Abstract
Objective:
The objective was to explore psychosocial experiences of closed loop technology for adults, children, and adolescents with type 1 diabetes and their parents taking part in two multicenter, free-living, randomized crossover home studies.
Methods:
Participants using insulin pump therapy were randomized to either 12 weeks of automated closed-loop glucose control, then 12 weeks of sensor augmented insulin pump therapy (open loop), or vice versa. Closed loop was used for 24 hours by adults and overnight only by children and adolescents. Participants completed the Diabetes Technology Questionnaire (DTQ) periodically and shared their views in semistructured interviews. This analysis characterizes the impact of the technology, positive and negative aspects of living with the device, alongside participants’ expectations, hopes, and anxieties.
Results:
Participants were 32 adults, age 38.6 ± 9.6 years, 55% male, and 26 children, mean age 12 years (range 6-18 years), 54% male. DTQ results indicated moderately favorable impact of, and satisfaction with, both open and closed loop interventions, but little evidence of a comparative advantage of either. Key positive themes included perceived improved blood glucose control, improved general well-being, particularly on waking, improved sleep, reduced burden of diabetes, and visibility of data. Key negative themes included having to carry around the equipment and dislike of the pump and second cannula (ie, sensor) inserted.
Conclusions:
Overall, participants reported a positive experience of the closed loop technology. Results are consistent with previous research with size of equipment continuing to be a problem. Progress is being made in the usability of the closed-loop system.
Keywords: closed loop, artificial pancreas, psychosocial, type 1 diabetes
Despite the rapid advancements in insulin pump technology and the ongoing development of more physiological insulin preparations, the currently available therapeutic options do not facilitate optimal glycemic control for many people with type 1 diabetes (T1D). The emergence of continuous glucose monitoring (CGM), which enables users to monitor real-time interstitial glucose readings and receive alarms for impending hypo- or hyperglycemia, support users in optimizing insulin therapy. Several recent studies have shown a clinical benefit of CGM and flash glucose monitoring (FGM) on reduction in HbA1c, in those participants that are able to meet the demands of using the device.1-5
The development of a closed loop system that combines continuous glucose monitoring with computer-based algorithm dictated insulin delivery, may support further improvements in glycemic control while reducing the risk of hypoglycemia. The vital component of such a system, also known as an artificial pancreas (AP), is a computer-based algorithm. The role of the control algorithm is to translate, in real time, the information it receives from the real-time CGM and to compute the amount of insulin to be delivered by the insulin pump.6 With the potential to provide a realistic treatment option for people with T1D, the usability and impact of psychosocial factors require evaluation to determine the realization of benefit from use of this technology.7 The purpose of this study, performed alongside two biomedical clinical trials, was to explore the hopes, expectations and experiences of those taking part in those trials.
Methods and Participants
Adults aged ≥18 years and children and adolescents aged 6-18 years using insulin pump therapy for at least six months, with HbA1c ≥7.5% (58 mmol/mmol) and ≤10% (86 mmol/mmol) (in pediatric study HbA1c was ≤10%) based on analysis from central laboratory or equivalent participated in the trial.
The study adopted an open-label, multicenter, randomized, two-period crossover design at Cambridge, UK, Profile Germany, and Graz, Austria.8 During closed loop intervention, adult participants used the closed loop system day and night while at home, work, and holidays. Meal bolus calculations were performed by participants using the standard bolus calculator. The pediatric study participants used the closed loop system overnight only while at home during school terms and holidays, reverting to open loop only during the day. Participants were instructed to initiate the system at home following their evening meal or at bedtime at latest, and to discontinue it before breakfast the next morning.
The study protocols were approved by independent research ethics committees. Both studies received approvals from regulatory authorities in the UK (Medicines & Health products Regulatory Agency), and the adult study was approved in Germany (Federal Institute for Drugs and Medical Devices) and Austria (Austrian Agency for Health and Food Safety). Written informed consent was acquired from all participants (or parents/guardians of minors) prior to any study-related procedures.
Identical commercially available insulin pumps (Dana R Diabecare, Sooil, Seoul, South Korea) and continuous glucose-monitoring devices (FreeStyle Navigator II, Abbott Diabetes Care, Alameda, CA, USA) were worn according to manufacturer’s instructions during the two treatment periods in the two trials. In the pediatric study, the control algorithm was residing on a tablet computer (Latitude 10, Dell, TX, USA), which was linked by cable to the CGM monitor overnight. In the adult study, the algorithm was running on a smartphone (Nexus 4, LG, South Korea), which wirelessly communicated with the study pump and the continuous glucose monitoring receiver.
