Abstract
Background
Bone-marrow mesenchymal stem cells (BMSCs) are pluripotent stem cells with potent self-renewal and differentiation ability that are widely used in transplantation of cell therapy. But the mechanism on microRNA (miRNA) regulating stem cell differentiation is complicated and unclear. The aim of this study was to investigate whether miR-199b-5p is involved in differentiation of cardiomyocyte-like cells and identify potential signal pathways in BMSCs.
Material/Methods
Mouse BMSCs were treated with 5-azacytidine and transfected by miR-199b-5p mimic and inhibitor, respectively. qRT-PCR was used to detect the expression of miR-199b-5p in BMSCs, 5-azacytidine treated BMSCs, and neonatal murine cardiomyocytes. The expression of cardiac specific genes and the HSF1/HSP70 signal pathway were examined by qRT-PCR or western blotting. The proliferation and migration of BMSCs were evaluated by CCK-8 assay and wound-healing assay.
Results
The expression of miR-199b-5p decreased gradually in the process of differentiation of BMSCs toward cardiomyocyte-like cells. The expression of cardiac specific genes and HSF1/HSP70 were increased in the miR-199b-5p inhibitor group; however, the miR-199b-5p mimic group presented an opposite result. Both the miR-199b-5p inhibitor group and the miR-199b-5p mimic group had no influence on BMSCs proliferation and migration. Using lentivirus vectors bearing HSF1 shRNA to silence HSF1 and HSP70, the anticipated elevated expression effect of cardiac specific genes induced by miR-199b-5p inhibitor was suppressed.
Conclusions
Downregulation of miR-199b-5p induced differentiation of BMSCs toward cardiomyocyte-like cells partly via the HSF1/HSP70 signaling pathway, and had no influence on BMSCs proliferation and migration.
MeSH Keywords: Cell Differentiation, HSP70 Heat-Shock Proteins, Mesenchymal Stromal Cells, MicroRNAs
Background
Cell-based therapies for myocardial repair and regeneration are currently being regarded as a promising treatment for heart failure and ischemic heart disease [1]. Many kinds of stem cells are used for these cell-based therapies, such as skeletal myoblasts, endothelial progenitor cells, bone-marrow mesenchymal stem cells, embryonic stem cells, endogenous cardiac stem cells, and so on. These stem cells can differentiate into cardiomyocytes and repair the damaged myocardium, thus improving cardiac function [2]. Among these cells, bone-marrow mesenchymal stem cells (BMSCs) have been widely studied for many years. Some studies have shown that BMSC-based cell therapies have a significant effect on improving ejection fraction and reducing scar size [3–5].
The mechanism by which MSC-based therapies repair the heart is complicated. Trans-differentiation of MSCs toward cardiomyocytes may be one of the major mechanisms [6]. The traditional method of inducing BMSCs toward cardiomyocytes in vitro is by using 5-azacytidine, a DNA demethylating agent [7,8]. But 5-azacytidine has low differentiation efficiency and cell toxicity, restricting its use to basic research in vitro. Recently, studies have focused on the role of miRNAs in regulating cell differentiation. It has been shown that a variety of miRNAs are involved in cardiac differentiation [9–11]. MiRNAs play a critical role in cell differentiation, which indicates that miRNAs should be investigated to clarify the relationship between miRNAs and cardiac differentiation. Martins et al. showed that miR-199b-5p had an effect on cardiac cellular signaling and gene expression [12]. Li et al. discovered that miR-199b-5p played an important maintenance role in cardiac development [13]. Our previous study confirmed miR-199b-5p can regulate angiogenesis in mouse myocardial microvascular endothelial cells [14]. However, the role of miR-199b-5p in cardiac differentiation has been not reported. In cardiomyocytes, heat shock transcription factor 1 (HSF1), and downstream effective protein heat shock protein 70 (HSP70) are considered to have a protective role in cell metabolism [15]. We previously demonstrated that HSF1 is partly regulated by miR-199b-5p in mouse myocardial microvascular endothelial cells [14]. Similarly, whether the regulatory effect can be exerted in BMSCs is worthy of study. Here, we aimed to investigate whether miR-199b-5p is involved in differentiation of cardiomyocyte-like cells and identify potential signal pathways in BMSCs.
Material and Methods
Culture of BMSCs
C57BL/6 mouse BMSCs were obtained from Cyagen Biosciences Corporation (Santa Clara, CA, USA) and were cultured with complete medium (DMEM/F12 (Corning, Manassas, VA, USA), 10% fetal bovine serum (Gibco, Grand Island, NY, USA), supplemented with 1% penicillin/streptomycin), and then were placed in an incubator at 37°C, 5% CO2. Quality tests included differentiation potential toward osteoblasts and adipocytes and chondrocytes and MSCs markers identification (90.8% CD44, 99.7% CD29, 87.15% Sca-1, and 1.3% CD117) of BMSCs; tests were conducted by cell supplier. When grown to 80–90% confluence, cells were passaged at a ratio of 1: 3 and about 50,000 cells/mL in a T25-flask. After abundant cultivation, the fourth passage of cells was used for the study experiments.
