Abstract
Background:
Patients with diabetes, especially pediatric ones, sometimes use continuous glucose monitoring (CGM) sensor in different positions from the approved ones. Here we compare the accuracy of Dexcom® G5 CGM sensor in three different sites: abdomen, gluteus (both approved) and arm (off-label).
Method:
Thirty youths, 5-9 years old, with type 1 diabetes (T1D) wore the sensor during a clinical trial where frequent self-monitoring of blood glucose (SMBG) measurements were obtained. Sensor was inserted in different sites according to the patient habit. Accuracy metrics include absolute relative difference (ARD) and absolute difference (AD) of CGM with respect to SMBG. The three sites were compared with ANOVA. If the test detected a difference, an additional pair-wise comparison was performed.
Results:
Overall, no accuracy difference was detected: the mean ARD was 13.3% (SD = 13.5%) for abdomen, 13.4% (12.9%) for arm and 12.9% (20.2%) for gluteus (P value = .83); the mean AD was 17.0 mg/dl (17.2 mg/dl) for abdomen, 17.2 mg/dl (17.1 mg/dl) for arm and 18.3 mg/dl (18.5 mg/dl) for gluteus (P value = .30). In hypo- and euglycemia ARD (P value = .87 and .15, respectively), and AD (P value = .68 and .37, respectively) were not statistically different. At variance, in hyperglycemia, a significant difference was detected between the two approved sites, abdomen and gluteus (ΔARD = −2.2% [CI = −4.2%, −0.1%], P value = .04), whereas the comparisons with the off-label location, arm-abdomen, and arm-gluteus were not significant.
Conclusions:
These results suggest that the accuracy of the sensor placed on the arm was not significantly different with respect to the two approved insertion sites (abdomen and gluteus). Larger, randomized trials are needed to draw final conclusions.
Keywords: accuracy evaluation, continuous glucose monitoring, insertion sites, type 1 diabetes, pediatric
This article focuses on the Dexcom® G5 (Dexcom, Inc, San Diego, CA) continuous glucose monitoring (CGM) sensor, one of the most used and accurate system available on the market. This sensor is approved to be inserted only on the abdomen or gluteus area,1 as clearly stated in the user manual.2 Nonetheless, patients with diabetes, especially pediatric ones, sometimes use CGM sensor in different positions from the approved ones, for example on their arm.
A number of studies assessed the accuracy of various CGM systems,3-5 particularly on prepubertal population with type 1 diabetes (T1D).6-9 Among them, Laffel9 evaluated the accuracy of the CGM system considered in this article in youths. Nevertheless, no comparative analysis between sensor insertion sites has been made yet, not even in adult patients.
In this article, the accuracy of the CGM sensor under study will be compared in relation to three different insertion sites: abdomen, gluteus and arm.
To do this, we used data available from the Pediatric Artificial Pancreas (PedArPan) project,6 where sensor-augmented pump (SAP) therapy was compared with the artificial pancreas (AP) in children during a camp. In this study, youths wore the sensor on buttocks and abdomen as well as on the arm, depending on their habits, thus offering the possibility to perform a comparison between the three insertion sites.
Methods
Dataset
In the PedArPan project,6 a total of 30 T1D children, 5 to 9 years old, completed an outpatient, closed-loop trial during a summer camp. Data, collected in the 30 pediatric subjects (19 boys and 11 girls), are reported in Del Favero et al:6 age, 7.6 years (SD 1.2 years); body weight, 26.0 kg (6.1 kg); height, 123 cm (8 cm); BMI, 16.9 kg/m2 (2.1 kg/m2), BMI z-score, 20.1 (0.9); HbA1c, 7.3% (0.9%) (57 mmol/mol [10 mmol/mol]); duration of diabetes, 4.7 years (1.6 years); pump users for 3.3 years (1.9 years); and total daily insulin, 20.3 units (6.2 units) (0.8 units/kg per day [0.2 units/kg per day]). An average of 38.4 (SD = 11.1) self-monitoring of blood glucose (SMBG) measurements was available for each CGM session. No specific schedule for SMBG sampling was used: they were instead collected according to a protocol designed to mimic the usual clinical practice, that is, before each meal, 2 hours after the meal, before and after physical exercise, and/or to verify any hypo/hyper alarm issued by the AP or by the CGM sensor.
In this study, three days with the AP—based on a children-specific version of the modular model predictive control (MMPC) algorithm10,11 and running on the diabetes assistant (DiAs) wearable platform12—were compared with 3 days of parent-managed SAP therapy, showing feasibility and safety of the AP. Both interventions lasted 72 hours and were separated by a 24-hour washout period.
Capillary blood glucose (BG) concentration (self-monitoring of blood glucose, SMBG) was frequently measured using the Accu-Chek® Aviva Combo BG meter (Roche Diabetes Care AG, Burgdorf, Switzerland). Patients wore the Dexcom® G4 Platinum Share CGM system equipped with the software 505 (also known as G4AP, Dexcom, Inc, San Diego, CA, USA), that can be considered equivalent to Dexcom® G5 for our purposes (see the Discussion for details).
