Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) is often complex and involves long fluoroscopic times, with significant radiation exposure to medical staff. We investigated protective effects of an additional attached lead shielding device. The lead shielding device covered with the X-ray tube table (0.125 mm lead equivalent) during ERCP procedures. Fluoroscopy scatter radiation, with or without the lead shielding device, was measured using an acrylic phantom and a radiation survey meter. Measurements (25 points) were made at 50 cm intervals, at both 90 and 150 cm above the floor. We created radiation maps, with and without the additional lead shielding device. Moreover, we monitored annual staff exposure to radiation, before and after inclusion of the shielding device. Without additional shielding, exposure doses at the physician’s position, 90 and 150 cm above the floor, were 1940 and 4040 (μSv/h) respectively. In contrast, with the shielding device, corresponding exposures were 270 and 450 (μSv/h) at 90 and 150 cm, respectively. Scattered radiation was decreased by 86.1% at 90 cm or 88.9% at 150 cm. However, with additional lead shielding in the middle, rather than hung over the operating table, scattered radiation was decreased by only ~10%. The staff’s annual dose equivalents (DEs) were 12.2–29.8 mSv/year without and 3.8–8.4 mSv/year with lead shielding. With lead shielding, dose equivalent values for the staff were decreased by 41.0–76.5%. Thus, with additional lead shielding, properly used, scattered radiation would be decreased by ~90%, thus decreasing exposure doses to medical staff during ERCPs.
Keywords: endoscopic retrograde cholangiopancreatography, additional lead shielding devices, scattered radiation, physician’s dose, radiation map
INTRODUCTION
In recent years, the use of endoscopic retrograde cholangiopancreatography (ERCP) has increased, along with prevalence of diseases of the gastrointestinal tract, such as bile duct carcinoma [1]. In ERCP, an endoscope with a gastric camera is placed from the patient’s mouth to the duodenum. Next, using a contrast medium under X-ray fluoroscopy, the catheter is inserted into the pancreatic bile duct. Interventional radiology procedures, such as ERCP, are often complex and involve long fluoroscopic analyses, resulting in significant radiation exposure to the operators [2–4]. Therefore, radiation protection for interventional radiology staff is an important issue [5–9].
Surgeons performing these procedures must be reminded that protective clothing, such as aprons, alone may not be sufficient protection, especially as ERCP is often performed by one specializing surgeon. At our hospital, about 140 ERCP procedures — including endoscopic retrograde biliary drainage (ERBD), endoscopic nasobiliary drainage (ENBD), endoscopic papillary balloon dilatation (EPBD) and endoscopic sphincterotomy (EST) — are performed annually. With this number of cases, exposure doses to operators are significant. The International Commission on Radiological Protection (ICRP) stated that the threshold radiation dose during cataract surgery (0.5 Gy) is lower than exposures currently occurring. During ERCP, crystalline lens exposure is also important [10].
Based on this background, we investigated reduction of scattered radiation doses to the physician (an endoscopist) and to the rest of the staff. We compared doses during ERCP procedures, before and after installation of an additional lead shielding device. Although there were reports of additional lead shielding devices being used during cardiac catheterization procedures [11–14], there have been few studies examining scattered radiation doses received by medical staff during ERCP [15].
In this study, we examined how an additional lead shielding device, attached to the operating room table, could further protect the physician and staff, especially nurses, from exposure.
