieved. Studies included healthy volunteers and patients with migraine, ED, type 2 diabetes, stroke, pulmonary hypertension, Becker muscular dystrophy and subarachnoid haemorrhage. Most studies used middle cerebral artery flow velocity to estimate CBF. Few studies employed direct measurements of tissue perfusion. Resting CBF velocity was unaffected by phosphodiesterase-5 inhibitors, but cerebrovascular regulation was improved in ED, pulmonary hypertension, diabetes, Becker's and a group of healthy volunteers. This evidence suggests that phosphodiesterase-5 inhibitors improve responsiveness of the cerebral vasculature, particularly in disease states associated with an impaired endothelial dilatory response. This supports the potential therapeutic use of phosphodiesterase-5 inhibitors in vascular cognitive impairment where CBF is reduced. Further studies with better resolution of deep CBF are warranted. The review is registered on the PROSPERO database (registration number CRD42016029668).
Keywords: Cerebral blood flow, dementia, phosphodiesterase-5 inhibitors, small vessel disease, vascular cognitive impairment
Introduction
Cerebral small vessel disease (SVD) is present in up to 70% of older adults1 and is the commonest cause of vascular cognitive impairment, which contributes up to 20% of dementia diagnoses.2,3 The overall clinical impact of SVD is significant, affecting cognition, mood, function and quality of life in older people.4–7 There are no licensed symptomatic treatments for vascular cognitive impairment.3,8 Furthermore there are no licensed disease modifying agents for SVD, with current interventions limited to controlling risk factors for vascular disease in general.3,4,8
Cognition declines over time as SVD advances.2,9–11 Cerebral blood flow (CBF) is reduced in SVD in grey and white matter, with particularly severe changes in subcortical areas.1,12–14 Whether reduced CBF is a cause or a consequence of SVD is unclear. This issue is complex to resolve because standard magnetic resonance imaging (MRI) sequences may not be sufficiently sensitive to detect the earliest pathological changes in SVD.13 15 However, reduced CBF may occur prior to the onset of clinical symptoms of Alzheimer's disease (AD),16 and is the earliest observed neurological abnormality in mouse models of AD.17 The mechanisms by which reduced CBF might accelerate SVD pathogenesis are unclear, but reduced protein synthesis is a well-described effect of mild degrees of cerebral ischaemia.18
CBF regulation is dependent on intact endothelial and myocyte function in small penetrating arteries, which facilitates increased regional blood flow in response to demand, such as during cognitive activity.17 This process can be termed cerebrovascular regulation (CVR). Impaired CVR may be associated with vascular risk factors and vascular disease in general.19,20 However, impairment of endothelium-dependent CVR is postulated to be a specific pathological feature of SVD.21–24 Although data are limited there is some evidence that impaired CVR correlates with the burden of white matter hyperintensities, a key radiological marker of SVD.25 Similarly, haemodynamic pulsatility, a measure of vessel stiffening, has also been shown to strongly correlate with the degree of leukoaraiosis.26 Improving CBF and CVR by augmenting endothelial-myocyte signalling (and thus improving function of the neurovascular unit) therefore has potential as both symptomatic and disease-modifying treatment for SVD.8
Phosphodiesterase-5 (PDE5) inhibitors (PDE5i) are used in the treatment of pulmonary hypertension (PH) and erectile dysfunction (ED). Inhibition of PDE5 reduces breakdown of cyclic guanosine monophosphate (cGMP), leading to vascular smooth muscle relaxation in small blood vessels, as for example in the treatment of ED where PDE5i delay de-tumescence.27 In PH PDE5 is more active and expressed more abundantly in the pulmonary vasculature.28,29 PDE5i reduce this heightened activity (sildenafil increasing cGMP levels 5- to 10-fold) leading to vasodilation. In PH there appear to be further downstream effects, PDE5i leading to reduced DNA synthesis in myocytes and reduced smooth muscle proliferation.29
PDE5 mRNA and protein are expressed in brain tissue of humans and experimental animals.30–33 Western blot detects PDE5 in both meningeal and larger cerebral arteries of experimental rodents and human participants34,35 and immunohistochemical labelling shows that PDE5 is present in smooth muscle cells of small arterial vessels.36 PDE5 activity in SVD has not been reported. This raises the question whether PDE5i could augment vasodilation of cerebral blood vessels, and hence increase CBF and/or restore CVR in SVD.
The aim of this review is to synthesise published data from human studies on the effects of PDE5i on CBF and CVR in adults.
Materials and methods
Protocol: PRISMA guidelines were followed (please see the PRISMA checklist online Supplement).
Eligibility criteria and study selection: randomized clinical trials and observational studies investigating the effects of PDE5i on CBF in adult humans were considered for inclusion. Abstracts were considered if data were presented. All imaging modalities for directly or indirectly measuring CBF and CVR were included. Only papers available in English were included. Publications were excluded if they had no adult human data, did not use selective PDE5i, or had no data on measurements of CBF. Case reports presenting data from single subjects were excluded. For excluded studies please see Supplement Table 1.
Information sources: (1) Embase, Medline and the Cochrane Library database searches were performed initially up to November 2016, and then repeated to check for new publications on 4 August 2017. Results were reviewed independently by MMHP and JDI. (2) Reference lists of all included studies, as well as of relevant non-eligible studies were checked.
Search strategies can be found in the online Supplement SII.
Data extracted from individual studies were: participant characteristics, presence or absence of a control group, intervention (drug name, dose, duration and route of administration; any comparisons between resting state and other conditions), assessment modalities and outcomes.
Risk of bias in individual studies was assessed using the Cochrane bias assessment tool.
A qualitative narrative synthesis was performed because the very heterogeneous studies and data were unsuitable for meta-analysis.
Results
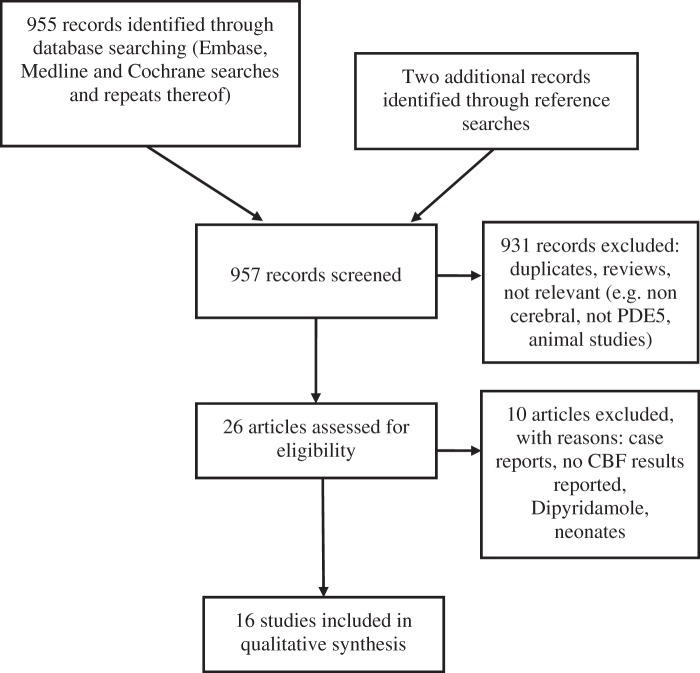
Sixteen studies with a total of 353 participants were included in the review (for PRISMA diagram see Figure 1). Summaries of the included papers are given in Table 1. Regarding the included studies: six studies were double blind randomised controlled studies (n = 103 participants).37–42 One was a randomised non-blinded study (n = 30), the two participant groups receiving two different medication protocols.43 Two studies with a total of 68 participants were controlled but not randomised or blinded.44,45 Seven observational studies with a total of 152 participants were included.46–52 Details of risk of bias are displayed in Table 2.