Participants were asked to complete the Diabetes Technology Questionnaire (DTQ) prior to randomization and again at the end of each crossover period. This validated measure8-10 asks respondents to rate the package of diabetes technology they are using, with scores ranging from 30-150; high scores indicating more favorable satisfaction with and impact of the device/system of interest. The DTQ was administered at the end of each crossover period, and compared to before the study. Higher scores reflect more favorable impact of and satisfaction with use of the system being rated. The 30 “Current” and “Change” items were supplemented with nine usability ratings of each glucose meter, insulin pump, CGM, or closed loop system in use at each measurement point. The alpha coefficients were, for adolescents, DTQ Current .89; DTQ Change .83 (compared with .95 and .94 in a prior sample of 151 adolescents); for adults, DTQ Current .92; DTQ Change .91. Sample questions cover fear or worry about low blood sugar during sleep and reacting to all of the blood sugar results.
Semistructured, qualitative interviews were conducted at baseline, midpoint, and study end for adult study and baseline plus end of closed loop phase for pediatric study with children and parents. All interviews were conducted individually by same interviewer at each time point at the sites, that is, KDB in the UK and VU in Germany and Austria. Interviewers were not directly part of the study team. Audiotapes were transcribed with all identifying details removed. The interviews explored participants’ experiences, their expectations and feelings immediately prior to starting the intervention. Then at the end of each phase participants were asked whether the technologies (closed loop and open loop) matched their expectation, whether any difficulties occurred, the benefits and downsides of living with the technologies throughout the duration of the trial and if there were any other aspects that had occurred as a consequence of trial participation. This mixed methods approach provided a cohesive, holistic psychosocial assessment while minimizing participant burden.
The interview questions were designed in collaboration with the clinical research team. The interview schedule was then piloted on six potential participants for usability, relevance, and acceptability. The feedback was positive with minor revisions suggested and the interview schedule was revised in line with these suggestions.
A thematic approach was used to analyze the data, informed by the method of constant comparison and involving concurrent data collection and analysis. A joint thematic analysis was used to compare interpretations and resolve any differences in interpretation to reach agreement on recurrent themes and findings.11 All three coders were experienced in qualitative methodology and independent from the study team. Saturation point was reached across the three sites with no further new themes emerging in any of the key themes across all sites for each study phase. Attrition rates of psychosocial substudy are reported in the results section. This analysis was used to develop a coding framework that captured original research questions and emerging findings. Thematic analysis identified key themes with a view to understanding participant experiences, exploring connections between themes and identifying how closed loop technology affects everyday living and factors important to quality of life in ways that are important to participants. Results are presented separately for sites to reflect similarities and differences between them.
Results
Diabetes Technology Questionnaire (DTQ)
The DTQ was completed by 26 adult participants at baseline, 22 following the closed loop phase and 18 following the open loop phase. The DTQ was completed by 20 children and adolescents at baseline, 16 following the closed loop phase, and 12 following the CGM phase of the study. Results are presented separately in Table 1 for children and adolescents and adults for the DTQ “Current” and “Change” items and then the usability ratings. Due to the small sample sizes and attrition, only descriptive analyses are reported.
Table 1.
Mean (SD) Usability Ratings Obtained From Pediatrics and Adults for Each Type of Device at the End of Each Study Phase.
| Adults |
Adolescents |
|||||
|---|---|---|---|---|---|---|
| BL | CL | CGM | BL | CL | CGM | |
| Glucose meter | 4.12 (0.73) | 3.74 (0.67) | 3.84 0.77 | 3.88 (0.65) | 3.84 (0.71) | 3.89 (0.74) |
| Insulin pump | 4.01 (0.62) | 3.37 (0.65) | 3.33 (0.54) | 3.60 (0.61) | 3.07 (0.58) | 3.33 (0.43) |
| Continuous glucose monitor | — | 3.55 (0.81) | 3.87 (1.03) | — | 3.84 (0.66) | 3.89 (0.51) |
| Closed loop system | — | 3.94 (0.91) | — | — | 3.65 (0.79) | — |
BL, baseline; CL, closed loop; CGM, open loop; —, no response.