MATERIALS AND METHODS
Phantom study
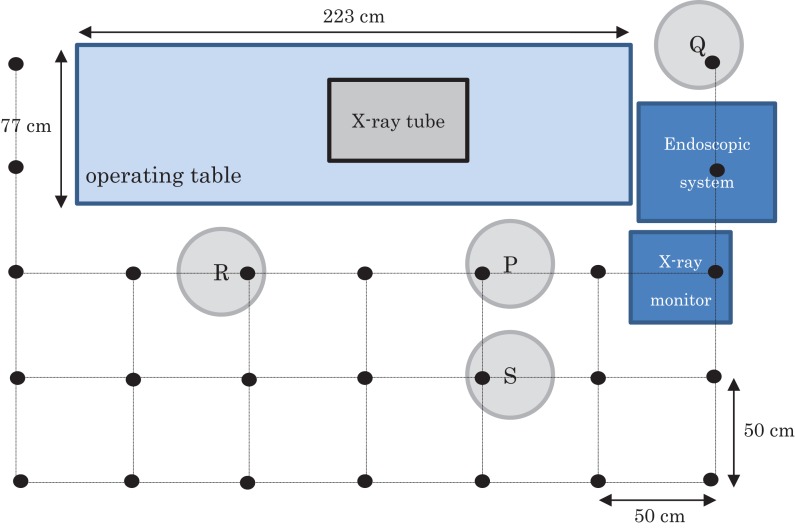
An X-ray unit (ZEXIRA; Toshiba Medical Systems, Tokyo, Japan) with an overcouch X-ray system was used (the operating table was 77 × 223 cm). The control system for this equipment sets the X-ray exposure automatically (91−92 kV, 2.2−2.3 mA). The image intensifier (I.I.) size was 10 in. Fluoroscopy-produced scattered radiation was measured using an acrylic phantom (30 × 30 × 20 cm). The measurement plan for the phantom study is shown in Fig. 1. The position of the X-ray tube was set, taking into account the expected position of the patient during an ERCP procedure. Measurements at 25 points were made at 50 cm intervals. In particular, the P point was the physician’s position, Q and R points were staff (nurses’) positions and S point was an assistant physician’s position. Measurements were made at each position at two distances above the floor, at 90 and 150 cm (90 cm corresponding to the approximate position of the gonads and 150 cm to that of the crystalline lens of the eye). These measurements were performed using a radiation survey meter (ICS-321; Hitachi-Aloka Medical, Ltd, Tokyo, Japan) with a measuring range of 1 μSv/h−300 mSv/h, both with and without the additional lead shielding device. This survey meter was used and calibrated on a regular basis. Moreover, each value was confirmed by performing the dose measurement a second time.
Figure 1.
The measurement arrangement plan of a phantom study. (Measurements were made at the positions of the black dots (each separated from the other by 50 cm.)) Positions of staff relative to the patient and X-ray tube during the ERCP procedure: P = Physician (endoscopist), Q = 1st nurse, R = 2nd nurse, S = assistant physician.
We examined whether the newly installed additional lead shielding device for ERCP would decrease the amount of scattered radiation, presenting the data visually with scattered radiation maps.
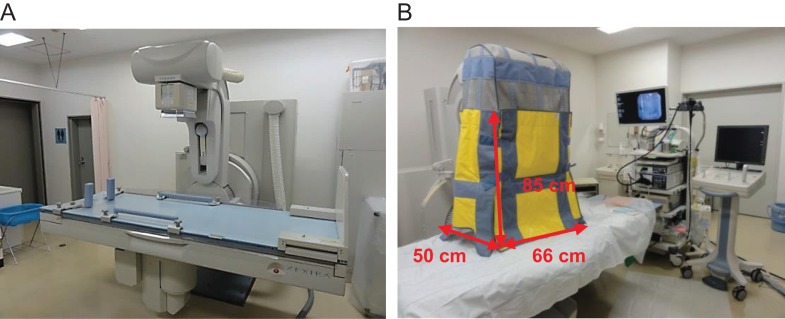
We used an additional lead shielding device (Hagoromo X-ray Protective Curtain, Maeda Co., Ltd, Tokyo, Japan: 0.125 mm lead equivalent) after July 2013. Its structure consists of four lead shielding sheets (the front and back are 85 × 66 cm and the two sides are each 85 × 50 cm), and it was hung down to the surface of the operating table during ERCP procedures (Fig. 2).
Figure 2.
Without shielding (A) and with shielding to the operating table (B). The front and back of the additional lead shielding are 85 × 66 cm and the two sides are each 85 × 50 cm.The upper part of the made of mesh (without the lead shield).
The upper part of the shield was made of mesh (without the lead shield). It weighed ~6 kg and could be folded in the middle. The focus image distance was 118 cm. We estimated the operating table height as being 85 cm above the floor level. We estimated the height of the operating table that is used clinically as being above the floor level. The scattered radiation dose reduction rate (%) was determined according to the equation (A—B/A) × 100, where A is the scattered radiation without shielding and B is that with shielding. After measuring the scattered radiation, we created a radiation map with the data obtained and SS-3030 software (S.S. Techno-Engineering Co., Nagoya, Japan). This is original software enabling creation of scattering radiation maps in an X-ray fluoroscopy room.