Figure 1.
PRISMA flow diagram; PDE5: phosphodiesterase 5; CBF: cerebral blood flow.
Table 1.
Overview of included studies.
| Study | Participants | Methods | Interventions | Endpoints | Outcomes |
|---|---|---|---|---|---|
| Al-Amran et al.44 | Diabetes vs. healthy males, 33–58 yrs old, N = 35 | Controlled trial, non blinded | Sildenafil (50 mg, once only, orally) | 50 min after dose: TCD to assess VMCA, side not specified (rest, breath holding and hyperventilation tests); CVR; FVD | No changes in VMCA. Sildenafil resulted in significant increase in CVR (from 0.74 ± 0.14 to 1.03 ± 0.14) and FVD (from 60.2 ± 4.96% to 74 ± 4.8%, p < 0.05). |
| Arnavaz et al.37 | Healthy males, 21–32 years old, N = 6 | Double blind, placebo controlled cross-over | Sildenafil (100 mg, once only orally) vs. placebo | TCD to assess VMCA, right hand side, at 30 and 60 min after dose | No differences / change (all velocities around 62 cm/s); no SEs (no headaches, no dizziness) |
| Chan et al.46 | Healthy adults (3 female), 34–60 years old, N = 10 | Observational, altitude study | Sildenafil (50 mg, once on day 1, once on day 3, orally) | TCD to assess VMCA, right hand side; Regional O2 saturation (rSaO2)) at 1 h and 2 h after dose (both 1 & 3 days after rapid ascent to 3480 m) | Day 1: SaO2 (83.9 ± 0.5 at baseline, 85.3 ± 0.4 at 1 h (p < 0.0001), 85.0 ± 0.5 at 2 h (p < 0.01) (all %)) and cerebral oxygenation (59.3 ± 1.3 at baseline, 62.7 ± 0.8 at 1 h ((p < 0.05), 65.3 ± 0.9 at 2 h (p < 0.0001) (all %)) Day 3: no change in SaO2 (baseline and post intervention values 86.7 ± 0.4 to 88.4 ± 0.5 (%)) but cerebral SaO2 increased (61.7 ± 0.9 at baseline, 65.0 ± 1.0 at 1 h ((p < 0.0001), 64.0 ± 0.9 at 2 h (p < 0.001), VMCA 65.3 ± 1.8 cm/s at baseline to 61.3 ± 1.5 cm/s (p < 0.01) at 1 h and 60.9 ± 1.7 cm/s (p < 0.0001) at 2 h). |
| Dhar et al.51 | Vasospasm post SAH (3 female), 40–74 years old, N = 6 | Observational | Sildenafil 30 mg i.v. over 30 min, 9 ± 2 days after SAH | 15O2-PET imaging 15 min after infusion Intracranial pressure Mean arterial pressure | ICP was unchanged; no change in global CBF (34.5 ± 7 ml/100 g/min at baseline vs. 33.9 ± 7 ml/100 g/min, p = 0.84) |
| Diomedi et al.38 | ED, 57.2 ± 8.4 and 56.6 ± 8.7 years old, N = 28 | Double blind placebo controlled trial | Sildenafil (50 mg, once only, orally) vs. placebo | TCD to assess VMCA, bilateral (rest and breath holding) 1 h after dose CVR | Sildenafil resulted in higher CVR vs. baseline condition (1.61 ± 0.45 vs. 1.37 ± 0.33; p = 0.02) and also vs. placebo (1.61 ± 0.45 vs. 1.39 ± 0.30; p = 0.04). |
| Jahshan et al.52 | Healthy males, 34 ± 2 years old, N = 14 | Observational | Sildenafil (100 mg, once only, orally) | TCD to assess VMCA, side not specified, 1 h after sildenafil; (rest, hyperventilation and hypercapnia, and during boluses of phenylephrine (25–200 µg), and during graded head-up tilting) End-tidal CO2 Blood pressure Electrocardiogram | Sildenafil attenuated the VMCA decrease to hyperventilation (36 ± 2.5 vs. 42 ± 2.5 cm/s, p < 0.05). Estimated cerebrovascular resistance was reduced in both conditions (1.32 ± 0.1 vs. 1.14 ± 0.07 on hypercapnia, 2.5 ± 0.2 vs. 1.95 ± 0/15 in hyperventilation, both p < 0.05) Sildenafil did not have effects on blood pressure increases elicited by phenylephrine nor on VMCA decreases due to head-up tilting. |
| Kruuse et al.39 | Healthy adults, 20–31 years old (4 female), N = 10 | Double blind cross-over trial | Sildenafil (100 mg, once only, orally) vs. placebo | TCD to assess VMCA, bilateral headache scores. Both every 15 min after dose for 2 h SPECT imaging at 60 min and 120 min | No effect on VMCA or regional CBF. No dilation of temporal or radial arteries. Headaches were induced with sildenafil. |
| Kruuse et al.40 | Migraine (all female), 37.3 ± 3.2 years old, N = 12 | Double blind cross-over trial | Sildenafil (100 mg, once only, orally) vs. placebo; 1 day washout | TCD to assess VMCA, bilateral Radial and temporal artery diameter Headache scores All above at baseline and every 15 min after dose for 3 h; SPECT imaging at baseline, 60 and 120 min post dose | No difference in VMCA Regional CBF and global CBF did not differ between treatment groups. No dilation of temporal or radial arteries. (No difference in pCO2) 10/12 after sildenafil vs. 2/12 after placebo got a headache. |
| Kruuse et al.47 | Healthy females, 23 ± 3 years old, N = 13 | Observational | Sildenafil (100 mg, once each visit, orally), 2 visits at least 1 week apart | fMRI Visual-evoked potentials (reversing checkerboard visual stimulus and hypercapnia) Both at 1 and 2 h post dose | No difference in responses was detected post sildenafil (both hypercapnia and visual stimulation elicited strong and consistent responses) |
| Lindberg et al.42 | Becker muscular dystrophy, 25–57 years old, N = 12 | Double blind cross-over trial | Sildenafil (20 mg TDS for 4 weeks) vs. placebo (TDS for 4 weeks), 2 week washout period | MRI (fMRI, ASL and MR angiography) BOLD imaging at end of each treatment period at similar time of day (interval in minutes or hours from last dosing not specified) | MRI BOLD responses were significantly increased after sildenafil vs. placebo (placebo: 0.16 ± 0.03, sildenafil: 0.38 ± 0.08, p = 0.0042) but not vs. baseline (baseline: 0.25 ± 0.09, NS) in patients with BMD. Such differences were also found in the BOLD visual response (sildenafil: 1.61 ± 0.20% vs. placebo 1.12 ± 0.16% (p < 0.03) vs. baseline: 1.67 ± 0.19% (NS)) No change in MCA diameter (MRA) No change in basal CBF (ASL MRI) or CO2 reactivity |
