Abstract
The novel system for self-monitoring of blood glucose (SMBG) PixoTest couples SMBG to a smartphone and does not require a separate glucose meter. The integrated system includes all components necessary for a glucose measurement, and owing to a colorimetric measurement principle, a smartphone camera can capture color changes and a software app calculates the corresponding glucose value. In the presented study, the system was evaluated in terms of system accuracy as described in ISO 15197:2013. It was shown to fulfill system accuracy requirements with 97-99% of results from three different reagent system lots within the accuracy limits and 100% of results within zone A of the consensus error grid.
Keywords: self-monitoring of blood glucose, technology, smartphone, ISO 15197:2013
Devices for self-monitoring of blood glucose (SMBG) are essential tools for people with diabetes. They should therefore be easy to integrate in daily life to facilitate SMBG. Results provided by the devices have to be accurate and reliable to enable adequate therapeutic decisions. An SMBG system that couples blood glucose (BG) measurements to an appropriate smartphone, PixoTest (iXensor Co Ltd, Taipei, Taiwan), has been developed recently. Accuracy of the BG results obtained with the novel technology was analyzed following ISO 15197:20131 (ISO 15197:2013 was harmonized in the European Union as EN ISO 15197:20152 with no changes regarding its requirements).
The PixoTest System
The investigated system is a novel CE-marked system for smartphone-based BG monitoring without requiring a separate meter (Figure 1, Table 1). The core of the system is the single-use test unit that contains a lancet and a test membrane in a small box. Via the Uni-Clip the test unit is attached to a smartphone in the correct location over the camera. The Uni-Clip is specific to the used smartphone; currently seven different smartphone models are applicable.
Figure 1.
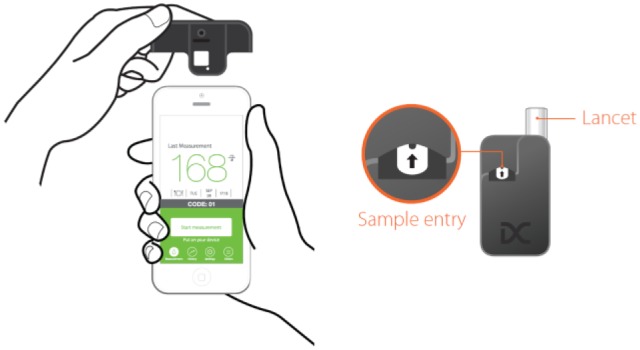
Smartphone with Uni-Clip (left) and test unit with test field and lancet (right).
Table 1.
Characteristics of the System.
| Sample | Capillary whole blood |
|---|---|
| Blood volume | ≥1.8 µla |
| Enzyme | Glucose oxidase |
| Method | Colorimetric analysis |
| Measuring range | 40-500 mg/dl (2.2-27.8 mmol/l) |
| Hematocrit | 20-60%b |
| Operating temperature | 10-35°C (50-95°F) |
At time of the study: ≥2 µl.
At time of the study: 30-55%.
To perform a measurement, the app is started, the test unit is placed on the smartphone, and a blood drop is gained by pressing the integrated lancet with the fingertip. The blood drop is then applied to the eyelet (the blood application channel) of the test unit and within 15 seconds the measurement result is displayed on the screen.
Prior to the first measurement and when opening a new box of test units, the system has to be calibrated with a standard card that is placed on the Uni-Clip after activating calibration mode in the app. This calibration process ensures that light and electronic conditions as well as sensor parts are suitable for testing. In addition, proper function of the system can be checked by measuring control solution.
The determination of BG concentration is implemented by the enzyme glucose oxidase and subsequent colorimetric analysis of the reaction product. When the blood drop is applied to the eyelet it is transferred to the reaction membrane where it reacts with glucose oxidase embedded in the membrane (Figure 2). The reaction results in a color change that is proportional to the concentration of glucose in the blood sample. Light that is provided from the liquid crystal display (LCD) of the smartphone is reflected from the reaction membrane and then analyzed photometrically via the camera module. The surrounding case protects from ambient light interference.
Figure 2.