Measurement of physician and staff exposure doses
The same X-ray unit and table that were used in the phantom study were used to measure the radiation exposure by the physician and staff under equivalent X-ray exposures and I.I. sizes as those used during ERCP. We compared the exposure doses to the physician, assistant physician and four nurses, without the additional lead shielding device during the first year (July 2012 − June 2013) and at 1 year after the device was in place (July 2013 − June 2014). The subjects each wore two personal optically stimulated luminescence dosimeters (Quixel badges; Nagase Landauer, Ltd, Tsukuba, Japan). One whole-body dosimeterwas worn under the lead apron on the chest (in men) or on the abdomen (in women). The other, positioned to measure exposure to the eye lens, was placed outside the lead apron on the neck collar, thus monitoring radiation doses in areas not protected by the lead apron. The manufacturer of the badges sent monthly reports of exposure doses to the staff. These reported exposure doses were added together at the end of 1 year, before and after installing the added protective device. Effective dose (ED) and dose equivalent (DE) values were calculated using equations from a previous Japanese study [16]: ED = 0.89 Hp(10)in + 0.11 Hp(10)out and DE = 1.00 × Hp(0.07)out, where Hp(10)in was the dose recorded by the dosimeter worn under the lead apron (0.35 mm lead equivalent) at the chest or abdomen (1 cm dose equivalent, under the lead apron); Hp(10)out was the dose received by the dosimeter worn outside the lead apron at the neck (1 cm dose equivalent, outside the lead apron); and Hp(0.07)out was the neck badge dose (70 μm dose equivalent, outside the lead apron).
RESULTS
Phantom study
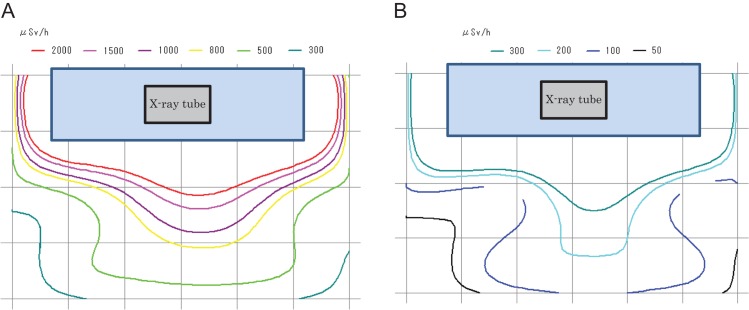
The scattered radiation doses, with and without additional lead shielding, are shown in Table 1. We also created scattered radiation maps, using software, to visualize the scattered dose of a predetermined measurement point (90 cm above the floor). These maps of the radiation doses without and with the additional lead shielding device, are shown in Fig. 3A and B, respectively.
Table 1.
Scattering doses (μSv/h) at the physicians’ (P, S) and nurses’ (Q, R) positions, and the maximum points, without (−) and with (+) lead shielding
| 150 cm | 90 cm | |||
|---|---|---|---|---|
| (−) | (+) | (−) | (+) | |
| P | 4040 | 450 | 1940 | 270 |
| Q | 890 | 135 | 495 | 185 |
| R | 2270 | 140 | 1170 | 134 |
| S | 1400 | 200 | 784 | 125 |
| maximum point | 4820 | 525 | 2310 | 342 |
P = physician, Q = 1st nurse, R = 2nd nurse, S = assistant physician.
Figure 3.
(A) Radiation map (without shielding 90cm in height above the floor). (B) Radiation map (with shielding 90 cm in height above the floor).
At 90 cm above the floor, the maximum dose without shielding was 2310 μSv/h. With the additional lead shielding device it was 342 μSv/h. Thus, the scattered radiation dose was decreased by 85.2%.
The dose at the physician’s position (P point, Fig. 1) without additional lead shielding was 1940 μSv/h. With the additional lead shielding device it was 270 μSv/h, i.e. a dose reduction of 86.1%.
The doses at the two nurses’ positions (Q and R) without additional lead shielding were 495 and 1170 (μSv/h), respectively. With the additional lead shielding device they were 185 and 134 (μSv/h), respectively. Thus, the scattered radiation was decreased by 62.6 and 88.5%, respectively.
The dose at the assistant physician’s position (S) without additional lead shielding was 784 μSv/h. With the additional lead shielding device it was 125 μSv/h, i.e. a dose reduction of 84.1% (Table 1).