| Lorberboym et al.48 | ED, 43–73 years old, N = 25 (n = 12 with PMH of stroke) | Observational | Sildenafil (50 mg once only, orally) | SPECT imaging 1 h post dose | Stroke patients showed more areas with diminished perfusion after sildenafil, and fewer areas with improved perfusion. Patients with vascular risk factors but no stroke had increased perfusion after sildenafil. |
| Lorberboym et al.43 | Stroke and ED, 41–75 years old, N = 30 | Randomised observational study | Tadalafil (20 mg once only or 5 mg OD for 1 week, both orally) | SPECT imaging 6 h post last / single dose | All had areas of reduced relative regional CBF in the affected hemisphere and some other areas after tadalafil. No significant difference was found between groups. |
| Mukherjee et al.49 | Vasospasm post SAH (gender not specified), 18–60 years old, N = 72 | Observational | Sildenafil (100–150 mg 4 hourly, i.v.) for 2–7 days | TCD to assess VMCA, side not specified (2 hourly) for 2–7 days | 8 sustained responders (≥40 cm/s decrease in MCV for ≥48 h), 4 transient responders |
| Rosengarten et al.45 | Pulmonary hypertension (6 female), 28–70 years old, N = 33 (11 patients, 22 controls) | Controlled study | Sildenafil (50 mg, once only, orally) and iloprost (2.8 mcg total dose, inhaled over 4 min); 2 h between iloprost and sildenafil Controls (did not receive medication) | TCD to assess VPCA, side not specified (visual stimulation), measured 1 h after sildenafil, exact interval not specified for iloprost Right heart catheterization | Attenuation parameter: baseline in pulmonary hypertension 0.55, CI 0.41-0.76, p = 0.02 for difference vs. controls; after sildenafil: 0.45; CI 0.34–0.54 vs. in controls 0.41; CI 0.36–0.49; p = 0.59); Rate parameter (pulmonary hypertension after sildenafil: 4.2 s; CI 2.6–5.6 s vs. in controls 2.8 s; CI 2.0–3.9 s; p = 0.32; baseline value in pulmonary hypertension only depicted graphically). Both substances lead to significant reduction of pulmonary arterial pressure and vascular resistance, as well as VPCA 60.6 cm/s (57.1–66.5) vs. 44.5 cm/s (38.3–51.3), p < 0.0001 |
| Van Osta et al.41 | Healthy adults: altitude reaction (6 female), 26–58 years old, N = 35 | Randomised double blind trial | Tadalafil (10 mg BD orally) vs. dexamethasone (8 mg BD orally) vs. placebo (BD orally), all for about 4 days in total | TCD to assess VMCA, right or left depending on signal quality, and ARI (as measure of CVR) after thigh cuff release; pCO2, pO2 and SaO2 Blood pressure (all assessed at 490 m and 4559 m) | Neither tadalafil nor dexamethasone had a significant effect on altitude VMCA or ARI, nor did average VMCA or ARI change with altitude. |
| Washington et al.50 | Vasospasm post SAH (6 female), 38–83 years old, N = 12 | Observational | Sildenafil (10 mg i.v. n = 5 & 30 mg i.v. n = 7, both once only) | Cerebral digital subtraction angiography (pre and 30 min post infusion) Neurological examination and vital signs monitored | 8 of 12 patients (67%) had angiographic improvement in vasospasm, with 3 of 5 (60%) from the low dose group and 5 of 7 (71%) from the high dose group (on average, 0.8 mm (62%) increase in vessel diameter in the most responsive vessel). One patient improved clinically (resolution of pronator drift). No adverse events. |
Table 2.
Assessment of risk of bias using Cochrane risk of bias assessment tool (low risk versus high risk versus unclear), max. score 8 (higher score meaning a lower risk of bias).
| Study | Score (out of 8) | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of study personnel | Assessor blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|---|---|
| Al-Amran et al.44 | 3 | High | High | High | High | High | Low | Low | Low |
| Arnavaz et al.37 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Chan et al.46 | 2 | High | High | High | High | High | Low | Low | High |
| Dhar et al.51 | 2 | High | High | High | High | High | Low | Low | High |
| Diomedi et al.38 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Jahshan et al.52 | 3 | High | High | High | High | High | Low | Low | Low |
| Kruuse et al.39 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Kruuse et al.40 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Kruuse et al.47 | 3 | High | High | High | High | High | Low | Low | Low |
| Lindberg et al.42 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Lorberboym et al.48 | 1 | High | High | High | High | High | Unclear | High | Low |
| Lorberboym et al.43 | 3 | Unclear | High | High | High | High | Low | Low | Low |
| Mukherjee et al.49 | 2 | High | High | High | High | High | Low | Unclear | Low |
| Rosengarten et al.45 | 2 | High | High | High | High | High | Low | Low | High |
| Van Osta et al.41 | 8 | Low | Low | Low | Low | Low | Low | Low | Low |
| Washington et al.50 | 2 | High | High | high | High | Unclear | Low | Low | High |
In addition to effects on CBF, we assessed papers for other reported effects of PDE5i. No cognitive assessments were performed in any of the trials, but no detrimental effects on cognition were reported. One trial in subarachnoid haemorrhage (SAH) reported a clinical improvement in one patient with resolution of pronator drift after administration of PDE5i.50 Reported side effects were consistent with the known effects of PDE5i, including headache, flushing and visual disturbance.49
A summary of the outcome measures used in the various studies is shown in Table 3.
Table 3.
