Requirements for blood glucose monitoring systems (BGMS) for self-testing are regulated, for example, in the international standard ISO 15197:20131 (harmonized in the European Union as EN ISO 15197:20152). Regarding measuring accuracy of a BGMS, ISO 15197:2013 specifies the following criteria: (1) compared to a traceable laboratory method at least 95% of BGMS results have to be within ±15 mg/dl at glucose concentrations <100 mg/dl and within ±15% at ≥100 mg/dl; (2) in a consensus error grid analysis at least 99% of results have to be within zones A and B.
This study was an ISO 15197:2013 accuracy evaluation at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (accredited by the Deutsche Akkreditierungsstelle GmbH (DAkkS) as testing laboratory according to DIN EN ISO/IEC 17025:2005 and 98/79/EC in terms of test procedures according to DIN EN ISO 15197) in compliance with all applicable regulatory requirements. The BGMS GlucoDr.auto™ AGM-4000 (All Medicus, Co, Ltd, Anyang-si, Republic of Korea) was tested. According to a statement of the manufacturer, this system is marketed in the UK as Glucozen.auto™ AGM-4000 (GlucoZen Ltd, Dudley, UK). Meters and three different lots of test strips were provided by the manufacturer. A YSI 2300 STAT Plus glucose analyzer (YSI Inc, Yellow Springs, OH, USA) that is traceable according to ISO 175113 was used for comparison measurements; trueness and precision were verified throughout the study. The BGMS was used according to its labeling and daily control measurements were performed. Each of the three test strip lots was tested in duplicate in 100 subjects; glucose concentrations of the capillary blood samples were distributed as specified in ISO 15197:2013. The accuracy criteria described above were applied to the BGMS and the relative bias4 was calculated.
Over the whole glucose concentration range, the BGMS had 98.5% (Lot 1), 97% (Lot 2) and 96% (Lot 3) of results within the limits stipulated by ISO 15197:2013 (Figure 1). Percentages of results within the limits were 98.3%, 95%, and 98.3%, respectively, for glucose concentrations <100 mg/dl and 98.6%, 97.9%, and 95%, respectively, for glucose concentrations ≥100 mg/dl. All results of the three test strip lots were within zones A and B of the consensus error grid. The relative bias was 2.7% (Lot 1) and 2.6% (Lot 2 and Lot 3).
Figure 1.
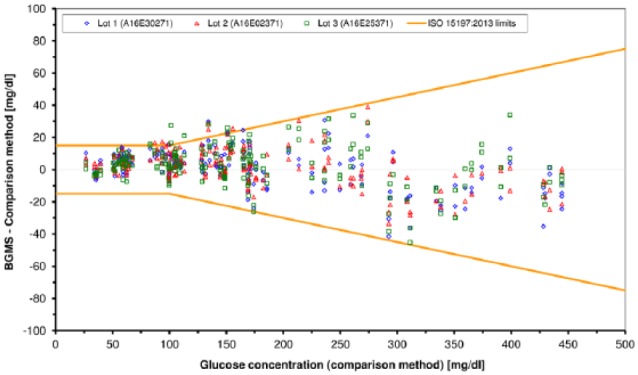
System accuracy for each individual lot: Absolute differences between BGMS results and comparison measurement results. For each lot, 200 data points are shown (100 samples measured in duplicate).
In this study, the system showed more than the minimally required 95% of results within the ISO 15197 system accuracy limits and did not show obvious variations between the evaluated test strip lots.
Acknowledgments
The authors would like to thank Delia Waldenmaier (IDT) for her support in writing the manuscript and Stefan Pleus (IDT) for data analysis and reviewing the manuscript.
Footnotes
Abbreviation: BGMS, blood glucose monitoring system.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GF is general manager of the IDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Bayer, Berlin-Chemie, Becton-Dickinson, Dexcom, LifeScan, Menarini Diagnostics, Novo Nordisk, Roche, Sanofi, Sensile, and Ypsomed. NJ, AB, UK, and CH are employees of the IDT.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by All Medicus Co, Ltd, writing of the manuscript was supported by GlucoZen Ltd and All Medicus Co, Ltd.
References
- 1. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2013. [Google Scholar]
- 2. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2013). EN ISO 15197:2015. [Google Scholar]
- 3. International Organization for Standardization. In vitro diagnostic medical devices—measurement of quantities in biological samples—metrological traceability of values assigned to calibrators and control materials. EN ISO 17511:2003. [Google Scholar]
- 4. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]