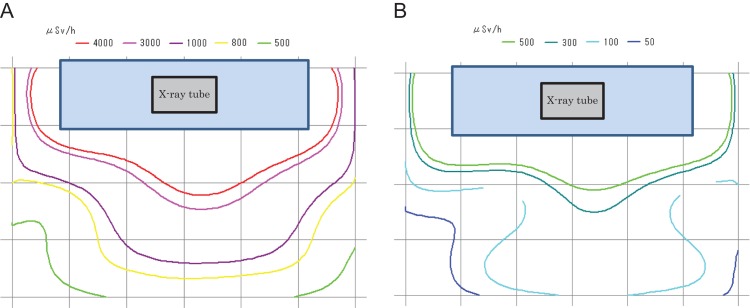
Scatter radiation maps without (Fig. 4A) and with (Fig. 4B) additional lead shielding devices were also generated for exposures 150 cm above the floor. These maps showed that the maximum dose without shielding was 4820 μSv/h. With the additional lead shielding device it was 525 μSv/h, i.e. a dose reduction of 89.1%.
Figure 4.
(A) Radiation map (without shielding 150 cm in height above the floor). (B) Radiation map (with shielding 150 cm in height above the floor).
The dose at the physician’s position (P point, Fig. 1), without additional lead shielding, was 4040 μSv/h. With the additional lead shielding device, it was 450 μSv/h, i.e. a dose reduction of 88.9%.
The doses at the two nurses’ positions (Q and R points, Fig. 1), without additional lead shielding, were 890 and 2270 (μSv/h), respectively. With the additional lead shielding device, they were 135 and 140 (μSv/h), i.e. dose reductions of 84.8 and 93.8%, respectively.
The dose at the assistant physician’s position (S point, Fig. 1), without additional lead shielding, was 1400 μSv/h. With the additional lead shielding device, it was 200 μSv/h, i.e. a dose reduction of 85.7% (Table 1).
Figure 5 shows the additional lead shielding that was not unfolded to hang down to the operating table. In a few cases, the ERCP procedure was performed with the shield remaining folded at the middle. In these cases, the scattered radiation was reduced by only ~10% at the physician’s position (Table 2). Thus, we concluded that the lead shielding should always be unfolded for greater protection.
Figure 5.
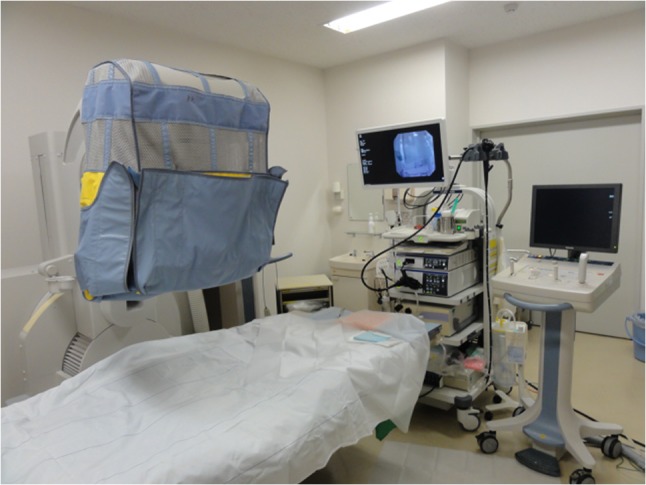
Additional lead shielding to the operating table not hung down (in the middle).
Table 2.
Scattering radiation at the physician’s position, without shielding or with the additional lead shielding device either installed properly (with) or not hung down to the operating table (in the middle)
| Height above the floor | 150 cm | 90 cm | ||
|---|---|---|---|---|
| μSv/h | Reducing rate (%a) | μSv/h | Reducing rate (%a) | |
| Without | 4040 | 1940 | ||
| With | 450 | 88.9 | 270 | 86.1 |
| In the middle | 3700 | 8.4 | 1720 | 11.3 |
aScatter radiation dose reduction rate (%) = [(without − (with or middle))/without] × 100.
Measurement of radiation doses to the physician and other staff
Table 3 shows physician and staff annual exposure radiation dose values (ED and DE) during ERCP procedures, before the additional lead shielding device (July 2012 through June 2013; n = 147: 80 men, 67 women; average patient age 70.1 years) and after the addition of the shielding device (July 2013 through June 2014; n = 144: 71 men, 73 women; average patient age 70.7 years).
Table 3.