Summary of outcome measures used in each included study – (indicated by √).
| Study | Summary | MCA velocity | Cerebrovascular reactivity | pCO2 | SPECT | Other |
|---|---|---|---|---|---|---|
| Al-Amran et al.44 | N = 18 diabetics N = 17 controls | √ | √ (TCD) | |||
| Arnavaz et al.37 | N = 6 | √ | ||||
| Chan et al.46 | N = 10 | √ | √ regional O2 Saturation | |||
| Dhar et al.51 | N = 6 | √ PET imaging | ||||
| Diomedi et al.38 | N = 14 sildenafil N = 14 placebo | √ | √ (TCD) | √ | ||
| Jahshan et al.52 | N = 14 | √ | √ (TCD) | √ | ||
| Kruuse et al.39 | N = 12 | √ | √ | |||
| Kruuse et al.40 | N = 12 (cross-over) | √ | √ | √ | ||
| Kruuse et al.47 | N = 13 | √ (fMRI) | √ | |||
| Lindberg et al.42 | N = 12 | √ (fMRI) | √ | √ MRI (ASL, MRA and MCA dimension) | ||
| Lorberboym et al.48 | N = 25 | √ | ||||
| Lorberboym et al.43 | N = 30 | √ | ||||
| Mukherjee et al.49 | N = 72 | √ | ||||
| Rosengarten et al.45 | N = 33 | √ | √ (TCD) | √ | √ right heart catheterization | |
| Van Osta et al.41 | N = 35 | √ | √ (TCD) | √ | ||
| Washington et al.50 | N = 12 | √ cerebral angiography | ||||
| N = 257 | N = 170 | N = 147 | N = 79 |
Healthy individuals
Four studies investigated the effect of sildenafil on CBF in healthy individuals. In two, sildenafil was compared to placebo in a double blind crossover design. The measure of CBF was mean blood flow velocity in the middle cerebral artery (VMCA) in one study,37 and an average of maximal velocities in the other,39 both determined by transcranial doppler imaging (TCD). Neither trial found changes in resting VMCA. In one of these studies39 single-photon emission computed tomography (SPECT) imaging with Xenon 133 inhalation was also used to assess regional CBF at rest, with no difference between sildenafil and placebo. In a later study using functional magnetic resonance imaging (fMRI), sildenafil did not affect changes in CVR triggered by hypercapnia and visual stimuli.47
In contrast, a study with 14 healthy male volunteers found that 1 h after sildenafil (100 mg) CVR was improved, in that VMCA decreases associated with hyperventilation were attenuated. Estimated cerebrovascular resistance (calculated as mean blood pressure divided by mean VMCA) was reduced in both the hypercapnia and hyperventilation (i.e. hypocapnia) conditions.52
Migraine
A double blind crossover trial40 investigated the effect of sildenafil on resting VMCA as well as resting global and regional CBF (using SPECT) in 12 migraineurs. Radial and temporal artery diameters were also measured. Ten subjects experienced a migrainous headache after sildenafil, and only two subjects after placebo. There were no changes in global or regional CBF, VMCA, or in radial or temporal artery diameter.
High altitude
In a double blind trial 35 healthy individuals were randomly allocated to tadalafil 10 mg twice daily, dexamethasone 8 mg twice daily or placebo.41 VMCA and an Acute Mountain Sickness (AMS) score were measured at an altitude of 490 m and approximately 20 h after a two-day ascent to 4559 m. The cerebral autoregulation index (ARI) was used to assess CVR. ARI was calculated from the rate of restoration of VMCA following an acute spell of hypotension induced by the sudden release of bilateral thigh cuffs inflated to 30 mmHg above systolic blood pressure for 3 min.53 Neither tadalafil nor dexamethasone had a significant effect on VMCA or ARI. AMS score improved with dexamethasone and tended to be better with tadalafil than placebo.
In an uncontrolled study (n = 10) sildenafil 50 mg was given one and three days after rapid ascent to 3480 m.46 Measurements were taken prior to dosing and at 1 and 2 h post-dosing on each day. On day one, systemic oxygen saturation (SaO2) and cerebral oxygenation increased after sildenafil, with unchanged VMCA and end-tidal partial pressure of carbon dioxide (pCO2).
On day three, systemic SaO2 did not change after sildenafil, but cerebral oxygenation again increased despite a reduction in VMCA.
Diabetes mellitus
One study investigated 18 subjects with type two diabetes mellitus and 17 age-matched healthy controls.44 Subjects with a history of cerebrovascular disease were excluded. VMCA, cerebrovascular reactivity assessed using breath holding54 and full range of vasodilation (FVD) were measured 50 min after a single dose of sildenafil (50 mg). FVD was derived from a comparison between breath holding (leading to increased pCO2 and vasodilation, the parameters commonly used to assess cerebrovascular reactivity) and hyperventilation (leading to decreased pCO2 and vasoconstriction);54 (see Table 4 for formulae for cerebrovascular reactivity and FVD). VMCA was significantly increased by breath holding and decreased by hyperventilation in both the diabetic and healthy control groups, but the response was attenuated in those with diabetes. While these responses did not vary following sildenafil administration in the healthy control group, the diabetic group showed significant improvements in cerebrovascular reactivity and FVD with sildenafil.
Table 4.
Cerebrovascular reactivity and full range of vasodilation (FVD) formulae.
| Parameter | Formula |
|---|---|
| Cerebrovascular reactivity | (VMCA hypercapnia − VMCA baseline) / VMCA baseline |
| FVD | 100 × [VMCA hypercapnia − VMCA hypocapnia]/ VMCA rest |
VMCA: middle cerebral artery velocity.
ED without known cerebrovascular disease
One double-blind randomised controlled trial compared sildenafil (50 mg) with placebo38 in 28 men with ED but no history of cerebrovascular disease. The measure of CVR used was cerebrovascular reactivity (VMCA response to breath holding). No difference was found in resting VMCA 1 h post-dosing with sildenafil compared to placebo. However, sildenafil significantly improved cerebrovascular reactivity.
ED with stroke and/or SVD
Two studies investigated the effects of PDE5i on CBF in patients with cerebrovascular disease and ED.43,48 Both used pre- and post-dose SPECT imaging to assess regional CBF. The initial study48 enrolled 25 male patients with ED, of whom 12 had a history of ischaemic stroke 1–5 years prior to study participation. Among the other 13 participants, eight had non-territorial focal areas of diminished CBF on baseline SPECT, and five had diffuse CBF abnormalities suggestive of SVD. The extent and exact location of white matter intensity lesions were not reported. One hour after oral administration of sildenafil (50 mg) the post-stroke group showed more areas with relatively diminished perfusion, and fewer areas of relatively increased perfusion, compared to non-stroke patients, although there was variation between subjects within the group. It was not possible to identify a clear pattern from the Brodman areas that were listed as showing a significant difference (not localised to one hemisphere or a particular region).
In the second study43 all participants had ED and a history of large vessel ischaemic (n = 6 right MCA, n = 9 left MCA) or small vessel ischaemic stroke (n = 15; all participants had more than one lesion) that had occurred 3–36 months prior to the study. Lesion volumes were not reported. Participants were randomised to either single dose tadalafil (20 mg) or daily dosing of tadalafil (5 mg) for seven days. SPECT was performed at baseline and 6 h after the final (or single) dose.