Isolation and culture of neonatal murine cardiomyocytes
All the animal experiment procedures were approved by the Animal Care and Use Committee and Animal Ethics Committee of Tongji University and were in compliance with the guidelines of the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health. We used the hearts from 2-day to 3-day old C57BL/6 mice to isolate and culture cardiomyocytes as previously described [16], with some modifications. Briefly, neonatal mice were sacrificed, and their hearts were quickly removed and transferred into precooled 4°C Hanks’ balanced salt solution (HBSS, Sigma-Aldrich, St. Louis, MO, USA) and then were cut into pieces. Heart tissue was digested by 50 mL 0.125% trypsin (Gibco, Grand Island, NY, USA) in a 150-mL conical flask with a magnetic stirrer (100 rpm, eight minutes, 37°C). After three minutes’ standing, the supernatant (about 10 mL, containing the cardiac cells) was transferred into a 15-mL centrifuge tube and centrifuged at 168 g for five minutes. Cardiac cells were resuspended with 3 mL complete medium after discarding the supernatant, and then were transferred into a 50-mL centrifuge tube. After replenishment with 10 mL 0.125% trypsin, we repeated the digestion procedure until there was no visible heart tissue in the conical flask. All collected medium containing cardiac cells was transferred into 6 cm dishes and placed in an incubator at 37°C, 5% CO2 for two hours. According to cells’ differential adhesion, cardiomyocytes are slower adherent than other cardiac cells. The non-attached cells were cardiomyocytes in dishes. The medium only containing cardiomyocytes in dishes were collected and centrifuged (168 g, five minutes) in a 50-mL centrifuge tube. Finally, cardiomyocytes were resuspended after discarding the supernatant and were plated in six-well plates with about 50,000 cells/mL for the study experiments.
β-galactosidase staining and inducing differentiation of BMSCs
The aging status of BMSCs was detected by senescence-associated β-galactosidase staining kit (Beyotime, Shanghai, China) after one day and three days of cell passage. Briefly, BMSCs were washed twice and fixed with 4% paraformaldehyde for 15 minutes and then incubated overnight at 37°C in an incubator with working solution (containing 0.05 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). To verify the differentiation potential of BMSCs toward cardiomyocyte-like cells, 5-azacytidine solution (10 mmol, Sigma-Aldrich, St. Louis, MO, USA) was added to medium at a 10 μmol final concentration for 24 hours in six-well plates. The 5-azacytidine treated cells were collected for the following cardiac specific factors detection as well as other experiment groups at these time points: three days, six days, nine days, and 12 days after cell transfection.
Transfection of miRNA and shRNA
We used lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA) to transfect miR-199b-5p mimic and its inhibitor (200 nM, Ruibo, Guangzhou, China) to upregulate and downregulate miR-199b-5p level. Briefly, using the manufacturer’s instruction, when cells reached 60–80% confluence, they were transfected with miR-199b-5p mimic or its inhibitor-lipid complex (1: 1 ratio). After three days of transfection, cells were harvested to verify the upregulation or downregulation. The downregulation of HSF1 in BMSCs was generated by the infection of lentiviral vector bearing shRNA HSF1, which was purchased from HanBio Biotechnology Corporation (Shanghai, China). According to manufacturer’s instructions, cells were infected by lentiviral bearing shRNA len-EGFP-puro-HSF1 or shRNA len-EGFP (as a shRNA control) for a variety of multiplicity of infection (MOI) with 30–40% cell confluence. The stable cell lines obtained from 200 MOI infected cells were used for experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNAs were extracted from BMSCs and differentiated cardiomyocyte-like cells by TRIzol reagent (Takara, Shiga, Japan) according to manufacturer’s instructions. For mRNAs reverse transcription, the relevant cDNAs were synthesized using PrimeScript™ RT reagent Kit (Takara, Shiga, Japan). The qRT-PCR was conducted with SYBR Premix Ex Taq II (Takara, Shiga, Japan) under the following conditions: 95°C for 30 seconds, 95°C for 5 seconds and 60°C for 34 seconds with 40 cycles. For miRNA RTq-PCR, Bulge-Loop™ miRNA qRT-PCR Starter Kit (Ruibo, Guangzhou, China) was used under the same conditions. The primers of miR-199b-5p and cardiac specific markers [17,18] (NKx2.5 GATA4, MEF2c, and β-MHC) and their related internal controls (U6 and GAPDH) are listed in Table 1. The difference in gene expression was evaluated using the threshold cycle difference between relevant genes, and the internal controls using the method of relative gene expression=2−ΔΔCT.
Table 1.
Gene primers sequences.