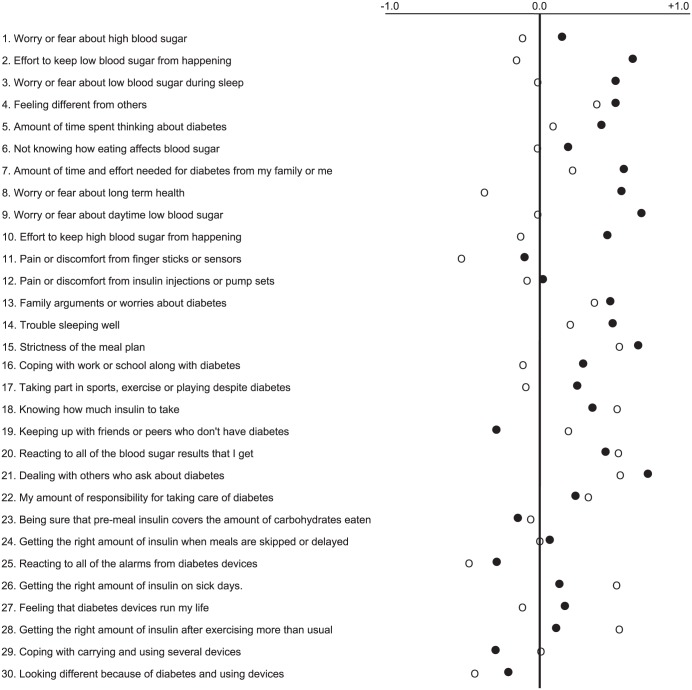
One method of comparing closed loop and open loop DTQ results is by calculation of difference scores between scores on the same DTQ items between each study phase and the corresponding score at Baseline. Figure 1 portrays these results with the combined adolescent and adult data. Difference scores were more positive after closed loop than open loop on 22 items while scores for 8 items scores were more negative or unchanged. Difference scores that fell into the negative range were obtained from adults on 17 items following the open loop phase and 6 items following the closed loop phase. Inferential statistical analyses were not done due to the small sample size.
Figure 1.
Mean differences in scores on DTQ Current items between the open loop and closed loop phases relative to Baseline scores. Positive differences imply favorable change in ratings of the impact of, and satisfaction with, the respective regimens. Filled circles represent closed loop and open circles represent open loop.
DTQ “Change” Items
Distributions of scores on the DTQ Change items were moderately positive and quite similar following both the open loop (mean, SD = 3.65, 0.74 for adults; 3.39, 0.50 for pediatrics) and closed loop phases (3.62, 0.81 for adults; 3.41, 0.46 for pediatric participants). Compared with adults, pediatric participants reported slightly less positive perspectives on changes experienced after both the open loop and closed loop phases.
Usability Ratings
Usability ratings for the individual components of the open and closed loop systems are presented separately for adults and pediatric participants in Table 1. Focusing on participants’ ratings of usability of the closed loop system, adults rated the closed loop system more positively (mean, SD = 3.94, 0.91) than did pediatric participants (3.65, 0.79). For both adults and pediatric participants, the usability items with the least favorable ratings were those related to “Size, weight, appearance and fashion issues” and “Use during sports, exercise and bathing.”
Interview Results
Adult demographic data are presented in Table 2. Twenty-six pediatric participants and 25 parents participated in the study and initial interviews (one participant, aged 18 years, preferred not to have a parent interviewed). Twenty-two parents and 14 children completed the second interviews, the younger children didn’t particularly want to be interviewed. Interview duration for pediatric participants ranged from 7 to 23 minutes (mean 15 minutes), and interview duration for parents ranged from 8 to 52 minutes (mean 22 minutes). Duration of diabetes ranged from 1.3 years to 11.0 years (mean 4.7 years); age of participants ranged from 6.6 years to 18.8 years (mean 12 years); there were 14 male (54%) and 12 female (46%) pediatric participants.
Table 2.
Demographic Data.
| Cambridge | Graz | Profil | |
|---|---|---|---|
| Adult participants | 11 | 10 | 11 |
| Gender (% male) | 55 | 50 | 55 |
| Age range (mean) | 27-60 (38.6 ± 9.6) | 32-57 (44.4 ± 7.3) | 21-54 (40.3 ± 9.7) |
| Db duration range (mean) | 8-34 (22.3 ± 8.8) | 10-35 (20.1 ± 8.8) | 6-49 (20.9 ± 11.4) |
| Interview duration range (mean) | 14-50 (27) | 15-38 (25) | 15-45 (21) |
Content Analysis: Pre/Poststudy
Participant expectations prestudy are summarized in Tables 3 and 4. Post–closed loop experience key themes are reported in Tables 5 and 6.