Physician exposure doses (mSv/year; per 1 year), before and after installation of the additional lead shielding device
| Before (July 2012–June 2013) | After (July 2013–June 2014) | ||
|---|---|---|---|
| n = 147 | n = 144 | ||
| Physician | ED | 6.3 | 4.5 |
| DE | 12.9 | 4.6 | |
| Ns1 | ED | 8.5 | 1.0 |
| DE | 12.2 | 7.2 | |
| Ns2 | ED | 4.4 | 1.5 |
| DE | 29.8 | 7.0 | |
| Ns3 | ED | 4.9 | 0.8 |
| DE | 17.0 | 8.4 | |
| Ns4 | ED | 1.6 | 0.4 |
| DE | 12.9 | 3.8 |
The physician’s ED was 6.3 mSv/year before addition of the lead shielding device and 4.5 mSv/year with shielding, a dose reduction of 28.6%. The DE was 12.9 mSv/year before using the additional lead shielding device and 4.6 mSv/year with shielding, i.e. a dose reduction of 64.3%.
The assistant physician did not return the badge, so this individual’s ED and DE values could not be determined.
The four nurses’ EDs were 8.5, 4.4, 4.9 and 1.6 mSv/year before using the additional lead shielding device, and 1.0, 1.5, 0.8 and 0.4 mSv/year with shielding, i.e. dose reductions of 65.9−88.2%. Their DEs were 12.2, 29.8, 17.0 and 12.9 mSv/year before using the additional lead shielding device and 7.2, 7.0, 8.4 and 3.8 mSv/year with shielding, i.e. dose reductions of 41.0−76.5%.
DISCUSSION
ERCP procedures have lower risks for patients than surgical procedures, and their wide acceptance has led to their increased use [1]. This is despite the fact that the radiation doses during ERCP are higher than those of any commonly performed diagnostic radiographic imaging [9, 17, 18]. Therefore, radiation protection for the physicians performing ERCP is very important.
Overcouch X-ray systems have higher radiation exposure doses at the level of the physician’s eyes, and these may exceed the ICRP limit [17]. There are two types of radiation effects, stochastic and deterministic. The ED is used to estimate the risk of stochastic effects (for example cancer), and the regulatory effective dose limit (that is, 20 mSv/year averaged over 5 consecutive years, 100 mSv over 5 years and 50 mSv in 1 year) is used to ensure that the occurrence of stochastic effects is maintained within acceptable levels [19]. To prevent the occurrence of stochastic effects, the radiation dose must be as low as is reasonably achievable, while ensuring that the procedure is of diagnostic utility and efficiently performed. The DE value is used to estimate the risk of deterministic effects (for example, cataracts), and the regulatory DE limit (e.g. for the eye lens, 150 mSv/year) is used to ensure that deterministic effects, causing tissue reactions, are avoided [19]. Results from only one physician were included in our study.
Protective clothing must also be worn by those performing ERCP. Nurses can stand at a distance from patients to protect themselves from scattered radiation, but doctors cannot. Therefore protective aprons are necessary for doctors including nurses. Moreover, we believe that further protection against scattered radiation can be achieved by using additional lead shielding in ERCP. Muniraj et al. [17] conducted a randomized, double-blind study of 100 therapeutic ERCP procedures. Patients were randomly assigned to groups with lead-free radiation-attenuating drapes (n = 50) or identical sham drapes (n = 50). The physician wore a personal dosimeter on the left collar, at the level of the left eye. The lead-free radiation-attenuating drapes blocked ≥90% of scattered radiation, thereby decreasing exposure to below the established limits for the eye lens.
Minami et al. [15] used radiation-attenuating curtains mounted on the X-ray tube. Patients were chosen randomly for endoscopic procedures, and radiation doses to the endoscopists were measured with electronic pocket dosimeters placed outside the protective apron. When protective curtains were not used, the mean radiation dose to the endoscopists was 340.9 μSv per procedure. With the protective curtains, the dose was 42.6 μSv per procedure.
Our mean radiation doses (dose equivalents on the neck collar) per procedure to the endoscopist (physician) were lower than those in a previous study, 87.8 μSv (12.9 mSv/year/147) without additional lead shielding, and 31.9 μSv (4.6 mSv/year/144) with additional lead shielding, respectively. We calculated doses per procedure from the annual exposure dose value for the physician. Compared with the dose reduction ratio in the previous study, which was approximately one-eighth, our results showed an approximate dose reduction ratio of one-third. The discrepancy in the results was likely caused by differences in measuring methods, or because in our study the additional lead shielding did not hang down to reach the operating table.