| Primers | Direction | Sequences (5′-3′) |
|---|---|---|
| Nkx2.5 | Forward | AAGCAACAGCGGTACCTGTC |
| Reverse | ACTTGTAGCGACGGTTCTGG | |
| GATA4 | Forward | ATGCCTGTGGCCTCTATCAC |
| Reverse | TGGTGGTAGTCTGGCAGTTG | |
| MEF2c | Forward | TCCATCAGCCATTTCAACAA |
| Reverse | AGTTACAGAGCCGAGGTGGA | |
| β-MHC | Forward | CAGCAGTTCTTCAACCACCA |
| Reverse | TCTCGATGAGGTCAATGCAG | |
| GAPDH | Forward | GCCATCACTGCCACTCAGAA |
| Reverse | GGCATGTCAGATCCACAACG | |
| miR-199b-5p | (RT primer) | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTG |
| Forward | GGCCCAGTGTTTAGACTACC | |
| Reverse | CAGTGCGTGTCGTGGAGT | |
| U6 | (RT primer) | AACGCTTCACGAATTTGCGT |
| Forward | GTGCTCGCTTCGGCAGCACATATAC | |
| Reverse | AAAAATATGGAACGCTTCACGAATTTG |
CCK-8 assay and wound-healing assay
To detect stem cell proliferation, the cells of four groups (normal control cells, 5-azacytidine treated cells, miR-199b-5p mimic, and inhibitor transfected cells) were seeded in 96-well plates at 37°C, 5% CO2, with 1,000 cells/200 μL density per well. Then 10 μL of CCK-8 reagent (Beyotime, Shanghai, China) was added to three wells in each group after one, three, six, nine, and 12 days, respectively. After the cells were incubated for four hours in the incubator, the optical density (OD) of wells at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA) and the growth curve was drawn according to the OD value. To detect stem cell migration, the cells of the four groups were seeded in six-well plate at 37°C, 5% CO2, with 100,000 cells/2 mL density per well. When the monolayer cells were 100% confluent, the wounds were created by manually scraping the cell monolayer with a 200 μL pipet tip. Then the cells were washed three times and serum-free medium was added into wells. Microscopy was used to record the stem cells migration of the four groups after 0 and 24 hours (before the time point, miR-199b-5p mimic or inhibitor group transfected for three days, and 5-azacytidine treatment group treated for one day), respectively. The cell migration distance on the wound was analyzed and quantified by comparing different time points.
Western blotting analysis
The proteins expression of HSF1 and HSP70 and the cardiac specific markers GATA4 and β-MHC were measured by western blotting using relevant primary antibodies and secondary antibodies. Primary antibodies included rat anti-HSF1 antibody (ab61382, 1: 1,000), mouse anti-HSP70 antibody (ab2787, 1: 1,000), rabbit anti-GATA4 antibody (ab134057, 1: 1,000), mouse anti-β-MHC (heavy chain cardiac myosin) antibody (ab50967, 1: 1,000) and mouse anti-GAPDH antibody (ab8245, 1: 1,000). All the primary antibodies were purchased from Abcam Corporation (Cambridge, MA, USA). Secondary antibodies included fluorescent-dye conjugated anti-rat, anti-mouse, and anti-rabbit IgG secondary antibodies (1: 2,000, Cell Signaling, Danvers, MA, USA). Briefly, cells were harvested by RIPA lysis buffer (Weiao, Shanghai, China) containing a protease inhibitor cocktail (Selleck, Houston, TX, USA). Cell lysates were separated by SDS-PAGE (10%) and the proteins were transferred to PVDF membranes (Weiao, Shanghai, China). After being sealed, the membranes were incubated with primary antibodies at 4°C overnight, and were incubated with relevant secondary antibodies. GAPDH was used as an internal control. The images were collected and analyzed by Odyssey (LI-COR Biosciences, Lincoln, NE, USA) and ImageJ 1.4 analysis software (National Institutes of Health, Bethesda, MD, USA), respectively.
Statistical analysis
For technical replicates, all experiments were repeated three times (n=3). For biological replicates, the independent procedures were repeated three times. Data represented the average of three biological replicates and were presented as mean ±SD. GraphPad Prism 5.0 software (San Diego, CA, USA) was used to analyze the data. Multi-group comparison was performed by one-way ANOVA followed by a Tukey’s test for post hoc analysis. A value of p<0.05 was considered statistically significant. Significance was described by asterisks, * p<0.05.
Results
Characteristics and differentiation potential of BMSCs in study mice
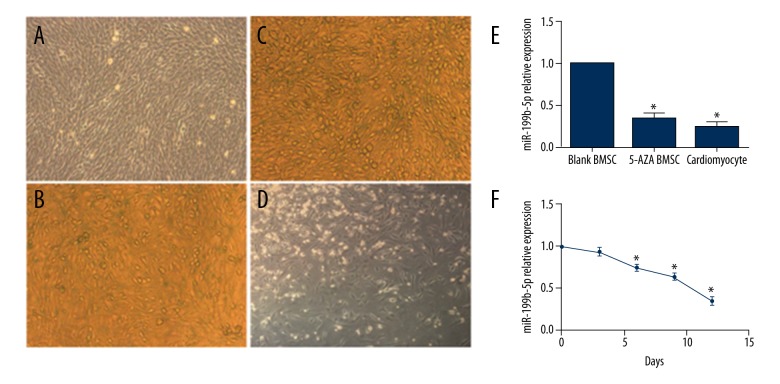
The fourth passage of BMSCs showed the typical mesenchymal stem cell characteristics. Microscopy showed cells spread on the plate with spindle shape (Figure 1A). At day 1 and day 3 after cell passage, only a few of the BMSCs were dyed with the β-galactosidase staining (Figure 1B, 1C) and there were no significant differences at these time points, which meant the BMSCs were in good status and conducive to cell differentiation. To verify the cardiac differentiation potential of BMSCs, cells were treated with 5-azacytidine. At day 12, the BMSCs presented a cardiomyocyte-like shape (Figure 1D). Therefore, the 5-azacytidine group was regarded as a positive control in this study and the BMSCs could be used in study experiments.
Figure 1.