Table 3.
Pediatric Prestudy Expectations.
| Participant |
Parent |
|||||
|---|---|---|---|---|---|---|
| Pos | Neg | N/A | Pos | Neg | N/A | |
| Impact on sleep | — | — | — | 7 | — | 15 |
| Impact on usual routine | 3 | 11 | 8 | 8 | 12 | 2 |
| Feel safe | 11 | 2 | 8 | 13 | 2 | 7 |
—, no response.
Table 4.
Adult Prestudy Expectations.
| Hopes for CL study participation | Cambridge (N = 11) n (%) |
Profil (N = 10) n (%) |
Graz (N = 11) n (%) |
|---|---|---|---|
| Improved diabetes control | 9 (82) | 4 (40) | 4 (36) |
| Future health benefits | 7 (64) | — | 4 (36) |
| More information/better monitoring of blood glucose | 5 (45) | 3 (30) | 2 (18) |
| Greater understanding/improved awareness of diabetes | 5 (45) | 3 (30) | 4 (36) |
| Technological advancement | 3 (27) | 3 (30) | — |
| Improved sleep/feel better | 2 (18) | — | 4 (36) |
| Improved HbA1c | 2 (18) | 5 (50) | 5 (45) |
| Better outcomes during sports/work | — | — | 2 (18) |
—, no response.
Table 5.
Pediatric Study Post–Closed Loop Data Experience.
| Participant |
Parent |
|||||
|---|---|---|---|---|---|---|
| Pos | Neg | N/A | Pos | Neg | N/A | |
| Impact on sleep | 11 | 3 | 6 | 16 | 4 | 1 |
| Impact on usual routine | 11 | 8 | 1 | 13 | 5 | 3 |
| Graphs on tablet helpful | 8 | 3 | 9 | 19 | — | 2 |
| Felt safe | 13 | — | 7 | 18 | — | 3 |
| Would recommend | 15 | — | 5 | 18 | — | 3 |
| Would keep | 14 | 1 | 5 | 16 | 1 | 4 |
| Met expectations | 9 | 1 | 10 | 18 | 2 | 1 |
Pos, positive/yes; Neg, negative/no; N/A, not applicable or not answered; —, no response.
n = 20 participants and n = 21 parents.
Table 6.
Adult Perspective.
| Question | Closed Loop |
||
|---|---|---|---|
| Y | N | NS | |
| Felt safe | 26 | 1 | 5 |
| Would recommend CL | 22 | 1 | 9 |
| Would keep CL | 21 | 3 | 8 |
| More time and effort | 16 | 9 | 7 |
Y, yes; N, no; NS, not sure/no answer.
Thematic Analysis
Pediatric Participants
Participants reported their subjective “best” aspects about closed as perceived improved blood glucose control (n = 6) and feeling better, especially on waking (n = 4). The “best” aspects about closed loop for parents were confidence/reassurance (n = 14), improved sleep (n = 5), and perceived improved blood glucose control (n = 11). Both parents and participants routinely reported the benefit of “waking up on a good number” and that it had an enduring positive effect during the following day of improved blood glucose control:
“Went to sleep with good levels and in the morning they were always the same and steady” (parent 001).
“It did help a lot bringing down my blood sugar and keeping it steady around the 5.8 mark” (child 024).
“I think having my blood sugars in target for a long period of time” (child 014).
“The confidence knowing that this machine could control her overnight” (parent 004a).
“The whole next day his blood sugar would be great” (parent 009a).
Participant 011 summed up their experience of the trial by saying, “When I wake up I can just get on with getting ready for school and read my bloods. I don’t have to worry about it going down. . . . It was really good for me because it means I don’t have to wake up earlier. My blood sugars were good in the morning so it meant I didn’t have to sort anything out.” This reflected the views of the other participants to some degree in terms of the reduced burden of diabetes, the perceived improved blood glucose control, and generally feeling better on waking for the rest of the (following) day.