In the phantom study, the scattered radiation exposure doses at the physician’s position were 1940 μSv/h (90 cm above the floor) and 4040 μSv/h (150 cm above the floor). The scattered radiation exposure was greater at 150 cm than at 90 cm above the floor. Scattered radiation exposure was greater at 150 cm in our study because we used an overcouch X-ray system (near the tube).
With the additional lead shielding device, the scattered radiation produced by fluoroscopy, measured using the acrylic phantom and the survey meter in the physician’s position, was ~90% lower than that measured without the additional shielding.
According to the European Society of Gastrointestinal Endoscopy (EGSE) guideline (2012), shields of >0.5 mm lead-equivalent thickness should be positioned between the X-ray tube and the staff, including when mobile C-arm units are used. Staff radiation exposures may be decreased by >90% by using radiation protection shields located between the X-ray tube and staff [20].
In our study, we used additional lead shielding of 0.125 mmPb, and appear to have achieved protective effects comparable with those reported for 0.5 mmPb lead equivalent, as suggested by the EGSE guidelines.
Also in the phantom study, the additional lead shielding device was highly effective against the scattered radiation produced by fluoroscopy. However, if the additional lead shielding was not unfolded to hang down to the operating table, scattered radiation was decreased by only ~10% (Table 2). This indicated that, if the additional lead shielding is not used properly, staff members are much less protected from the scattered radiation coming from the patient. Hence, it is necessary to ensure that the additional lead shielding device is unfolded and reaches the operating table.
To the best of our knowledge, only a few reports have described use of additional lead shielding during ERCP procedures. There have also been few previous reports on the scattered radiation doses received by operators and other medical staff during ERCP.
The radiation maps have added clarity to the distribution of the scattered radiation distribution, e.g. the radiation dose at the physician’s position was decreased to about one-ninth. Such information should lead to greater awareness about exposures to physicians and other staff, especially nurses [21]. When the measurement area was narrowed, more detailed radiation maps could be drawn. We believe that ours is the first report presenting a scattered radiation map for ERCP.
Previous studies also evaluated scattered radiation doses to the staff during ERCP. For example, O’Connor et al. [22] reported that the mean equivalent dose to the lens of a gastroenterologist’s eye was 0.09 mSv per ERCP procedure with an overcouch X-ray tube. Doses to staff eyes during ERCP could potentially exceed the revised ICRP limit per annum when an overcouch X-ray tube is used. Sulieman et al. [23] reported that the mean radiation dose to the first operator was 0.27 mGy to the eye lens and 0.32 mGy to the chest. The mean radiation dose to the nurse at chest level was 0.19 mGy.
At the end of 1 year, we compared ED values for the physician and staff, with and without the additional lead shielding device. Before using the lead shielding device, the ED value for the physician was 6.3 mSv/year and those for the four nurses were 8.5, 4.4, 4.9 and 1.6 mSv/year. Moreover, the DE (to the eye lens) for the physician was calculated at 12.9 mSv/year and for the nurses at 12.2, 29.8, 17.0 and 12.9 mSv/year. With use of the additional lead shielding device, the mean ED for the physician was 4.5 mSv/year and those for the nurses were 1.0, 1.5, 0.8 and 0.4 mSv/year. Moreover, the DE (to the eye lens) was calculated at 4.6 mSv/year for the physician and at 7.2, 7.0, 8.4 and 3.8 mSv/year for the four nurses.
In addition, ED and DE values were significantly higher before installation of the lead shielding device than after during other common radiological procedures such as in the Video-Fluoroscopic Swallowing Study [24, 25]. Hence, additional lead shielding devices were highly effective for decreasing exposure doses to both physicians and staff. In our hospital, ERCP procedures (including ERBD, ENBD, EPBD and EST) are implemented in about 140 cases per year. If the same personnel were present for more procedures without additional protection, their exposures might exceed dose limits. With additional lead shielding, however, exposures would remain below dose limits, even for 500 ERCP procedures, according to our results and those of others [17].
The positions of the nurses are usually at points Q and R (Fig. 1). It was suggested, however, that their exposure was increased because they inevitably move toward the X-ray tube when the patient moves [15]. Chida et al. [26] reported that algorithms based on single dosimetry may underestimate the ED in certain cases and they recommended double dosimetry.