Reduced relative CBF was found in the stroke-affected hemisphere as a whole versus the unaffected hemisphere after tadalafil, in both treatment arms, particularly in the zones bordering the infarct. However, smaller cortical and subcortical areas showed mixed effects (with areas of increased and decreased flow on either side), with no clear region or side-specific pattern. No significant difference was found between the two stroke groups (small vessel versus large vessel) or the two dosing regimens.
Subarachnoid haemorrhage
Three uncontrolled studies investigated the effects of sildenafil during the acute phase of SAH.49–51 One study49 enrolled 72 patients with vasospasm refractory to 24 h of hypertension, hypervolaemia and haemodilution (HHH) therapy. All patients had had their aneurysms surgically secured and had received nimodipine. Following administration of 100–150 mg of intravenous sildenafil every 4 h (for 2–7 days) eight showed sustained (>48 h) reduction in VMCA (>40 cm/s), and five had intermittent reductions.
In another study50 12 patients with angiogram proven vasospasm received intravenous sildenafil (10–30 mg). Angiography was repeated 30 min after infusion. Eight patients had an improvement in vasospasm.
In a further study 15O2-positron emission tomography (PET) imaging was performed pre- and post-intravenous sildenafil in six patients with digital subtraction angiography proven SAH.51 No changes in regional or global CBF were found after sildenafil.
None of these studies had a control group or control condition.
Pulmonary hypertension
In 11 adults with severe PH, inhaled iloprost and 50 mg oral sildenafil were administered consecutively (with a washout period).45 Flow velocity of the posterior cerebral artery (VPCA) was recorded at baseline (where it was significantly lower in those with PH versus controls), and in response to a visual stimulation paradigm. Pulmonary arterial pressure and systemic vascular resistance were also assessed. The 22 healthy control participants underwent the visual stimulation and VPCA measurements but were not given the study medication and did not have right heart catheterisation. In subjects with PH both agents reduced pulmonary and vascular resistance parameters. The attenuation parameter (which represents dampening of flow variation due to the vessel wall) and the time rate parameter (which represents the initial steepness of CBF velocity increase) were also assessed. Sildenafil administration was associated with normalization of both of these in PH. In contrast, iloprost slightly worsened the time rate parameter.
Becker muscular dystrophy (BMD)
In BMD the dystrophin protein is absent or truncated leading to loss of neuronal nitric oxide synthase (nNOS) and nitric oxide (NO) production, causing functional ischaemia in skeletal muscle, and possibly also in cerebral vascular myocytes.42 A randomised double blind cross-over trial of 12 participants with BMD compared the effect of sildenafil with placebo in a four-week treatment block.
CVR in response to sensory stimulation was examined using blood oxygenation-level dependent (BOLD) functional MRI. Compared to placebo, sildenafil significantly increased BOLD signal in the somatosensory cortex in response to median nerve stimulation. Increased BOLD response was also observed in the visual cortex following a flickering checkerboard stimulus.
MCA circumference and CO2 reactivity, measured using MR phase contrast mapping and MR angiography, were unaffected by sildenafil.
Discussion
Summary of findings
In the studies retrieved resting CBF was not affected by PDE5i in health or disease states37–42,44,45,51 with the possible exception of SAH.49,50 In contrast, CVR in states of acute challenge, such as hyperventilation or stimulatory tasks, was improved by PDE5i. This was observed in clinical conditions associated with impaired endothelial vasodilatory responses,38,42,44,45 and also in one study of healthy volunteers.52
Potential mechanisms
Vascular smooth muscle tone regulation is complex, with inputs from neurons, glia, interneurons and perivascular nerves.55,56 Contractile messengers include the sympathetic neurotransmitters norepinephrine and neuropeptide Y. Sympathetic stimulation via beta-adrenoceptors augments L-type calcium channel activity. Relaxant mediators include the parasympathetic transmitter acetylcholine, vasoactive intestinal peptide, calcitonin gene-related peptide and substance P. Many messengers converge on a key signalling pathway: endothelial derived NO.55,56 NO activates soluble guanylate cyclase in myocytes, which converts guanosine triphosphate to cGMP. As cGMP concentration increases, smooth muscle tone is reduced and the vessel dilates.55–57
Altered NO-cGMP signalling has been shown to be a feature of PH29 and ED,27 and is augmented in both conditions by PDE5i treatment. A similar mechanism may also apply to the cerebral circulation. This is supported by the fact that endothelial cells have an important role in CBF regulation.22,25 Vessel wall morphology and function are affected in SVD.22,58,59 The PDE5i-mediated improvement in CVR noted here, in individuals with diseases affecting vascular function38,44,45,48 may reflect augmented responsiveness of the endothelium and neurovascular unit to local metabolic or neurogenic stimuli.
The study investigating BMD42 supports this theory, describing ‘functional ischaemia’ due to reduced function of nNOS, rather than endothelial NOS (eNOS), and showing augmented responses to stimuli following treatment with sildenafil (see below).42 Although a different subtype of NOS is implicated, these findings support a role for the NO-cGMP signalling pathway in CVR.
In PH, where endothelial PDE5 is expressed more abundantly than in healthy lung,29,60 the vasodilatory effects of PDE5i (leading to reductions in pulmonary arterial pressure) are well-described.27,61–64 Furthermore, PDE5i attenuate vascular remodelling and right ventricular hypertrophy, as well as improving functional status in PH.29,60,65,66
It is interesting that a similar response was found in one study in healthy males assessed by TCD in response to hypercapnia and hypocapnia,52 but not in a study of healthy females using fMRI,47 or in the healthy control group in another study.44 Differences in imaging modalities and different sildenafil doses (100 mg in the first two of these studies, 50 mg in the third) may explain these divergent findings. The effectiveness of the hypercapnic stimulus may have varied between studies; only Jahshan et al.53 confirmed a rise in pCO2 and were also the only authors to report an effect of PDE5i on CVR in healthy volunteers. Furthermore, all three of these studies had small sample sizes.
SVD is also associated with proximal vessel stiffness and pulsatility; increased MCA pulsatility has been proposed to damage the microvasculature by transmitting higher pressure differences between systole and diastole to the cerebral small arteries.26 Of the studies here, only one investigated haemodynamic pulsatility, using PCA attenuation parameter (a measure of dampening due to vessel wall attenuation) and time rate parameter (representing the initial steepness of blood velocity increase).45 Both measures were improved by sildenafil in participants with PH. This raises the question whether PDE5i might delay the progression of SVD by mitigating the deleterious effects of raised large artery pulsatility on the small vessels.
The lack of change in basal CBF following PDE5i, in the papers retrieved here, may reflect the absence of a stimulus. Another explanation is that the techniques used were insufficiently sensitive to detect modest changes in resting CBF in relevant brain regions (subcortical nuclei and white matter).