Per protocol, a sensor was inserted in the afternoon of day 0 (arrival at the camp) and kept for about 3 days, then a new sensor was inserted in the afternoon of day 4 (wash-out) and kept for other 3 days. Data from 08:00 of day 1 to 08:00 of day 4 and from 08:00 of day 5 to 08:00 of day 8 were available for the analysis.
Since no difference in sensor accuracy was found between SAP and AP,6 here we considered data from the two different therapies together.
In the two study sessions, patients inserted the CGM sensor in different locations according to their habit. As a result, the 60 sensor sessions (30 patients, two sessions each) were distributed as follow: 11 youth wore the sensor in the abdomen, 19 in the arm and 30 in the gluteus.
Accuracy Outcomes
SMBG measurements and CGM readings were downloaded from the devices, carefully synchronized at the beginning of the experiment. Each available SMBG measurement was matched with the CGM reading at same time.
The metrics used to assess the sensor accuracy were absolute relative difference (ARD) and absolute difference (AD), evaluated on all the available CGM-SMBG data pairs, regardless the patient in which they were collected.
We also computed the percentage of data points falling in zone A of Clarke Error Grid (CEG A), and the percentage of data matching the International Organization for Standardization (ISO) 15197:2013 15/15%, that is, the percentage of data falling within either 15 mg/dl from the SMBG measurement if the SMBG < 100 mg/dl or within 15% of SMBG if SMBG > 100 mg/dl. We considered also other ranges, specifically the 5/5%, 10/10% and 20/20% ranges.
In addition, we also evaluated the accuracy in three glycemic regions: euglycemia (70 mg/dl < SMBG ≤ 180 mg/dl), hypoglycemia (SMBG ≤ 70 mg/dl), and hyperglycemia (SMBG > 180 mg/dl).
To evaluate patient-level variability of the accuracy, for each patient we calculated the mean ARD (MARD), the median ARD (MedARD), the mean AD (MAD), and the median AD (MedAD). Then, we analyzed the distribution of these metrics on the 60 sensor sessions (30 patients, two sessions each). Similarly, for each patient we computed also the other aforementioned metrics (ISO 5/5%, 10/10%, 15/15%, 20/20% and CEG A) and analyzed their distribution on the 60 sensor sessions.
We reported both mean (SD) values and median [25th, 75th] percentile of the AD and ARD distributions, to allow a comparison with other similar works.
All the other normally distributed metrics are reported as mean (SD) values, while nonnormally distributed ones as median [25th, 75th] percentile. Normality was assessed with Lilliefors test.
Statistics
To detect a difference in the accuracy metrics among the different insertion sites a three-arm analysis of variance (ANOVA) was used. The Kruskal-Wallis test was used in place of the ANOVA, whenever one of the analyzed distributions was nonnormal.
If ANOVA/Kruskal-Wallis tests detected a difference between the insertion sites, the three pair-wise comparisons (abdomen vs arm, arm vs gluteus, and abdomen vs gluteus) were performed using an unpaired t-test or a Wilcoxon rank sum test. A correction procedure for multiple comparisons was considered.
Results of these comparisons are presented as Δ [CI], that is, difference between two mean/median accuracy in the two wearing-positions and [confidence interval boundary at 95%] on the estimated Δ. Furthermore, the P values associated to the comparison are reported.
Statistical analysis was performed with Matlab R2015b software (The MathWorks, Inc, Natick, MA, USA) using the Statistics 10.1 toolbox. All P values were two-tailed. P values <.05 were considered statistically significant.
Results
Table 1 reports the accuracy metrics evaluated on all the available original CGM-SMBG data pairs, regardless of the patient in which they were collected.
Table 1.
Accuracy Metrics Evaluated on All Available CGM-SMBG Data Pairs, Regardless of the Patient in Which They Were Collected.
| ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|
| Metric | Unit | All | Abdomen | ARM | Gluteus | P value | ||
| Overall | Matched pairs | # | 2291 | 413 | 762 | 1116 | ||
| ARD | Mean (SD) | % | 13.1 (16.9) | 13.3 (13.5) | 13.4 (12.9) | 12.9 (20.2) | .83 | |
| Median [25-75] percentile | % | 9.4 [4.3, 17.5] | 9.7 [4.5, 17.1] | 9.9 [4.3, 18.1] | 9.0 [4.2, 17.1] | |||
| AD | Mean (SD) | mg/ml | 17.7 (17.8) | 17.0 (17.2) | 17.2 (17.1) | 18.3 (18.5) | .30 | |
| Median [25-75] percentile | mg/ml | 13.0 [6.0, 23.0] | 13.0 [6.0, 22.0] | 12.0 [5.0, 24.0] | 13.0 [6.0, 23.0] | |||
| ISO 5/5% | % | 30.4 (—) | 28.3 (—) | 32.2 (—) | 30.0 (—) | |||
| ISO 10/10% | % | 55.9 (—) | 54.7 (—) | 55.0 (—) | 56.9 (—) | |||
| ISO 15/15% | % | 72.5 (—) | 73.4 (—) | 72.6 (—) | 72.0 (—) | |||
| ISO 20/20% | % | 82.9 (—) | 83.1 (—) | 81.8 (—) | 83.7 (—) | |||
| CEG A zone | % | 81.2 (—) | 81.1 (—) | 80.2 (—) | 81.9 (—) | |||
| Hypoglycemia | Matched pairs | # | 166 | 26 | 75 | 65 | ||
| ARD | Mean (SD) | % | 19.2 (45.1) | 16.7 (14.0) | 16.7 (15.4) | 23.2 (69.7) | .87 | |
| Median [25-75] percentile | % | 12.9 [6.2, 21.7] | 13.1 [6.2, 24.6] | 12.9 [6.1, 21.2] | 12.7 [6.1, 20.3] | |||
| AD | Mean (SD) | mg/ml | 10.4 (14.6) | 9.9 (7.8) | 9.5 (7.6) | 11.6 (21.4) | .68 | |
| Median [25-75] percentile | mg/ml | 8.0 [4.0, 14.0] | 7.0 [4.0, 16.0] | 8.0 [4.0, 13.7] | 8.0 [4.0, 13.3] | |||
| ISO 5/5% | % | 30.1 (—) | 30.8 (—) | 30.7 (—) | 29.2 (—) | |||
| ISO 10/10% | % | 62.0 (—) | 53.8 (—) | 61.3 (—) | 66.2 (—) | |||
| ISO 15/15% | % | 78.9 (—) | 69.2 (—) | 81.3 (—) | 80.0 (—) | |||
| ISO 20/20% | % | 91.0 (—) | 92.3 (—) | 90.7 (—) | 90.8 (—) | |||
| CEG A zone | % | 91.0 (—) | 88.5 (—) | 90.7 (—) | 92.3 (—) | |||
| Euglycemia | Matched pairs | # | 1392 | 263 | 470 | 659 | ||
| ARD | Mean (SD) | % | 14.6 (13.2) | 15.8 (15.0) | 14.8 (13.5) | 14.0 (12.2) | .15 | |
| Median [25-75] percentile | % | 11.0 [4.8, 20.2] | 12.0 [5.9, 20.6] | 11.0 [4.9, 20.5] | 10.5 [4.2, 20.0] | |||
| AD | Mean (SD) | mg/ml | 16.2 (15.3) | 17.4 (18.5) | 16.1 (14.9) | 15.8 (14.2) | .37 | |
| Median [25-75] percentile | mg/ml | 12.0 [5.0, 22.0] | 12.0 [7.0, 22.0] | 12.0 [5.0, 22.0] | 12.0 [5.0, 21.7] | |||
| ISO 5/5% | % | 27.4 (—) | 22.4 (—) | 28.3 (—) | 28.7 (—) | |||
| ISO 10/10% | % | 49.1 (—) | 45.6 (—) | 49.6 (—) | 50.1 (—) | |||
| ISO 15/15% | % | 66.2 (—) | 66.5 (—) | 67.2 (—) | 65.3 (—) | |||
| ISO 20/20% | % | 77.2 (—) | 76.0 (—) | 76.6 (—) | 78.0 (—) | |||
| CEG A zone | % | 74.3 (—) | 73.4 (—) | 74.0 (—) | 74.8 (—) | |||
| Hyperglycemia | Matched pairs | # | 733 | 124 | 217 | 392 | ||