The “worst” reported aspects were size of the equipment (n = 5), sensors and having to have a second cannula (n = 3), pump (n = 3), and getting used to the system (n = 3). The “worst” reported aspects by parents were the pump (n = 7), the size of the system (n = 9), the sensors (n = 4), and technical difficulties with connectivity and calibration (n = 6).
“When it played up and the sensors weren’t connecting” (parent 016).
“We did struggle from the start getting used to it” (parent 025).
“The pump, that caused quite a lot of frustration” (parent 003a).
“The fact that she had to wear this great big sensor, the pumps and then this great big computer” (parent 004a).
Things participants would change focused on the size of the system (n = 7), the pump (n = 5), and connectivity/technical issues (n = 4).
Nineteen parents found the graphs and information on the tablet more useful than the children, and tended to look at/review that information more. One parent (008a) summarized it as “Definitely helpful, gave me reassurance that it was working. It helped with patterns with sugars and going forward the graphs could show you that you need to change the basal rate to fix it.” Another parent (011a) commented that it was “really helpful to be able to look back . . . and see she had been in target all night.”
Adults
Post–closed loop Cambridge
Overall, the Cambridge participants’ experiences of using the closed loop system were positive:
“It was good, gives you more freedom” (adult 001).
“It was brilliant, sad to see the back of it. I would have carried on with it forever” (adult 005).
“It copes better with illness than perhaps people expect it would” (adult 006).
There were few concerns prior to starting the trial. One participant was worried it would “make them feel like a sick person” and when asked following the trial whether this has in fact been the case, the participant reported, “yes because you were always fiddling with something. There was always something beeping at me.” Another participant was concerned about the visibility of diabetes and did report that to be the case following the trial. However, this participant went on to say that this had helped the person to be able to have that conversation and talk to people about it.
The “best” aspects about the CL system reported by participants were not having to think about it (diabetes control), for example, “I didn’t have to worry about big highs overnight because it would fix it for me” (010); “Feeling good all of the time . . . it takes away the daily grind” (003); improved blood glucose control, for example, “It shaved off the vast majority of the highs and lows” (007); and “being able to look at the graph and see the amount of insulin I was actually having in comparison to what I would be giving myself” (002)
Reported “worsts” were carrying around the devices and equipment as well as a dislike of the study pump (n = 3). One participant reported additional hypoglycemia episodes due to exercise.
Post–open loop Cambridge
Key positive themes were perceived improved blood glucose control, as well as being able to see the trend graphs on the CGM device.
“% in target was a lot better. Very pleased with the open loop, would do it again” (adult 001).
“Definitely made a big difference overnight and in the mornings” (adult 009).
Reported “bests” about the open loop system included having greater awareness of blood sugars:
“Useful for me to get some overview of what my glucose levels were like at different times of the day” (adult 008).
“Having the CGM and being able to read it every 5 minutes” (adult 007).
“I liked having the sensor and knowing what my blood sugars were” (adult 010).
Key negative themes were carrying the equipment around along with calibration and usability difficulties. One participant reported “I would quite like a bit of time off thinking about diabetes” now the trial had come to an end; another said “It wasn’t as good as I had hoped” (adult 002), and a third said “I had some problems with the infusion sets” (adult 007).
“Worsts” about the open loop system were the pump, set changes, and the alarms. Participants would change the pump and the size of all the equipment.
Post–closed loop Graz participants
The majority of participants had a positive experience, with no major concerns prior to study start. The control and the monitoring of the blood sugar, especially overnight, were reported as a relief. One participant cited glucose fluctuations, however these did not transpire, although the participant also commented that there remained room for improvement in blood glucose stability. Another was worried about problems during physical training; this was applicable because the participant had additional hypoglycemic episodes during sports. An experience similarly reported by other participants.
The “best” things about the closed loop reported by the participants were the following:
Improvement of the blood sugar control: “the 100% transparency of the blood sugar curve” (adult 016) and “it was very positive of course that you have the whole blood sugar curve at a glance” (adult 012).
A relief of diabetes treatment: “I could sleep during the whole night without worrying” (adult 011) and “It is great that you always know your blood sugar and that the closed loop system takes care of it” (adult 013).
Relating to the “worst” things about CL systems, the alarms were disturbing, usability was challenging, and there was a large number of necessary devices.
“Especially in the beginning, I had many alarms, . . . the technique should be more advanced” (adult 004).