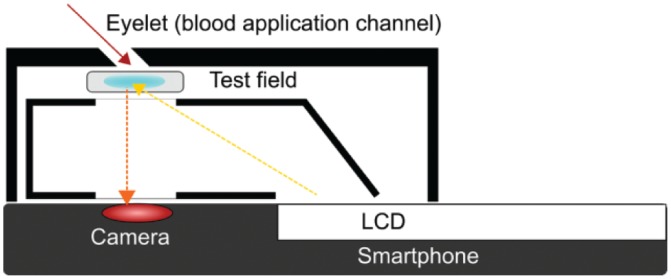
Technology inside the test unit.
The app is required for the performance of measurements but also serves as a logbook where values are stored and can be displayed graphically.
Methods
Accuracy of the system was evaluated based on ISO 15197:2013 clause 6.3, the international standard that describes requirements for BG monitoring systems for self-testing.1 The study was performed at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (accredited by the Deutsche Akkreditierungsstelle GmbH [DAkkS] as testing laboratory according to DIN EN ISO/IEC 17025:2005 and 98/79/EC in terms of test procedures according to DIN EN ISO 15197) according to all relevant regulatory requirements. The SMBG systems and smartphones (iPhone 6S, Apple Inc, Cupertino, CA) used in the evaluation were provided by the manufacturer of the system and adjusted and maintained according to the manufacturer’s labeling. Measurements with control solution and calibrations with the standard card were performed daily prior to the measurements. Measurements were performed under controlled laboratory conditions with capillary blood samples from 100 different subjects distributed into the glucose concentration categories stipulated by ISO 15197:2013. Blood samples were measured with each of three different reagent systems lots of the system, as well as with the glucose oxidase-based YSI 2300 STAT Plus glucose analyzer (YSI Inc, Yellow Springs, OH). This comparison method conforms to traceability requirements according to ISO 17511;3 trueness and precision were verified during the study procedures through internal and external quality control measures. For each lot, two measurements per subject with two different smartphones were performed (total n = 600 measurements). Absolute differences between the system and the comparison method as well as the relative bias according to Bland and Altman4 and the regression according to Passing and Bablok5 were calculated. According to ISO 15197:2013, at least 95% of the BGMS results of three reagent system lots shall be within ±15 mg/dl of the comparison method at BG concentrations <100 mg/dl, and within ±15% at higher glucose concentrations. As a second criterion, at least 99% of combined results of three reagent system lots shall be within zones A and B of the consensus error grid.
Results
The three tested reagent system lots had 97%, 98.5% and 99%, respectively, within the system accuracy limits of ISO 15197:2013 when compared against the glucose oxidase laboratory method (Figure 3, Table 2). All results were within zone A of the consensus error grid, indicating no effect on clinical action. Therefore, both system accuracy criteria of ISO 15197:2013 were fulfilled. The relative bias ranged from −1.0% to −0.6% between the three reagent system lots.
Figure 3.
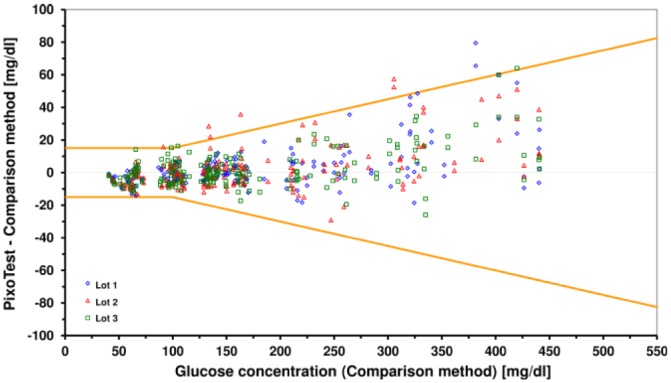
Difference plot with system accuracy results of the system compared to YSI 2300 STAT Plus glucose analyzer. Orange lines indicate system accuracy limits of ISO 15197:2013.
Table 2.