| ARD | Mean (SD) | % | 9.0 (8.6) | 7.3 (6.0) | 9.1 (9.0) | 9.5 (9.1) | .05 | |
| Median [25-75] percentile | % | 6.8 [3.4, 12.4] | 6.2 [2.7, 10.1] | 6.6 [3.0, 12.7] | 6.9 [4.0, 12.7] | |||
| AD | Mean (SD) | mg/ml | 22.1 (21.5) | 17.7 (15.2) | 22.2 (21.9) | 23.4 (22.8) | .03 | |
| Median [25-75] percentile | mg/ml | 16.0 [8.0, 30.0] | 14.0 [6.0, 24.0] | 16.0 [7.0, 30.0] | 17.0 [9.0, 31.0] | |||
| ISO 5/5% | % | 36.3 (—) | 40.3 (—) | 41.0 (—) | 32.4 (—) | |||
| ISO 10/10% | % | 67.4 (—) | 74.2 (—) | 64.5 (—) | 66.8 (—) | |||
| ISO 15/15% | % | 82.9 (—) | 88.7 (—) | 81.1 (—) | 82.1 (—) | |||
| ISO 20/20% | % | 92.1 (—) | 96.0 (—) | 89.9 (—) | 92.1 (—) | |||
| CEG A zone | % | 92.1 (—) | 96.0 (—) | 89.9 (—) | 92.1 (—) |
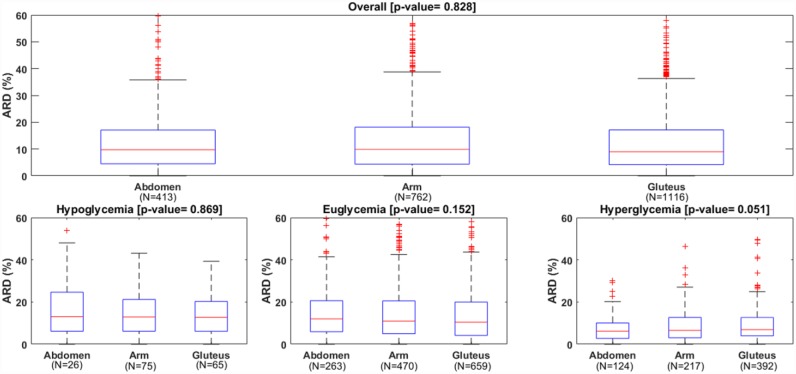
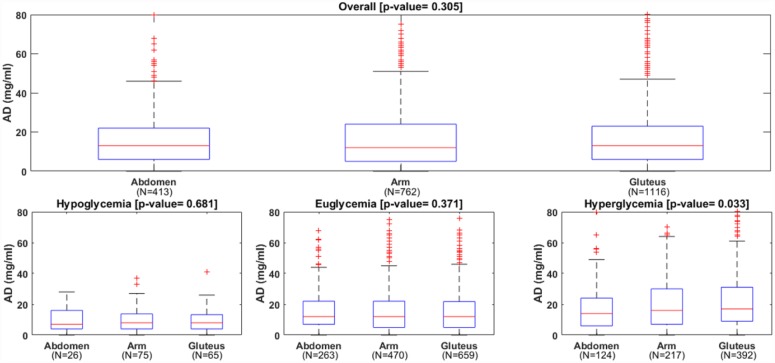
The overall mean/median of ARD were not significantly different in the three wearing locations: 13.3/9.7% for abdomen, 13.4/9.9% for arm and 12.9/9.0% for gluteus (P value = .83). Likewise, the overall mean/median of AD were similar: 17.0/13.0 mg/dl for abdomen, 17.2/12.0 mg/dl for arm and 18.3/13.0 mg/dl for gluteus (P value = .30). The boxplots of ARD and AD distribution are reported in Figures 1 and 2 to further illustrate these findings. The number of points matching the ISO criteria were also similar among the three locations.
Figure 1.
Distributions of absolute relative difference (ARD) of the CGM, overall (upper panel) and in each glycemic range (lower panels).
Figure 2.
Distributions of absolute difference (AD) of the CGM, overall (upper panel) and in each glycemic range (lower panels).
As detailed in Table 1, in hypo- and euglycemia the absence of statistically significant differences in ARD (P value = .87 and P value = .15, respectively) and AD (P value = .68 and P value = .37, respectively) was confirmed. On the contrary, statistical significant differences were found in the hyperglycemia: mean/median ARD was 7.3/6.2% for abdomen, 9.1/6.6% for arm and 9.5/6.9% for gluteus (P value = .05); mean/median AD changed from 17.7/14.0 mg/dl for abdomen, to 22.2/16.0 mg/dl for arm and to 23.4/17.0 mg/dl for gluteus (P value = .03). Thus, for hyperglycemia the pair-wise comparison was performed, showing no significant difference between arm versus gluteus (P = .86 for ARD and P = .78 for AD) and between arm versus abdomen (P = .16 for ARD and P = .14 for AD). The comparison of the two approved sites showed that abdomen was significantly better than gluteus: mean ARD difference was ΔARD = −2.2% [−4.2%/−0.1%] (P value = .04); mean AD difference was ΔAD = −5.8 mg/dl [−11.0 mg/dl/−0.6 mg/dl] (P value = .02).