Relating to the devices: “And the next bad thing was of course the bulkiness of the devices. You have to carry them with you all the time” (adult 012).
Post–open loop Graz
The “best” reported aspect of open loop was the continuous overview and the possibility to have trend graphs of the blood sugar values. The participants reported:
“[The best aspect] is the constant monitoring of the blood sugar level, you have a continuous data stream. When you measure by yourself with finger pricking, you only have single values and no curve” (adult 003).
“My expectations were even surpassed I have a much better overview on my blood sugar level” (adult 007).
The “worst” aspects were the usability of the pump as well as the alarms and calibration of the CGM device.
Post–closed loop Profil
Most participants reported positive experiences with closed loop therapy, with a key positive theme being steadiness of blood sugar. Improved quality of life was also reported. Only one participant reported severe problems during closed loop usage because of difficulties in adjustment of blood glucose. This participant also experienced technical problems.
Reported “best” aspects were the following:
Relief in diabetes treatment: “I actually did not have to do anything anymore and it was amazing that the pump worked that well” (adult 014), another participant added: “It was an absolute improvement to my previous pump therapy” (adult 018).
Steadiness of blood glucose: “Also the closed loop worked well, especially at night, it was very good for me. I often have hypoglycemia, which did not occur when I had the CL system.” (adult 011). One participant reported, “Generally, my blood sugar was very stable and stayed stable for several hours” (adult 013).
Despite feeling safe whist using the closed loop system, all participants reported downsides, mostly related to failures of technology and problems with the equipment and disturbing alarms.
“Closed loop is a great thing. Unfortunately I had a few problems with system crashes” (adult 018).
“However, the nights were terrible, I had alarms constantly and I could not get sleep during the nights” (adult 008). Another participant reported, “I mean there have been days where it worked very well, but, well, not all the time” (adult 004).
Post–open loop Profil
Positive aspects during open loop treatment were perceived improvement of blood glucose control and the good overview of the blood sugar values. Hypoglycemia warning alarms were a particular benefit for the patients. The “best” things were the visibility of blood sugar values: “I constantly had a very simple control of the blood glucose curve without measuring by myself” (adult 004).
Key negative themes were the pump and the alarms of CGM system. Two participants reported several hypoglycemic episodes during open loop treatment. After termination of the trial some participants complained about problems with blood sugar fluctuations.
Discussion
DTQ scores indicated that both the open and closed loop systems yielded favorable impact, usability, and satisfaction ratings from adults and children. Participants rated both the open and closed loop systems favorably on an absolute level and on a relative level for impact and satisfaction (but not usability) in comparison with their previous diabetes technology. Children’s ratings of the closed loop system were consistently less favorable than those of adults, perhaps because children used that system only overnight rather than 24 hours a day or because children perceived greater inconvenience or intrusion. The least favorable usability ratings were reported for size, weight, and appearance of the closed loop system and its use during sports, exercise, and bathing. Generalizability of such subjective results is not possible due to small participant numbers across three countries.
Prior to the trial start, participants were not concerned about taking part and were generally optimistic about the potential benefits of participation and the closed loop system. The only perceived downside for parents was the potential additional burden on daily routines however this concern was not realized and most parents reported a positive impact in this regard. Similarly, 16 parents and 11 children reported a positive impact on sleep throughout the trial, consistent with previous research.10 Chronic sleep deprivation is widespread for parents of children with T1D10 with a consequential negative impact on well-being. Having the reassurance that the system is taking care of the diabetes, with associated reduced burden of diabetes management, throughout the trial was reported with 18 parents and 13 children reporting feeling safe while using the equipment.
Overall, participants reported positive aspects of taking part, however in line with previous studies there were challenges with connectivity and portability of the closed loop system. This was consistently reported across study sites. Improved blood glucose control was the primary benefit reported by participants and having greater control over diabetes in terms of being able to see what was happening. These were themes reported for both the closed loop and open loop phase of the trial across all study sites. The burden of diabetes self-management is relentless and people with diabetes (PWD) seek solutions and technologies that will alleviate that burden.12
All participants reported having experienced some technical or usability difficulties with the equipment, however these were most often associated with the insulin pump and connectivity challenges rather than with the closed loop technology itself. Alarms appeared to be a continuous problem, particularly overnight, contributing toward disturbed sleep.