Among the included studies, 10 used TCD to measure CBF velocity in the middle cerebral artery (VMCA37–41,44,46,49,52) or posterior cerebral artery (VPCA45). Although TCD is non-invasive, affordable, portable and readily repeatable, and gives ‘real time’ readings of flow velocity, it has important limitations. TCD assesses flow velocity in one large vessel only, and thus only indirectly the overall blood supply to the brain as a whole. Importantly, it does not report on the smaller, deep brain vessels implicated in SVD, and the distribution of flow across these. Further, it is likely incorrect to assume that constant VMCA implies no change in blood volume delivered to the brain. Specifically, this inference must assume a constant diameter of the vessel measured. In contrast, in patients with SAH, decreased VMCA is interpreted as MCA dilatation reflecting greater global CBF.49,50 Even very small changes in vessel diameter imply very significant changes in flow at a given velocity as vessel resistance is related to vessel radius to the power of four. Although accurate measurement of vessel diameter would thus be key to determining blood flow, none of the TCD studies retrieved here directly measured MCA diameter; one study measured radial and temporal artery diameter (both unchanged), and calculated MCA diameter from SPECT assessment of CBF and VMCA.40 MCA diameter assessed by magnetic resonance angiography (MRA) in the BMD study was unchanged,42 and increased in some participants in one of the SAH studies, where it was measured by digital subtraction angiography.50 In light of these reports, we believe that assumptions regarding MCA diameter should be viewed with caution.
Measurement of CBF can also be performed using SPECT, PET, fMRI and arterial spin labelling (ASL) MRI, which allow resolution of regional CBF. The studies using hexamethylpropyleneamineoxime (HMPAO) or Xenon SPECT.39,40 functional47 and cross-sectional perfusion MRI42 found no change in regional and global CBF at rest following PDE5i treatment in healthy individuals, migraineurs or BMD. After stimulation, the BMD patients showed an increase in regional response with sildenafil (measured using BOLD fMRI).42 In two studies using HMPAO SPECT in individuals with prior stroke, a relative reduction in regional CBF in and around the stroke lesion was reported, with increase in some other brain areas.43,48 The authors ascribe this to post-stroke dysfunction of cerebral auto-regulation, leading to a ‘steal phenomenon’ whereby healthy brain is able to achieve a greater vasodilatory response at the expense of ischaemic areas.67 However, infarcted areas will not regain blood flow. Around the infarct, there may also be areas of oligaemia, which have only limited supply from collateral vessels.68–70 Underlying pathology and mechanisms in large vessel strokes are different to those in SVD, so these findings may not be generalisable. Furthermore, measurements of reduction in relative flow in areas immediately adjacent to infarct areas may also be due to sub-optimal spatial resolution of SPECT, with voxels overlapping the infarcted area, i.e. partial volume effect.71,72
It is not currently known whether changes in CVR reviewed here affect cognitive performance, or alter disease progression in SVD.
In vascular cognitive impairment there is a lack of consensus regarding disease classification and diagnostic criteria, and the exact relation between pathology and cognition has not been determined. Patient groups in trials are thus often heterogeneous (including ‘pure’ SVD, lacunar infarcts in ‘strategic’ positions, larger infarcts, bleeds, and amyloid-related pathology) so that it has been difficult to identify therapeutic targets and treatments.3,4 An attempt to reduce cognitive decline in SVD through blood pressure control and antiplatelet treatment was negative in a phase III study monitoring participants for a mean of three years.73
Pre-clinical studies of the effects of PDE5i in cerebrovascular disease are limited by the lack of a robust animal model of SVD.74 However, studies of PDE5i in animal models of neurodegeneration have shown promising results. A study using sildenafil found improved memory task performance in a mouse model of AD.75 In a similar mouse model tadalafil improved water maze performance and reduced Tau phosphorylation after 12 weeks of treatment.76 Object recognition task performance was also improved by PDE5i in male Wistar rats.77 Acute stroke studies in rodents found improved outcome with PDE5i, apparently due to neurorepair.78–81
Studies of the effects of PDE5i on cognition in humans are limited. A study of sildenafil in 10 healthy males found enhanced event-related potentials, but no changes in auditory attention and word recognition.82 In another study of six healthy participants sildenafil enhanced performance in a simple reaction time test, but not on other measures (including short term memory and divided attention).83
Beneficial neurorestorative and functional effects of sildenafil have been observed in two case reports. In a subject with a history of occipital infarcts, visual field defects improved following sildenafil treatment,84 and in an individual with spastic quadriplegia, mild motor restoration was found after administration of sildenafil (the details of this are not further specified).85
Whether the cognitive effects observed in the two single-dose studies in healthy individuals82,83 can be sustained with longer-term administration is unclear. However, the case reports of single subjects with disease states (spastic quadriplegia and stroke) reported improvements in symptoms lasting seven85 to over 12 months84 in motor and visual symptoms, respectively.
Limitations
The review is limited by the relative paucity of research studies and variability in data quality. Of the included studies, only six were double blind randomised controlled trials (three cross-over trials, three with a control group) with a total of 103 participants.37–42 Individually these trials had small sample sizes, ranging from six37 to 35 (divided into three treatment and control groups).41 None of the remaining studies were blinded and only a further two had control groups.44,45 Results should thus be interpreted with caution.
The participant groups in the studies included here are very heterogeneous with a variety of brain and/or systemic pathologies and hence potentially disparate disease mechanisms. Protocols of intervention, PDE5i used, route of administration and outcome measures differed between studies, with a range of techniques used to measure CBF.
It should also be noted that most of the retrieved studies only assessed acute (often single) dosing of PDE5i. Exceptions to this are the BMD study, where sildenafil was given for four weeks prior to assessment,42 the stroke and ED study comparing once only tadalafil with a seven day regimen43 and one of the altitude trials, where PDE5i was given for about four days.46 Nevertheless, none of the interventional studies investigated periods longer than a number of weeks. One of the altitude studies, however, showed attenuation of some of the observed changes on day three versus day one.46 This raises the question whether any short-term effects on CVR observed will be maintained with longer-term administration.
Whether or not PDE5i had been used prior to study intervention by participants is not reported, particularly in the ED38,43,48 and PH studies,45 where this may be expected (the study in PH included participants ‘using or not using sildenafil’48). Only three studies explicitly state that subjects were not using40,47 or were not previous users50 of PDE5i.
Across all studies, of the 353 participants only 53 were female (with one of the SAH studies not specifying gender49), thus females are significantly under-represented, particularly in the studies investigating disease groups. Due to these factors comparison across studies is imperfect and findings cannot easily be generalised.
Conclusions
Our systematic review of the literature suggests that PDE5i affect CBF in certain clinical conditions. Measures of CVR, but not basal CBF, are improved by PDE5i especially in disorders characterised by an impaired endothelial dilatory response. This may be because deficient NO-mediated signalling has disproportionate effects on brain microvascular responsiveness compared to the resting state. Although PDE5i did not produce increases in resting CBF that were detectable with middle cerebral artery insonation, a possible action on resting CBF at the level of the small arterioles remains untested.