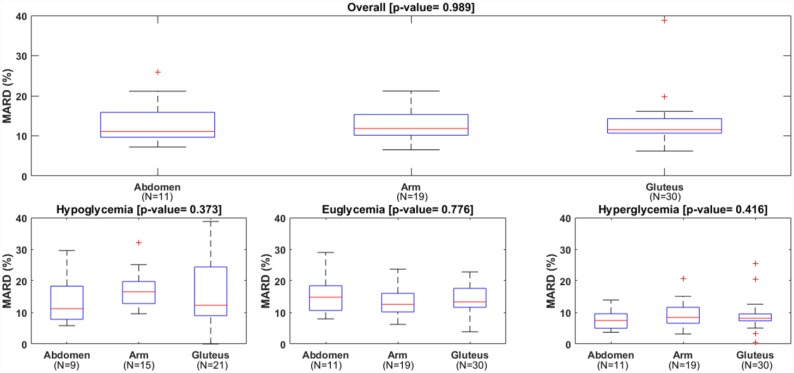
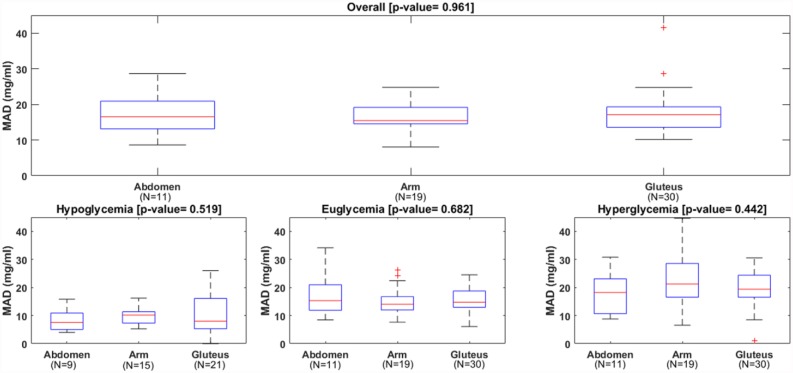
Table 2 reports the patient-level analysis. All metrics were computed for each sensor session and, then, their distribution across the 60 sessions was considered. Overall MARD and MAD were not different among wearing locations: median MARD was 11.1% for abdomen, 11.8% for arm and 11.5% for gluteus (P value = .99); median MAD was 16.5 mg/dl for abdomen, 15.4 mg/dl for arm and 17.1 mg/dl for gluteus (P value = .96). The boxplots of MARD and MAD distribution among the patients, reported in Figures 3 and 4, confirmed these findings. In the same way, overall MedARD and MedAD were not different among wearing locations: median MedARD was 10.0% for abdomen, 10.2% for arm and 8.6% for gluteus (P value = .85); median MedAD was 14.0 mg/dl for abdomen, 11.5 mg/dl for arm and 13.3 mg/dl for gluteus (P value = .40). The number of points matching the ISO criteria was also similar in the three locations.
Table 2.
Patient-Level Analysis: The Metrics Are Computed for Each Patient and Then the Distribution of Across the 60 Sensor Sessions (30 Patients, Two Sessions Each) Is Reported.
| ANOVA |
|||||||
|---|---|---|---|---|---|---|---|
| Metric | Unit | All | Abdomen | Arm | Gluteus | P value | |
| Overall | Matched pairs | # | 38.2 (11.0) | 37.5 (12.1) | 40.1 (12.4) | 37.2 (9.9) | |
| MARD | % | 11.5 [10.4, 15.1] | 11.1 [9.6, 15.9] | 11.8 [10.1, 15.4] | 11.5 [10.7, 14.3] | .99 | |
| MedARD | % | 9.2 [7.4, 11.8] | 10.0 [7.3, 11.9] | 10.2 [6.9, 11.9] | 8.6 [7.5, 11.0] | .85 | |
| MAD | mg/ml | 17.0 [13.8, 19.4] | 16.5 [13.1, 20.9] | 15.4 [14.5, 19.1] | 17.1 [13.6, 19.3] | .96 | |
| MedAD | mg/ml | 13.0 [10.0, 15.3] | 14.0 [10.0, 16.5] | 11.5 [9.1, 14.0] | 13.3 [11.0, 15.5] | .40 | |
| ISO 5/5% | % | 31.5 [22.6, 38.8] | 26.2 [18.3, 39.4] | 32.0 [23.1, 39.2] | 32.7 [24.0, 36.7] | .65 | |
| ISO 10/10% | % | 57.5 [48.8, 63.5] | 54.1 [47.1, 62.9] | 53.8 [48.3, 66.2] | 58.4 [50.0, 63.0] | .74 | |
| ISO 15/15% | % | 73.4 [66.7, 81.8] | 72.0 [59.4, 84.7] | 76.5 [66.7, 84.7] | 73.4 [66.7, 78.3] | .93 | |
| ISO 20/20% | % | 85.2 [77.4, 91.2] | 84.0 [72.7, 95.1] | 85.7 [80.2, 91.4] | 84.9 [77.8, 90.0] | .97 | |
| CEG A zone | % | 84.2 [74.5, 89.0] | 84.0 [71.9, 94.2] | 85.7 [79.5, 89.9] | 84.2 [75.0, 87.5] | .91 | |
| Hypoglycemia | Matched pairs | # | 3.7 (2.3) | 2.9 (1.7) | 5.0 (2.7) | 3.1 (1.9) | |
| MARD | % | 14.0 [10.3, 19.3] | 11.2 [7.8, 18.3] | 16.5 [12.8, 19.8] | 12.3 [9.0, 24.4] | .37 | |
| MedARD | % | 13.2 [7.7, 17.9] | 10.9 [6.5, 18.1] | 16.1 [11.1, 17.9] | 9.4 [6.1, 18.9] | .38 | |