The questionnaire data and qualitative interview findings yielded a relatively consistent perspective of participants’ psychosocial reactions to use of the closed and open loop systems. Both the DTQ and interview findings showed that participants were generally favorably disposed toward both systems, while the interview results tended to reveal somewhat stronger satisfaction with use of the closed loop system compared with the DTQ results. In terms of usability issues, both the DTQ and interview methods suggested that participants were least satisfied with structural/logistical aspects of the technology, implying that integration of the closed loop system into a single device and miniaturizing the device would be well received by patients.
The presence of increased hypoglycemic events at Profil and Graz, in particular associated with sports related activities remains a concern for adults. Overnight success of closed loop systems is widely reported, with positive results in 24 hour trials.13 Fear of hypoglycemia is widely reported by PWD and closed loop systems must be designed with consideration for the prevention of both fear and experience of hypoglycemia. This issue was not raised as a concern by adult participants at Cambridge, nor by pediatric participants. In fact, improved sleep as a consequence of greater confidence in optimized blood glucose control was reported by pediatric and parent participants.
Participants who had taken part in previous trials, reported a clear improvement in the size and connectivity of the equipment, however expressed a preference for only one device. This is consistent with previous research reporting the preferences reported by people when thinking about artificial pancreas/closed loop systems.9 It is clear that the closed loop technology is progressing at a remarkable pace and patient reported outcomes support the clinical data in this regard.
Limitations of the current study include small participant numbers and the current difficulty of demonstrating clinically and statistically significant differences between any two successive stages in the evolution of fully automated closed loop insulin delivery. The differences between successive stages are by necessity incremental and small, with the expense and intensity of early clinical trials thus far prohibiting enrollment of large samples. Concurrent evaluation of metabolic and psychosocial effects are essential to ensure the uptake and continued use of such systems by people with T1D to meet their own personal needs. The INSPIRE study group is currently developing novel measures to achieve this goal and ensure that people with T1D receive appropriate training and support in the new systems for successful use.
Conclusions
Overall, participants reported a positive experience of the closed loop technology. Results were consistent with previous research in terms of benefits and downsides of the technology. Progress is clearly being made in the usability of the closed loop system toward a marketable device.
Footnotes
Abbreviations: AP, artificial pancreas; CGM, continuous glucose monitoring; CL, closed loop; DTQ, Diabetes Technology Questionnaire; FGM, flash glucose monitoring; GRZ, Graz; JDRF, Juvenile Diabetes Research Association; PRO, Profil; PWD, people with diabetes; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JDRF (22-2011-668) and Seventh Framework Programme of the European Union (ICT FP7- 247138). Additional support for the Artificial Pancreas work by National Institute for Health Research Cambridge Biomedical Research Centre and Wellcome Strategic Award (100574/Z/12/Z). Abbott Diabetes Care supplied discounted continuous glucose monitoring devices, sensors, and details of communication protocol to facilitate real-time connectivity. Diasend provided discounted platform for data upload.
References
- 1. Deis D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730-2732. [DOI] [PubMed] [Google Scholar]
- 2. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-U1465. [DOI] [PubMed] [Google Scholar]
- 3. Kordonouri O, Pankowska E, Rami B, et al. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53(12):2487-2495. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: Results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10:377-383. [DOI] [PubMed] [Google Scholar]
- 5. Bolinder J, Antuna R, Geelhoed-Dujvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. [DOI] [PubMed] [Google Scholar]
- 6. Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23:1-12. [DOI] [PubMed] [Google Scholar]
- 7. Barnard KD, Hood KK, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther. 2015;17(4):295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. NEJM. 2015; 373:2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wysocki T, Reeves G, Kummer M, Ross JL, Yu M. Psychometric validation of the Diabetes Technology Questionnaire. Diabetes. 2015. (suppl 2):A633. [Google Scholar]
- 10. Barnard KD, Wysocki T, Allen JM, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Diabetes Res Care. 2014;2:000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neuendorf KA. The Content Analysis Handbook. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 12. Barnard KD, Pinsker JE, Oliver N, Astle A, Eyal D, Kerr D. Future artificial pancreas technology for type 1 diabetes: what do users want? Diabetes Technol Ther. 2015;17(5): 311-315. [DOI] [PubMed] [Google Scholar]
- 13. Russell S. Progress of artificial pancreas devices towards clinical use: the first outpatient studies. Curr Opin Diabetes Obes Metab. 2015;22(2): 106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]