Future studies using high resolution, blood-flow specific cross-sectional imaging techniques as surrogates of deep CBF measurements (e.g. ASL MRI86) are warranted to explore effects of PDE5i at the arteriolar level. Future studies should also test whether PDE5i mediated vascular changes are correlated with cognitive function, using tools that assess appropriate cognitive features such as attention, information processing speed and executive function, which are particularly affected in SVD.3,4,9,87
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by Alzheimer's Society (UK) and Alzheimer's Drug Discovery Foundation (ADDF grant number 20140901).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JDI, AHH, TRB, JBM and CK have received research funding from Alzheimer's Society (UK) and ADDF to carry out a clinical trial of the effects of a PDE5i on CBF.85 MMHP is supported by this funding.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001; 357: 169–175. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol 2007; 113: 349–388. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JT, Thomas A. Vascular dementia. Lancet 2015; 386: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003; 2: 89–98. [DOI] [PubMed] [Google Scholar]
- 5.Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 2009; 339: b2477–b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol 2007; 20: 390–397. [DOI] [PubMed] [Google Scholar]
- 7.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Cieslak A, Barber P, et al. Therapeutic strategies and drug development for vascular cognitive impairment. J Am Heart Assoc 2017; 6: e005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence AJ, Brookes RL, Zeestraten EA, et al. Pattern and rate of cognitive decline in cerebral small vessel disease: a prospective study. PloS ONE 2015; 10: e0135523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt R, Berghold A, Jokinen H, et al. White matter lesion progression in LADIS: frequency, clinical effects, and sample size calculations. Stroke 2012; 43: 2643–2647. [DOI] [PubMed] [Google Scholar]
- 11.Brickman AM, Siedlecki KL, Muraskin J, et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 2011; 32: 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009; 5: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016; 36: 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markus HS, Lythgoe DJ, Ostegaard L, et al. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry 2000; 69: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeestraten EA, Benjamin P, Lambert C, et al. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel disease. PloS One 2016; 11: e0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruitenberg A, den Heijer T, Bakker SLM, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005; 57: 789–794. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 18.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol 1994; 36: 557–565. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson SF, Doubal FN, Shuler K, et al. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke 2010; 41: e434–442. [DOI] [PubMed] [Google Scholar]
- 20.Hooper WC, Catravas JD, Heistad DD, et al. Vascular endothelium summary statement I: health promotion and chronic disease prevention. Vasc Pharmacol 2007; 46: 315–317. [DOI] [PubMed] [Google Scholar]
- 21.Khan U, Hassan A, Vallance P, et al. Asymmetric dimethylarginine in cerebral small vessel disease. Stroke 2007; 38: 411–413. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease: endothelial cells and SVD. Brain Pathol 2015; 25: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marstrand JR, Garde E, Rostrup E, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 2002; 33: 972–976. [DOI] [PubMed] [Google Scholar]
- 25.Blair GW, Doubal FN, Thrippleton MJ, et al. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: a systematic review. J Cereb Blood Flow Metab 2016; 36: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb AJS, Simoni M, Mazzucco S, et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 2012; 43: 2631–2636. [DOI] [PubMed] [Google Scholar]
- 27.Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res 2004; 16: S4–S7. [DOI] [PubMed] [Google Scholar]
- 28.Tuder RM, Marecki JC, Richter A, et al. Pathology of pulmonary hypertension. Clin Chest Med 2007; 28: 23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharton J, Strange JW, Møller GMO, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 2005; 172: 105–113. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson TM, Sher E. The role of phosphodiesterases in hippocampal synaptic plasticity. Neuropharmacology 2013; 74: 86–95. [DOI] [PubMed] [Google Scholar]
- 31.Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 2010; 59: 367–374. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu-Albergine M, Rybalkin SD, Rybalkina IG, et al. Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J Neurosci 2003; 23: 6452–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Staveren WCG, Steinbusch HWM, Markerink-Van Ittersum M, et al. mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol 2003; 467: 566–580. [DOI] [PubMed] [Google Scholar]
- 34.Kruuse C, Rybalkin SD, Khurana TS, et al. The role of cGMP hydrolysing phosphodiesterases 1 and 5 in cerebral artery dilatation. Eur J Pharmacol 2001; 420: 55–65. [DOI] [PubMed] [Google Scholar]
- 35.Kruuse C, Khurana TS, Rybalkin SD, et al. Phosphodiesterase 5 and effects of sildenafil on cerebral arteries of man and guinea pig. Eur J Pharmacol 2005; 521: 105–114. [DOI] [PubMed] [Google Scholar]
- 36.Hainsworth A, Vasita E, Andoh J, et al. Phosphodiesterase 5 (PDE5) as a potential therapeutic target in small arteries of the aged human brain. Alzheimers Dement 2014; 10: P880. [Google Scholar]
- 37.Arnavaz A, Aurich A, Weissenborn K, et al. Effect of sildenafil (Viagra) on cerebral blood flow velocity: a pilot study. Psychiatry Res 2003; 122: 207–209. [DOI] [PubMed] [Google Scholar]
- 38.Diomedi M, Sallustio F, Rizzato B, et al. Sildenafil increases cerebrovascular reactivity: a transcranial Doppler study. Neurology 2005; 65: 919–921. [DOI] [PubMed] [Google Scholar]
- 39.Kruuse C, Thomsen LL, Jacobsen TB, et al. The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow 2002; 22: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 40.Kruuse C, Thomsen LL, Birk S, et al. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain 2003; 126: 241–247. . [DOI] [PubMed] [Google Scholar]
- 41.Van Osta A, Moraine J-J, Melot C, et al. Effects of high altitude exposure on cerebral hemodynamics in normal subjects. Stroke 2005; 36: 557–560. [DOI] [PubMed] [Google Scholar]
- 42.Lindberg U, Witting N, Jørgensen SL, et al. Effects of sildenafil on cerebrovascular reactivity in patients with Becker muscular dystrophy. Neurotherapeutics 2017; 14: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorberboym M, Makhline E, Lampl Y. Regional cerebral blood flow following single-dose and continuous-dose tadalafil after stroke. Acta Neurol Scand 2014; 130: 380–386. [DOI] [PubMed] [Google Scholar]
- 44.Al-Amran FG, Zwain AA, Hadi NR, et al. Autonomic cerebral vascular response to sildenafil in diabetic patient. Diabetol Metab Syndr 2012; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosengarten B, Schermuly RT, Voswinckel R, et al. Sildenafil improves dynamic vascular function in the brain: studies in patients with pulmonary hypertension. Cerebrovasc Dis 2006; 21: 194–200. [DOI] [PubMed] [Google Scholar]