| MAD | mg/ml | 8.4 [6.4, 11.5] | 7.5 [5.0, 10.9] | 10.2 [7.3, 11.4] | 8.0 [5.3, 16.1] | .52 | |
| MedAD | mg/ml | 7.5 [4.9, 11.0] | 7.5 [4.0, 11.3] | 9.0 [6.1, 10.9] | 6.0 [4.0, 12.3] | .69 | |
| ISO 5/5% | % | 33.3 [0.0, 50.0] | 50.0 [25.0, 75.0] | 25.0 [14.3, 43.3] | 20.0 [0.0, 50.0] | .33 | |
| ISO 10/10% | % | 57.1 [50.0, 80.8] | 50.0 [47.5, 75.0] | 50.0 [50.0, 60.0] | 66.7 [25.0, 100.0] | .57 | |
| ISO 15/15% | % | 80.0 [65.0, 100.0] | 66.7 [57.5, 100.0] | 80.0 [71.4, 97.5] | 83.3 [62.5, 100.0] | .97 | |
| ISO 20/20% | % | 90.9 (19.2) | 95.6 (13.3) | 89.7 (15.2) | 89.7 (23.7) | .72 | |
| CEG A zone | % | 90.6 (19.7) | 93.7 (13.8) | 87.5 (18.3) | 91.5 (23.0) | .73 | |
| Euglycemia | Matched pairs | # | 23.2 (8.7) | 23.9 (9.7) | 24.7 (9.7) | 22.0 (7.8) | |
| MARD | % | 13.2 [11.1, 17.7] | 14.8 [10.6, 18.4] | 12.6 [10.1, 16.0] | 13.3 [11.6, 17.6] | .78 | |
| MedARD | % | 11.0 [7.6, 14.3] | 12.7 [9.9, 15.5] | 8.9 [6.5, 14.0] | 10.7 [8.2, 13.8] | .32 | |
| MAD | mg/ml | 14.7 [12.7, 18.9] | 15.3 [11.9, 21.0] | 14.0 [12.0, 16.7] | 14.7 [13.0, 18.7] | .68 | |
| MedAD | mg/ml | 11.0 [9.0, 15.0] | 13.0 [9.9, 17.2] | 10.0 [8.0, 13.7] | 11.0 [10.0, 15.5] | .25 | |
| ISO 5/5% | % | 27.8 [16.7, 37.3] | 19.0 [14.2, 31.0] | 23.5 [16.6, 42.0] | 29.0 [23.3, 35.0] | .44 | |
| ISO 10/10% | % | 50.8 [37.7, 61.6] | 42.9 [34.4, 62.1] | 54.5 [37.8, 65.9] | 51.7 [38.9, 57.1] | .50 | |
| ISO 15/15% | % | 68.2 [55.6, 78.0] | 69.0 [53.9, 77.8] | 74.2 [63.3, 81.8] | 64.6 [54.2, 74.1] | .31 | |
| ISO 20/20% | % | 80.9 [66.7, 88.2] | 82.8 [62.8, 96.0] | 83.3 [74.3, 88.1] | 79.1 [66.7, 87.0] | .77 | |
| CEG A zone | % | 75.3 [65.5, 85.2] | 75.9 [62.1, 91.0] | 81.8 [73.3, 86.4] | 74.3 [65.4, 84.4] | .66 | |
| Hyperglycemia | Matched pairs | # | 12.2 (8.0) | 11.3 (8.5) | 11.4 (7.5) | 13.1 (8.4) | |
| MARD | % | 8.2 [6.4, 9.9] | 7.4 [5.0, 9.6] | 8.4 [6.6, 11.6] | 8.2 [7.3, 9.5] | .42 | |
| MedARD | % | 7.0 [5.4, 9.1] | 6.0 [5.0, 9.1] | 8.6 [5.0, 9.8] | 6.6 [5.5, 7.9] | .23 | |
| MAD | mg/ml | 19.5 [16.0, 24.9] | 18.2 [10.6, 23.1] | 21.2 [16.5, 28.5] | 19.4 [16.5, 24.4] | .44 | |
| MedAD | mg/ml | 17.0 [11.5, 21.0] | 13.5 [11.0, 18.7] | 21.0 [13.5, 24.0] | 17.0 [11.5, 18.0] | .13 | |
| ISO 5/5% | % | 35.0 [23.8, 50.0] | 42.9 [22.8, 50.0] | 33.3 [26.3, 56.7] | 34.3 [23.5, 48.7] | .82 | |
| ISO 10/10% | % | 68.3 [56.9, 81.5] | 75.0 [57.5, 85.7] | 61.5 [50.0, 82.5] | 70.6 [60.0, 76.2] | .35 | |
| ISO 15/15% | % | 83.3 [79.3, 100.0] | 90.9 [80.0, 100.0] | 83.3 [71.7, 96.1] | 82.8 [80.0, 100.0] | .70 | |
| ISO 20/20% | % | 92.1 (12.5) | 94.2 (8.9) | 88.8 (17.0) | 93.5 (10.1) | .37 | |
| CEG A zone | % | 92.1 (12.5) | 94.2 (8.9) | 88.8 (17.0) | 93.5 (10.1) | .37 |
Figure 3.