Some characteristics and differentiation potential of mouse BMSCs and miR-199b-5p expression under different cells and time. (A) The fourth passage of BMSCs (100×). (B, C) β-galactosidase staining at day 1 and day 3 after BMSCs passage (100×). (D) Cell morphology at day 12 with 5-azacytidine treatment (100×). (E) Expression level of miR-199b-5p in BMSCs, 5-azacytidine treated BMSCs at day 12 and cardiomyocytes. (F) Expression level of miR-199b-5p in 5-azacytidine treated BMSCs at 0, 3, 6, 9, 12 days; * p<0.05, n=3.
MiR-199b-5p was associated with differentiation of BMSCs toward cardiomyocyte-like cells
To study whether miR-199b-5p is involved in the cardiac differentiation of BMSCs, the expression of miR-199b-5p was compared among BMSCs, 5-azacytidine treated BMSCs, and cardiomyocytes (Figure 1E). The result showed that lower expression of miR-199b-5p was present in cardiomyocytes (0.25-fold versus control, p<0.05) and 5-azacytidine treated BMSCs (0.35-fold versus control, p<0.05), compared with normal control BMSCs. Next, the miR-199b-5p level was detected during differentiation of BMSCs toward cardiomyocyte-like cells (Figure 1F). The expression of miR-199b-5p decreased gradually during the process of differentiation of BMSCs toward cardiomyocyte-like cells (p<0.05), which indicated that one of endogenous miRNAs, miR-199b-5p, may play an important role in differentiation of BMSCs toward cardiomyocyte-like cells.
Downregulation of miR-199b-5p induced the differentiation of BMSCs toward cardiomyocyte-like cells
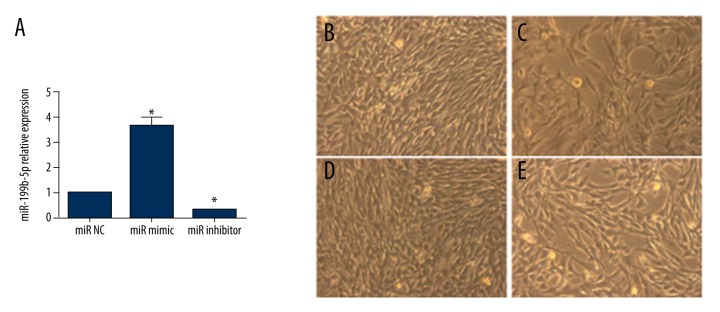
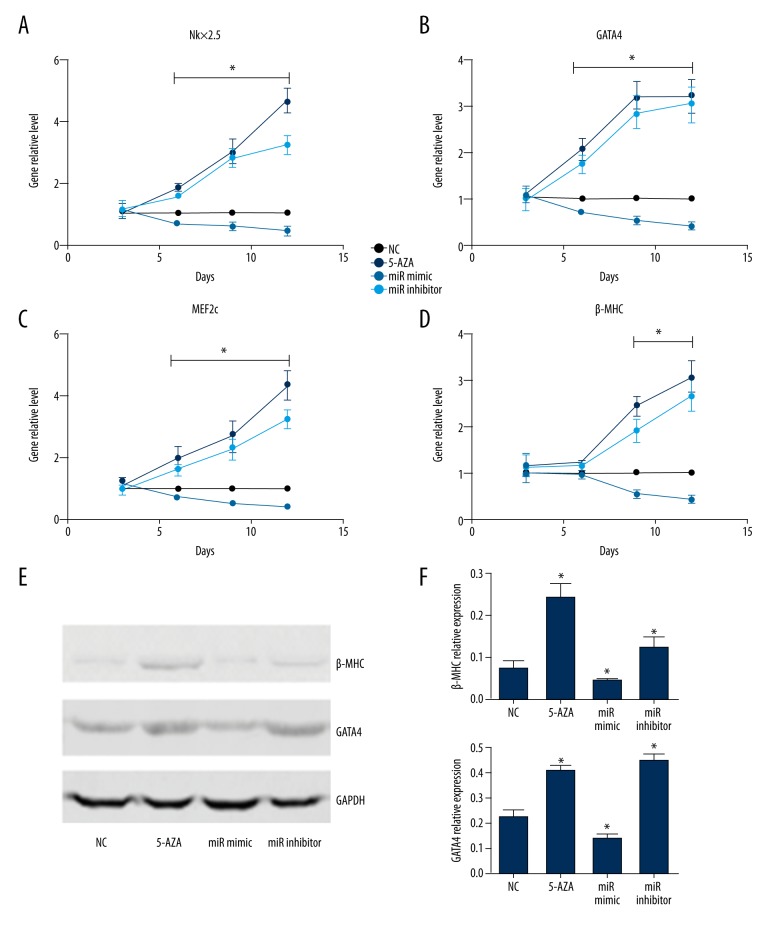
To investigate the specific role of miR-199b-5p in the differentiation of BMSCs toward cardiomyocyte-like cells, miR-199b-5p mimic and inhibitor were used to upregulate and downregulate miR-199b-5p level. After three days of transfection, the level of miR-199b-5p was obviously upregulated (3.6-fold versus control, p<0.05) or downregulated (0.3-fold versus control, p<0.05. Figure 2A). Then, the cells morphology was observed at day 12 and miR-199b-5p inhibitor group was similar to the 5-azacytidine treated group in the four groups (Figure 2B–2E). To detect the gene level of cardiac specific markers Nkx2.5, GATA4, MEF2c, and β-MHC, we chose four time-points: three days, six days, nine days, and 12 days after miR-199b-5p mimic and inhibitor transfection. At day 3, there was no significant change in these markers level. The cardiac-early specific markers Nkx2.5, GATA4, and MEF2c were elevated in the 5-azacytidine treated group and the miR-199b-5p inhibitor group at day 6 and sustained at a higher level at day 9 and day 12. The level of cardiac-later specific marker β-MHC started to increase at day 9 (p<0.05, Figure 3A–3D). The protein level of GATA4 and β-MHC at day 12 also showed similar results (p<0.05, Figure 3E, 3F). At the same time, miR-199b-5p mimic group presented an opposite result (p<0.05, Figure 3E, 3F). The aforementioned results indicated that the downregulation of miR-199b-5p can induce the differentiation of BMSCs toward cardiomyocyte-like cells.