System Accuracy Results for Each Individual Reagent System Lot (n = 200 per Lot).
| Within limits of | All glucose concentrations |
Glucose concentrations <100 mg/dl |
Glucose concentrations ≥100 mg/dl |
Consensus error grid |
Relative bias | Regression | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ±15 mg/dl/15% | ±15 mg/dl | ±10 mg/dl | ±5 mg/dl | ±15% | ±10% | ±5% | Zones A+B | |||
| Lot 1 (I16D01A) | 99% | 100% | 92.9% | 69.9% | 98.6% | 93.8% | 70.8% | 100% | −0.6% | y = 1.05x–6.09 |
| Lot 2 (I16D02A) | 97% | 98.3% | 93.1% | 50% | 96.5% | 88.7% | 65.5% | −1.0% | y = 1.05x–7.58 | |
| Lot 3 (I16D03A) | 98.5% | 98.3% | 89.7% | 48.3% | 98.6% | 94.4% | 66.2% | −0.9% | y = 1.05x–7.70 | |
Discussion
There has been a lot of progress in technology in the past years, and diabetes technology is expected to follow to benefit from these innovations and to maintain acceptance among the users. The novel technology of the investigated system allows BG measurements without a traditional glucose meter. Due to the colorimetric measurement principle, glucose concentration can be detected via a smartphone camera. Because the lancet is included in the test unit, no separate lancing device is required. The necessity for fewer additional devices and the possibility to perform the measurements more discreetly might be very valuable for the users. Adherence to regular SMBG, which is important for a good glycemic control, might thus be increased by such an integrated system. But more important is the measurement quality of the system, as the patients rely on the values provided by the device and eventually calculate insulin doses based on these values. ISO 15197:2013 therefore stipulates minimum requirements for the analytical accuracy of SMBG systems. Compliance with this standard is commonly used to obtain a CE mark which is obligatory for distributing a system in the European Union. The evaluated system fulfilled with the tested reagent system lots and the used smartphone the accuracy criteria and only showed minor differences in relative bias between the evaluated lots. Its accuracy is comparable to that of traditional SMBG systems currently available.6,7 The tested system is CE-marked for seven different smartphone models, however, results of this study are limited to the tested model.
In conclusion, the novel SMBG system offers a possibility for smartphone-based BG monitoring and provided accurate and reliable results in this study. It might thus be a valuable support for patients with diabetes.
Acknowledgments
The authors would like to thank Stefan Pleus (IDT) for data analysis and for reviewing the manuscript, and the laboratory personnel of the IDT for performing the study.
Footnotes
Abbreviations: BG, blood glucose; LCD, liquid crystal display; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NJ, AB, DR, and CH are employees of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsge-sellschaft mbH an der Universität Ulm, Ulm, Germany (IDT). GF is general manager of the IDT, which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Bayer, Berlin-Chemie, Becton-Dickinson, Dexcom, LifeScan, Menarini Diagnostics, Novo Nordisk, Roche, Sanofi, Sensile, and Ypsomed. CC is an employee of iXensor Co Ltd.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Performance of the study and writing of the manuscript were funded by iXensor Co Ltd.
References
- 1. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. EN ISO 15197:2013. [Google Scholar]
- 2. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. EN ISO 15197:2015. [Google Scholar]
- 3. International Organization for Standardization. In vitro diagnostic medical devices—measurement of quantities in biological samples—metrological traceability of values assigned to calibrators and control materials. EN ISO 17511:2003. [Google Scholar]
- 4. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 5. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J Clin Chem Clin Biochem. 1983;21(11):709-720. [DOI] [PubMed] [Google Scholar]
- 6. Freckmann G, Link M, Schmid C, Pleus S, Baumstark A, Haug C. System accuracy evaluation of different blood glucose monitoring systems following ISO 15197:2013 by using two different comparison methods. Diabetes Technol Ther. 2015;17(9):635-648. [DOI] [PubMed] [Google Scholar]
- 7. Link M, Schmid C, Pleus S, et al. System accuracy evaluation of four systems for self-monitoring of blood glucose following ISO 15197 using a glucose oxidase and a hexokinase-based comparison method. J Diabetes Sci Technol. 2015;14(9):1041-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]