Distributions of mean absolute relative difference (MARD) of the CGM, overall (upper panel) and in each glycemic range (lower panels).
Figure 4.
Distributions of mean absolute difference (MAD) of the CGM, overall (upper panel) and in each glycemic range (lower panels).
No statistically significant differences in median MARD, median MAD, median MedARD, and median MedAD were also found for the three glycemic ranges.
Discussion
A clinically relevant question is whether or not wearing a CGM sensor on the arm ensures comparable accuracy with respect to other insertion sites. In fact, although the sensor considered in this paper was approved only to be inserted in the abdomen or in the gluteus, it is sometimes used on the arm. To the best of our knowledge, this study is the first attempt to investigate this question.
CGM data were collected using the Dexcom® G4 with software 505 that can be considered equivalent to Dexcom® G5 for the purpose of this paper: sensing and signal processing technologies are identical in the two sensor models, while they only differ from data transmission hardware (the first requires an ad hoc receiver, while the latter allows direct data transmission and processing on patient’s smartphone).
Overall, no statistically significant difference was found for the three wearing locations. Similarly, no difference was found in hypo- or euglycemia, while statistical difference was found in the hyperglycemic range between the two approved sites, in particular sensors placed in the abdomen performed better than those on gluteus.
Our findings in terms of sensor accuracy are in good agreement with those of Laffel,9 the only other available work assessing accuracy of the sensor considered here in pediatric subjects with T1D. There mean/median ARD were found 13/10% when CGM was compared against SMBG and CEG A was 83%; similarly, here, mean/median ARD were 13.1/9.4% and CEG A was 81.2%.
It should be noted that Laffel9 assessed the CGM object of the study, by evaluating its accuracy against both Yellow Springs Instruments (YSI) (YSI BG analyzer; Yellow Springs, OH) and SMBG collected with Bayer [Whippany, NJ] Contour™ Next USB BG meter.
As expected, the evaluation of CGM against SMBG leads to slightly larger MAD and MARD with respect to CGM against YSI, about 30% more in the first case since the uncertainty in the CGM measurement “adds-up” with the uncertainty of the SMBG measurement. Therefore, when comparing our findings with those of Laffel,9 it is important to refer to their CGM versus SMBG comparison.
Another difference with the analysis of Laffel9 is the definition of the glycemic regions: Laffel defined five glycemic regions based on the CGM values (ie, 40 mg/dl ≤ CGM ≤ 60 mg/dl, 60 mg/dl < CGM ≤ 80 mg/dl, 80 mg/dl < CGM ≤ 180 mg/dl, CGM > 180 mg/dl and CGM > 250 mg/dl), while here we resorted to a more common segmentation in three glycemic regions based on the SMBG value: SMBG ≤ 70 mg/dl, 70 mg/dl < SMBG ≤ 180 mg/dl, SMBG > 180 mg/dl.
This report suffers of a number of limitations, including the lack of randomization, the relative small sample size, the different number of patient using the sensor in the three insertion sites.
Furthermore, the accuracy was evaluated against SMBG instead of YSI. Nonetheless, this issue affects all the arms equally, so that its impact on the conclusions is expected to be limited.
Finally, the small sample size does not allow us to reliably perform other subanalysis of potential interest, for example with respect to day of sensor wear time or day- vs night-time. This interesting investigation should be performed on larger datasets collected using protocols specifically designed to analyze CGM accuracy.
Conclusion
The sensor object of the present work, when placed on the arm exhibits an accuracy that is not statistically significantly different from the accuracy in the two approved insertion sites (abdomen and gluteus). Larger, randomized trials are needed to draw final conclusions.
Acknowledgments
The authors thank the patients and their families for participating in the study.