Figure 2.
The level of miR-199b-5p after three days of miR-199b-5p transfection and cell morphology at day 12. (A) The level of miR-199b-5p after three days of miR199b-5p mimic or inhibitor transfection. (B) BMSCs as control without any treatment. (200×) (C) BMSCs were treated with 5-azacytidine (200×). (D) BMSCs were transfected with miR-199b-5p mimic. (200×) (E) BMSCs were transfected with miR-199b-5p inhibitor. (200x); * p<0.05, n=3.
Figure 3.
Cardiac specific markers miRNA level and the protein level of GATA4 and β-MHC. (A–D) Nkx2.5, GATA4, MEF2c, and β-MHC miRNA level at days 3, 6, 9, and 12 after cell transfection or 5-azacytidine treatment, respectively. (E) Western blotting showed the expression of GATA4 and β-MHC at day 12 after cell transfection or 5-azacytidine treatment. (F) GATA4 and β-MHC relative expression; * p<0.05, n=3.
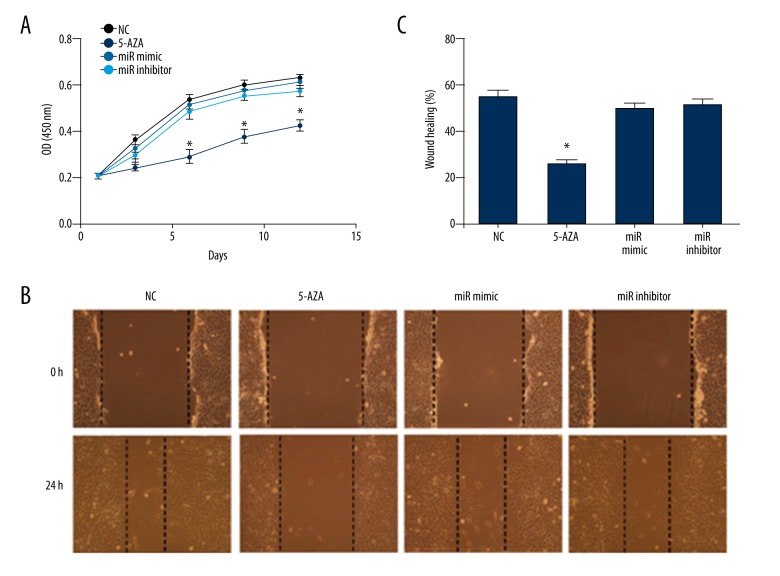
Upregulation or downregulation of miR-199b-5p had no influence on BMSCs proliferation and migration
The low expression of miR-199b-5p had an effect on inducing BMSCs toward cardiac differentiation, but whether the level change of miR-199b-5p influenced the physiological function of BMSCs during the induce process is not known. Thus, we investigated the function of BMSCs proliferation and migration. For stem cell proliferation, we determined the cell growth curve at different time points during the induction process. Compared with the normal control group, the level change of miR-199b-5p did not show a significant OD value difference, and the 5-azacytidine treatment led to a significant OD value decrease in the CCK-8 assay (p<0.05, Figure 4A), which indicated that as exogenous inducers, 5-azacytidine inhibited BMSCs proliferation, but the mimic or inhibitor of miR-199b-5p had no effect. Similarly, we compared the stem cell migration ability of four groups during the first 24 hours of induced differentiation by wound-healing assay. After 24 hours, the BMSCs of the normal control group almost extended to the center of the wound and the mimic or inhibitor group of miR-199b-5p presented a similar result, but few cells reached to the center of wound in the 5-azacytidine treatment group after 24 hours (Figure 4B) and relative quantitative analysis showed the same results (p<0.05, Figure 4C). These results indicated that the exogenous expression change of miR-199b-5p had no cell toxicity during the differentiation of BMSCs and the method of downregulation of miR-199b-5p might have a better potential application value than 5-azacytidine in MSCs therapy.
Figure 4.
BMSCs proliferation and migration. (A) Cell growth curve in normal control BMSCs, 5-azacytidine treated BMSCs, miR-199b-5p mimic and inhibitor transfected BMSCs in CCK-8 assay. (B) Cell migration in the aforementioned four groups at 0 hours and 24 hours. (100×) (C) Quantitative analysis of wound healing by cells migration distance in the aforementioned groups; * p<0.05, n=3.