- 46.Chan CWM, Hoar H, Pattinson K, et al. Effect of sildenafil and acclimatization on cerebral oxygenation at altitude. Clin Sci (Colch) 2005; 109: 319–324. [DOI] [PubMed] [Google Scholar]
- 47.Kruuse C, Hansen AE, Larsson HBW, et al. Cerebral haemodynamic response or excitability is not affected by sildenafil. J Cereb Blood Flow 2009; 29: 830–839. [DOI] [PubMed] [Google Scholar]
- 48.Lorberboym M, Mena I, Wainstein J, et al. The effect of sildenafil citrate (Viagra) on cerebral blood flow in patients with cerebrovascular risk factors. Acta Neurol Scand 2010; 121: 370–376. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee KK, Singh SK, Khosla VK, et al. Safety and efficacy of sildenafil citrate in reversal of cerebral vasospasm: a feasibility study. Surg Neurol Int 2012; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Washington CW, Derdeyn CP, Dhar R, et al. A phase I proof-of-concept and safety trial of sildenafil to treat cerebral vasospasm following subarachnoid hemorrhage. J Neurosurg 2016; 124: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhar R, Washington C, Diringer M, et al. Acute effect of intravenous sildenafil on cerebral blood flow in patients with vasospasm after subarachnoid hemorrhage. Neurocrit Care 2016; 25: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahshan S, Dayan L, Jacob G. Nitric oxide-sensitive guanylyl cyclase signaling affects CO2-dependent but not pressure-dependent regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol 2017; 312: R948–R955. [DOI] [PubMed] [Google Scholar]
- 53.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 54.Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke J Cereb Circ 1992; 23: 668–673. [DOI] [PubMed] [Google Scholar]
- 55.Dunn KM, Nelson MT. Neurovascular signaling in the brain and the pathological consequences of hypertension. AJP Heart Circ Physiol 2014; 306: H1–H14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995; 268: C799–822. [DOI] [PubMed] [Google Scholar]
- 57.Kitazono T, Faraci FM, Taguchi H, et al. Role of potassium channels in cerebral blood vessels. Stroke J Cereb Circ 1995; 26: 1713–1723. [DOI] [PubMed] [Google Scholar]
- 58.Hassan A. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003; 126: 424–432. [DOI] [PubMed] [Google Scholar]
- 59.Deplanque D, Lavallee PC, Labreuche J, et al. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: a case–control study. Int J Stroke 2013; 8: 413–421. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, Mason NA, Morrell NW, et al. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 2001; 104: 424–428. [DOI] [PubMed] [Google Scholar]
- 61.Lepore JJ, Maroo A, Pereira NL, et al. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol 2002; 90: 677–680. [DOI] [PubMed] [Google Scholar]
- 62.Dishy V. The effect of sildenafil on nitric oxide–mediated vasodilation in healthy men. Clin Pharmacol Ther 2001; 70: 270–279. [DOI] [PubMed] [Google Scholar]
- 63.Halcox JPJ, Nour KRA, Zalos G, et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002; 40: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 64.Elhwuegi A. The wonders of phosphodiesterase-5 inhibitors: a majestic history. Ann Med Health Sci Res 2016; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tantini B, Manes A, Fiumana E, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol 2005; 100: 131–138. [DOI] [PubMed] [Google Scholar]
- 66.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 67.Reinhard M, Rutsch S, Hetzel A. Cerebral autoregulation in acute ischemic stroke. Perspect Med 2012; 1: 194–197. [Google Scholar]
- 68.Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis 2003; 17: 1–6. [DOI] [PubMed] [Google Scholar]
- 69.Fisher M, Bastan B. Identifying and utilizing the ischemic penumbra. Neurology 2012; 79: S79–S85. [DOI] [PubMed] [Google Scholar]
- 70.Bandera E, Botteri M, Minelli C, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke 2006; 37: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 71.Grade M, Hernandez Tamames JA, Pizzini FB, et al. A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015; 57: 1181–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med 2008; 60: 1362–1371. [DOI] [PubMed] [Google Scholar]
- 73.Pearce LA, McClure LA, Anderson DC, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hainsworth AH, Allan SM, Boltze J, et al. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 2017; 15: 16. [DOI] [PMC free article] [PubMed]
- 75.Puzzo D, Staniszewski A, Deng SX, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. J Neurosci 2009; 29: 8075–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.García-Barroso C, Ricobaraza A, Pascual-Lucas M, et al. Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology 2013; 64: 114–123. [DOI] [PubMed] [Google Scholar]
- 77.Rutten K, Van Donkelaar EL, Ferrington L, et al. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology 2009; 34: 1914–1925. [DOI] [PubMed] [Google Scholar]
- 78.Menniti FS, Ren J, Coskran TM, et al. Phosphodiesterase 5A inhibitors improve functional recovery after stroke in rats: optimized dosing regimen with implications for mechanism. J Pharmacol Exp Ther 2009; 331: 842–850. [DOI] [PubMed] [Google Scholar]
- 79.Gao F, Sugita M, Nukui H. Phosphodiesterase 5 inhibitor, zaprinast, selectively increases cerebral blood flow in the ischemic penumbra in the rat brain. Neurol Res 2005; 27: 638–643. [DOI] [PubMed] [Google Scholar]
- 80.Zhang RL, Chopp M, Roberts C, et al. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS ONE 2012; 7: e48141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ölmestig JNE, Marlet IR, Hainsworth AH, et al. Phosphodiesterase 5 inhibition as a therapeutic target for ischemic stroke: a systematic review of preclinical studies. Cell Signal 2017; 38: 39–48. [DOI] [PubMed] [Google Scholar]
- 82.Schultheiss D, Müller SV, Nager W, et al. Central effects of sildenafil (Viagra) on auditory selective attention and verbal recognition memory in humans: a study with event-related brain potentials. World J Urol 2001; 19: 46–50. [DOI] [PubMed] [Google Scholar]
- 83.Grass H, Klotz T, Fathian-Sabet B, et al. Sildenafil (Viagra): is there an influence on psychological performance? Int Urol Nephrol 2001; 32: 409–412. [DOI] [PubMed] [Google Scholar]
- 84.Schlindwein P, Eicke BM, Stoeter P, et al. Sildenafil improves scotoma after posterior cerebral infarctions: a case report. J Neurol 2010; 257: 674–677. [DOI] [PubMed] [Google Scholar]
- 85.Cocchiarella A. Partial motor restoration upon administration of sildenafil: a case study. Dev Neurorehabil 2012; 15: 39–43. [DOI] [PubMed] [Google Scholar]
- 86.Pauls MMH, Clarke N, Trippier S, et al. Perfusion by arterial spin labelling following single dose tadalafil in small vessel disease (PASTIS): study protocol for a randomised controlled trial. Trials 2017; 18: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brookes RL, Hollocks MJ, Khan U, et al. The brief memory and executive test (BMET) for detecting vascular cognitive impairment in small vessel disease: a validation study. BMC Med 2015; 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.