Footnotes
Abbreviations: AD, absolute difference; ANOVA, analysis of variance; AP, artificial pancreas; ARD, absolute relative difference; BG, blood glucose; CEG A, zone A of Clarke error grid; CGM, continuous glucose monitoring; DiAs, diabetes assistant; ISO, International Organization for Standardization; MAD, mean absolute difference; MARD, mean absolute relative difference; MedAD, median absolute difference; MedARD, median absolute relative difference; MMPC, modular model predictive control; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; YSI, Yellow Springs Instruments.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SF has nothing to disclose. SDF holds patent applications related to diabetes technology and is consultant of Dexcom, Inc (San Diego, CA, USA). RV has nothing to disclose. RB has received feed for consulting and advisory board from Roche Diagnostics, Sanofi Aventis, Eli Lilly, Medtronic, Lifescan, Theras Diabetes Care, Abbott Diabetes Care, and NovoNordisk. DI has nothing to disclose. IR has received fees for consulting from Roche Diagnostics, Eli Lilly, Sanofi Aventis, and Menarini. MM has nothing to disclose. RS has received fees for consulting from Abbott and Lilly. DB reports nonfinancial research support from Roche Diagnostics, Abbott Diabetes Care, and NovoNordisk during this study, has received lecture fees from Eli Lilly, LifeScan, Roche Diagnostics, and Sanofi Aventis, and has provided advisory services to Abbott. CC holds patent applications related to the study control algorithms and has received research support from Sanofi-Aventis and Adocia and nonfinancial research support from Dexcom and Roche Diagnostics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the Ministero dell’Istruzione, Università e Ricerca (Italian Ministry of Education, Universities and Research) through the project Learn4AP: Patient-Specific Models for an Adaptive, Fault-Tolerant Artificial Pancreas (SIR: Scientific Independence of Young Researchers initiative, project ID: RBSI14JYM2). Data collection was possible thanks to the support of private donors and associations for patients with diabetes. The Italian Research Foundation Società Italiana Diabetologia provided administrative support. Dexcom supported the study providing the CGM sensors and the related consumables, Roche Diagnostics with insulin pumps, glucometers, and related consumables, Abbott Diabetes Care with the free style ketonesmeters and related consumables.
The PedArPan Study Group: University of Padua, Padua, Italy: Claudio Cobelli, Angelo Avogaro, Daniela Bruttomesso, Simone Del Favero, Federico Boscari, Roberto Visentin, Roberta Calore, Yenny Leal Moncada, Silvia Galasso, Valeria Vallone, Alfonso Galderisi.
University of Pavia, Pavia, Italy: Lalo Magni, Mirko Messori, Federico Di Palma, Eleonora Losiouk, Giordano Lanzola.
University of Turin, Turin, Italy: Ivana Rabbone, Davide Tinti.
Hospital San Raffaele, Milan, Italy: Riccardo Bonfanti, Andrea Rigamonti.
Azienda Ospedaliera Universitaria Integrata of Verona, Verona, Italy: Alberto Sabbion, Marco Marigliano, Claudio Maffeis.
Second University of Naples, Naples, Italy: Dario Iafusco, Angela Zanfardino, Alda Troncone.
Bambino Gesù, Children’s Hospital, Rome, Italy: Riccardo Schiaffini.
University of Rome TorVergata, Rome, Italy: Novella Rapini.
University of Virginia, Charlottesville, VA, USA: Daniel Chernavvsky.
References
- 1. Safety Information on Dexcom Website, available at Placement section of https://www.dexcom.com/safety-information (last accessed April 19th, 2017)
- 2. Dexcom® G5 Mobile CGM System User Guide. 2016, Dexcom®, Inc. Available at: https://s3-us-west-2.amazonaws.com/dexcompdf/LBL013445-G5-Mobile-CGM-System-UG-US.pdf (last accessed April 19th, 2017).
- 3. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9(2):209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Steen SC, Rijkenberg S, Limpens J, Van der Voort PH, Hermanides J, DeVries JH. The clinical benefits and accuracy of continuous glucose monitoring systems in critically III patients-a systematic scoping review [published online ahead of print January 14, 2016]. Sensors (Basel). pii:E146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Facchinetti A. Continuous glucose monitoring sensors: past, present and future algorithmic challenges [published online ahead of print December 9, 2016]. Sensors (Basel). pii:E2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39(7):1180-1185. [DOI] [PubMed] [Google Scholar]
- 7. Elleri D, Allen JM, Tauschmann M, et al. Feasibility of overnight closed-loop therapy in young children with type 1 diabetes aged 3-6 years: comparison between diluted and standard insulin strength. BMJ Open Diabetes Res Care. 2014;2(1):e000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thabit H, Leelarathna L, Wilinska ME, et al. Accuracy of continuous glucose monitoring during three closed-loop home studies under free-living conditions. Diabetes Technol Ther. 2015;17(11):801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18(2):S223-S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patek SD, Magni L, Dassau E, et al. International Artificial Pancreas (iAP) Study Group. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59(11):2986-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toffanin C, Messori M, Di Palma F, et al. Artificial pancreas: model predictive control design from clinical experience. J Diabetes Sci Technol. 2013;7(6):1470-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keith-Hynes P, Mize B, Robert A, Place J. The diabetes assistant: a smartphone-based system for real-time control of blood glucose. Electronics (Basel). 2014;3(4):609-623. [Google Scholar]