Downregulation of miR-199b-5p induced the differentiation of BMSCs toward cardiomyocyte-like cells via HSF1/HSP70 signaling pathway
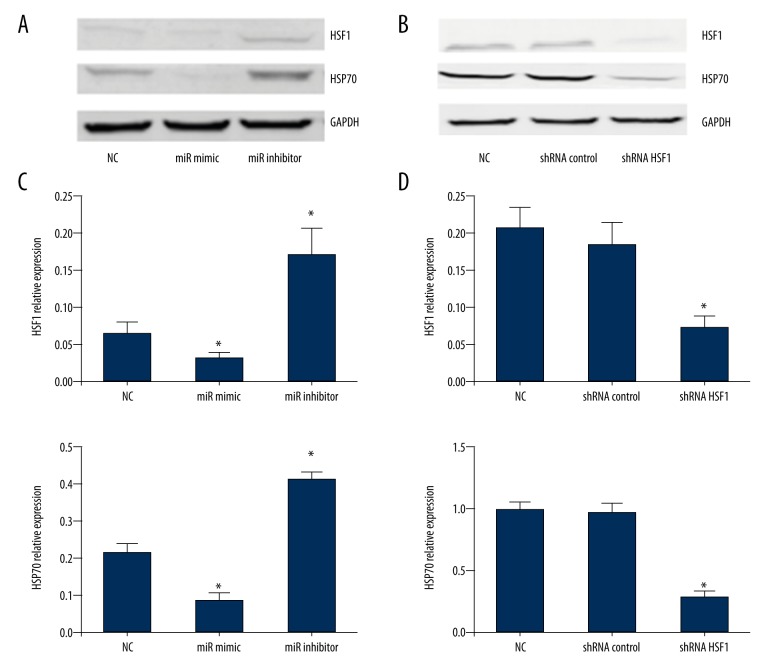
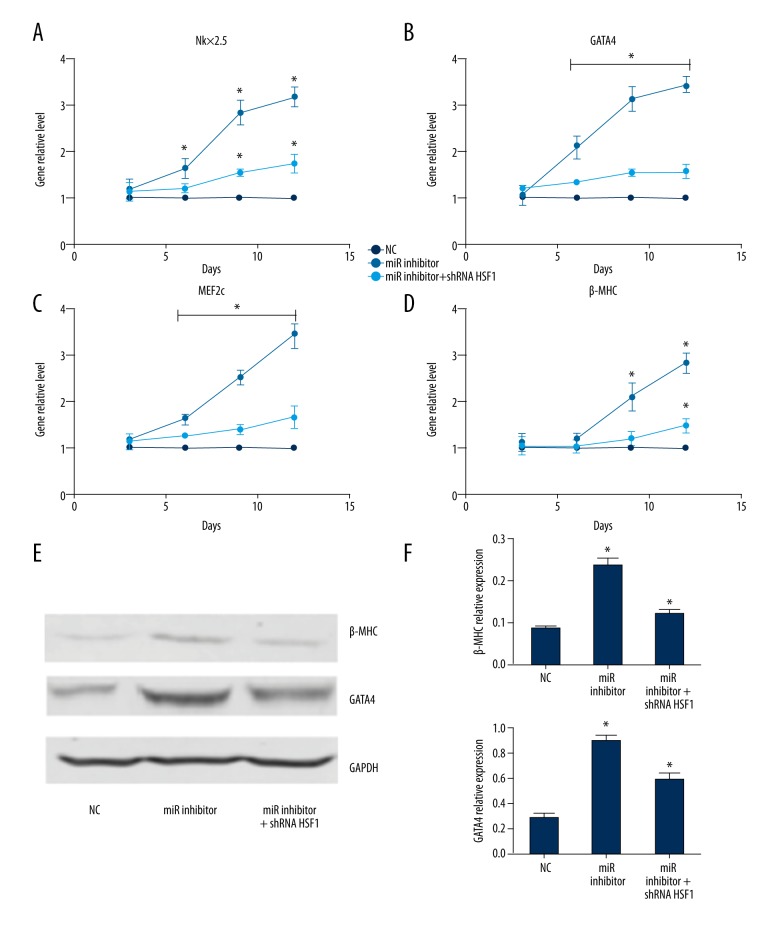
As we previously confirmed that HSF1 is partly regulated by miR-199b-5p in mouse myocardial microvascular endothelial cells [14], to explore whether the target regulation effect of miR-199b-5p on the expression of HSF1 protein was also consistent in BMSCs, we used miR-199b-5p mimic and inhibitor to upregulate and downregulate the miR-199b-5p level. Western blotting showed that downregulation of miR-199b-5p increased the expression of HSF1 protein in BMSCs. In addition, the HSF1 downstream relative protein HSF70 also presented a higher level (p<0.05, Figure 5A, 5C). Based on the induction effect of the downregulation of miR-199b-5p and its relationship with HSF1, we speculated that miR-199b-5p might exert its function via some signaling pathways and HSF1/HSP70 signaling pathway was one of them. To verify the hypothesis, lentiviral vector bearing shRNA HSF1 was used to downregulate the HSF1 gene. Western blotting showed the expression of HSF1 was obviously decreased in the stable cells line after using lentivirus bearing shRNA HSF1 infection (p<0.05, Figure 5B, 5D). To investigate whether the downregulation of HSF1 can affect the differentiation of BMSCs toward cardiomyocyte-like cells induced by downregulation of miR-199b-5p, we repeated the induction differentiation experiments in the stable cells line infected by HSF1 shRNA lentivirus (named miR-199b-5p inhibitor + shRNA HSF1 group). Interestingly, we found compared with miR-199b-5p inhibitor group in BMSCs, the elevated expression of cardiac specific genes was suppressed in miR-199b-5p inhibitor + shRNA HSF1 group (p<0.05, Figure 6A–6D). Relative proteins expression also showed the same suppression effect (p<0.05, Figure 6E, 6F). Which means HSF1/HSP70 pathway may play an important role in the process of downregulation of miR-199b-5p inducing differentiation of BMSCs toward cardiomyocyte-like cells.
Figure 5.
The protein level of HSF1 and HSP70. (A) The protein level of HSF1 and HSP70 after three days with HSF1 mimic or inhibitor transfection. (B) The protein level of HSF1 and HSP70 in the stable cells line infected by HSF1 shRNA or control lentivirus; * p<0.05, n=3.
Figure 6.
Cardiac specific markers Nkx2.5, GATA4, MEF2c, and β-MHC miRNA level and the protein level of GATA4 and β-MHC. (A–D) Nkx2.5, GATA4, MEF2c, and β-MHC miRNA level at 3, 6, 9, and 12 days in normal control BMSCs, miR-199b-5p inhibitor transfected group in BMSCs and miR-199b-5p inhibitor transfected group in BMSCs infected HSF1 shRNA lentiviral, respectively (E) The protein level of GATA4 and β-MHC at day 12 in the aforementioned three groups. (F) GATA4 and β-MHC relative expression; * p<0.05, n=3.
Discussion
With the rapid development of cell basic research, cell-based therapy has been used in a variety of clinical fields, such as in orthopedics, central nervous systems, and cardiovascular systems, and presents a promising future prospect [19-22]. The potential mechanisms of cell-based therapy are being studied including molecule signaling pathways that may have an important role in mediating miRNAs and their various functions. Among these signaling pathways, the classic Notch signaling pathway has a known effect on cell differentiation via miRNAs [23]. And miR-199b-5p has been shown to be related with Notch signaling pathway in cancer [24]. Here we demonstrated that the HSF1/HSP70 signaling pathway was partly involved in BMSCs differentiation toward cardiomyocytes mediated by miR-199b-5p.
In the traditional methods of inducing BMSCs differentiation toward cardiomyocytes, the DNA demethylating agent 5-azacytidine has an induction effect by regulating DNA demethylation and changing DNA stability and gene expression [25]. miRNAs exert their functions by regulating target genes and influencing gene expression [26]. Shen et al. confirmed that miRNA-1–2 can regulate differentiation of BMSCs toward cardiomyocytes via the WNT signaling pathway [9]. Similarly, Zhang et al. demonstrated that miR-499 has the induction effect of rat MSCs toward cardiac differentiation through wnt/β-catenin signaling pathway [10]. On the other hand, a synergic effect can be exerted by the combination of different miRNAs in cardiac differentiation [11]. In our study, we used miR-199b-5p mimic and inhibitor to upregulate and downregulate miR-199b-5p level, and downregulated miR-199b-5p induced BMSCs toward a cardiomyocyte-like cell phenotype with elevated cardiac specific markers levels. This change had no influence on BMSCs proliferation and migration. We observed that 5-azacytidine inhibited BMSCs proliferation and migration, which could support that 5-azacytidine might disturb the normal cell cycle by inducing DNA damage [27]. Different from the kind of mechanism, miR-199b-5p may not combine with growth-relative target genes, so the change of its expression level has no effect on BMSCs proliferation and migration. On the other hand, Chen et al. [28] discovered miR-199b which consists of miR-199b-5p and miR-199b-3p can modulate vascular cell fate during induced pluripotent stem cells differentiation by targeting the Notch Ligand Jagged1 and enhancing VEGF Signaling. Zhao et al. [29] indicated that miR-199b-5p can modulates osteogenesis in human BMSCs. In our study, we did not observe that the upregulation of miR-199b-5p induced osteogenesis in mouse BMSCs, which means miR-199b-5p can participate in regulating different cell growth status under different external conditions and different cell types.
As important molecules in the heat shock protein family, HSF1 and HSP70 play a critical role in anti-hypertrophy and anti-ischemia in cardiomyocytes [15,30]. Cell apoptosis and cell differentiation are usually considered to have a firm relationship in the cell physiological process and HSP70 may have an impact on cell differentiation by interacting with different key apoptotic proteins [31]. In our study, HSF1/HSP70 expression was regulated by miR-199b-5p and their downregulation influence BMSCs differentiation, which indicates HSF1/HSP70 has an effect on maintaining BMSCs stability. Meanwhile, it is reported that upregulation of HSF1/miR-34a/HSP70 results in a pro-survival phenotype in ischemic cardiomyocytes and improves Sca-1+ stem cell survival [32], which is in accordance with our result from another aspect.
We used a traditional BMSCs induction differentiation method to establish a positive control, thus it was easily observed how miR-199b-5p influenced BMSCs differentiation. However, the contractility of cardiomyocyte-like cells was not observed in all groups, which were observed in a co-culture model with cardiomyocyte [33]. This indicated that the appearance of contractility requires a complicated cell microenvironment. Also, we only conducted cardiomyocyte-like cell verification from morphology, gene, and protein levels. More effective methods should be applied. And last, we used miR-199b-5p inhibitor to transiently transfect, which may not be enough to maintain a long-time, low miR-199b-5p level in the BMSCs induction process. These aforementioned limitations suggest a need for additional research. Last, but not least, we only found the HSF1/HSP70 signaling pathway to be partly involved in BMSCs differentiation toward cardiomyocyte-like cells mediated by miR-199b-5p, more significant signaling pathways should be investigated in the future.
Conclusions
We concluded that the downregulation of miR-199b-5p induced differentiation of BMSCs toward cardiomyocyte-like cells, partly via the HSF1/HSP70 signaling pathway, and had no influence on BMSCs proliferation and migration, which provides a new research interest for MSCs-based heart regeneration therapy through the approach of miRNAs.
Footnotes
Conflict of interest
None.
Source of support: Project supported by National Natural Science Foundation of China (Grant No. 81470513) and Pudong New Area Health System Discipline Lead Development Program (Grant No. PWRd2017-14)
References
- 1.Madonna R, Van Laake LW, Davidson SM, et al. Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J. 2016;37(23):1789–98. doi: 10.1093/eurheartj/ehw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 3.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302–10. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs. autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308(22):2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61(23):2329–38. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 6.Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev. 2015;20(1):53–68. doi: 10.1007/s10741-014-9435-x. [DOI] [PubMed] [Google Scholar]
- 7.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu WR, Zhang XR, Qian H, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med. 2004;229(7):623–31. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Pan B, Zhou H, et al. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1–2 via WNT signaling pathway. J Biomed Sci. 2017;24(1):29. doi: 10.1186/s12929-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LL, Liu JJ, Liu F, et al. MiR-499 induces cardiac differentiation of rat mesenchymal stem cells through wnt/beta-catenin signaling pathway. Biochem Biophys Res Commun. 2012;420(4):875–81. doi: 10.1016/j.bbrc.2012.03.092. [DOI] [PubMed] [Google Scholar]
- 11.Pisano F, Altomare C, Cervio E, et al. Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. Stem cells (Dayton, Ohio) 2015;33(4):1187–99. doi: 10.1002/stem.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins PAD, Salic K, Gladka MM, et al. MiRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12(12):1220–31. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Liu L, Hou N, et al. miR-199-sponge transgenic mice develop physiological cardiac hypertrophy. Cardiovasc Res. 2016;110(2):258–67. doi: 10.1093/cvr/cvw052. [DOI] [PubMed] [Google Scholar]
- 14.Du P, Dai F, Chang Y, et al. Role of miR-199b-5p in regulating angiogenesis in mouse myocardial microvascular endothelial cells through HSF1/VEGF pathway. Environ Toxicol Pharmacol. 2016;47:142–48. doi: 10.1016/j.etap.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto M, Minamino T, Toko H, et al. Upregulation of heat shock transcription factor 1 plays a critical role in adaptive cardiac hypertrophy. Circ Res. 2006;99(12):1411–18. doi: 10.1161/01.RES.0000252345.80198.97. [DOI] [PubMed] [Google Scholar]
- 16.Song W, Lu X, Feng Q. Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res. 2000;45(3):595–602. doi: 10.1016/s0008-6363(99)00395-8. [DOI] [PubMed] [Google Scholar]
- 17.Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: Proliferation, migration, and differentiation. Stem Cells (Dayton, Ohio) 2006;24(1):23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 18.Arminan A, Gandia C, Bartual M, et al. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18(6):907–18. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 20.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: A review. J Neurosci Res. 2009;87(10):2183–200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 21.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862–71. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 22.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–29. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 23.Kwon C, Han Z, Olson EN, Srivastava D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates notch signaling. Proc Natl Acad Sci US A. 2005;102(52):18986–91. doi: 10.1073/pnas.0509535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won KY, Kim YW, Kim HS, et al. MicroRNA-199b-5p is involved in the Notch signaling pathway in osteosarcoma. Hum Pathol. 2013;44(8):1648–55. doi: 10.1016/j.humpath.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Rosca AM, Burlacu A. Effect of 5-azacytidine: Evidence for alteration of the multipotent ability of mesenchymal stem cells. Stem Cells Dev. 2011;20(7):1213–21. doi: 10.1089/scd.2010.0433. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Ahrens TD, Timme S, Hoeppner J, et al. Selective inhibition of esophageal cancer cells by combination of HDAC inhibitors and Azacytidine. Epigenetics. 2015;10(5):431–45. doi: 10.1080/15592294.2015.1039216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Margariti A, Kelaini S, et al. MicroRNA-199b modulates vascular cell fate during iPS cell differentiation by targeting the notch ligand Jagged1 and enhancing VEGF signaling. Stem Cells (Dayton, Ohio) 2015;33(5):1405–18. doi: 10.1002/stem.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao R, Li Y, Lin Z, et al. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3beta/beta-catenin signaling pathway. Biochem Biophys Res Commun. 2016;477(4):749–54. doi: 10.1016/j.bbrc.2016.06.130. [DOI] [PubMed] [Google Scholar]
- 30.Zou Y, Li J, Ma H, et al. Heat shock transcription factor 1 protects heart after pressure overload through promoting myocardial angiogenesis in male mice. J Mol Cell Cardiol. 2011;51(5):821–29. doi: 10.1016/j.yjmcc.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Lanneau D, de Thonel A, Maurel S, et al. Apoptosis versus cell differentiation role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1(1):53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Huang W, Meng W, et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: A critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells (Dayton, Ohio) 2014;32(2):462–72. doi: 10.1002/stem.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yu X, Lin Q, et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42(2):295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]