They reported that the threshold for developing a cataract, ~0.5 Gy, could be lower than current exposure doses; the dose limit to the lens is 20 mSv per year, averaged over defined 5 year periods, with no single year exceeding 50 mSv. The ICRP established guidelines for radiation protection for doctors and other medical staff [10]. In addition, wearing lead glasses remains useful for protecting the eye lens.
Deterministic effects are those occurring only above a certain dose threshold. Therefore, multiple levels of radiation protection, for example, thyroid neck shields and protective glasses, are required to minimize radiation exposure. It is believed that the dose limit of exposure to the lens would not be exceeded with protective glasses and additional lead shielding in place.
Oztas et al. [27] reported that the crystalline lens exposure for persons involved in ERCP procedures was 92 μSv/h per procedure. Because ~1850 procedures are performed annually, the 150 mSv/year dose limit to the crystalline lens would be exceeded. These investigators, therefore, recommended protective glasses.
Naidu et al. [28] estimated annual ED values, based on the results of 61 patients undergoing ERCP during a 2 month period. They reported that the dose to the operator during ERCP was 3.35−5.87 mSv, consistent with our results. They emphasized the need for measures to minimize radiation exposure during ERCP, recommending protective glasses or a thyroid protector.
Shin et al. [29] investigated use of radiation protection equipment during ERCP in Korea. Although use of protective aprons and thyroid protectors was high, use of protective eyeglasses was low (37.8%).
It is necessary to keep radiation exposure doses from fluoroscopy as low as possible to avoid radiation-induced skin injuries in patients undergoing interventional radiography [30–35]. When participating in an ERCP procedure, all staff should be aware of the radiation protection afforded by the inverse square law. Some medical staff, especially nurses, are somewhat protected from scattered radiation because of their distance from the source [21, 36]. Physicians, however, cannot be protected because they must remain close to the patient [37, 38]. The additional lead shielding device is highly effective in reducing the scattered radiation dose to physicians and other medical staff during ERCP. Our study had limitations. We analyzed data from only one hospital, and from only one physician and four nurses. We were unable to measure ED or DE values for the assistant physician. Finally, we did not take into consideration the fluoroscopy time or patient body weights in calculating doses to the staff. Although the survey meter used for measurement was regularly calibrated, there may be variations in such measurements.
CONCLUSIONS
We assessed whether a newly installed additional lead shielding device decreased exposure to scattered radiation for the operator and nurses during ERCP. The results of the phantom study showed a maximum decrease of 89.1% scattered radiation exposure with the additional lead shielding. Exposure radiation dosimetry results from the Quixel badges indicated that the DE doses were decreased by 64.3% (physician) and 41.0−76.5% (four nurses) by using an additional lead shielding device. We concluded that the additional lead shielding device was highly effective for reducing radiation exposure to both the physician and other staff members. If the additional lead shielding is used properly, exposure to scattered radiation can be decreased by ~90%.
ACKNOWLEDGEMENTS
We thank Mr Hiroo Chiba, Mr Hiroshi Uematsu and Mrs Chizuko Nagashima, of the Tohoku Medical and Pharmaceutical University Hospital, for their invaluable assistance.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Uppal DS, Wang AY. Advances in endoscopic retrograde cholangiopancreatography for the treatment of cholangiocarcinoma. World J Gastrointest Endosc 2015;7:675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tse F, Yuan Y, Bukhari M et al. Pancreatic duct guidewire placement for biliary cannulation for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database Syst Rev 2016;5:Cd010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thiruvengadam NR, Forde KA, Ma GK et al. Rectal indomethacin reduces pancreatitis in high- and low-risk patients undergoing endoscopic retrograde cholangiopancreatography. Gastroenterology 2016;151:288–97.e4. [DOI] [PubMed] [Google Scholar]
- 4. Cote GA, Zyromski NJ. Clarifying the role of endoscopic retrograde cholangiopancreatography in the treatment of patients with pancreatic fluid collections. Gastroenterology 2016;150:1243–45. [DOI] [PubMed] [Google Scholar]
- 5. Kachaamy T, Harrison E, Pannala R et al. Measures of patient radiation exposure during endoscopic retrograde cholangiography: beyond fluoroscopy time. World J Gastroenterol 2015;21:1900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao C, Thosani N, Kothari S et al. Radiation exposure to patients during ERCP is significantly higher with low-volume endoscopists. Gastrointest Endosc 2015;81:391–8. [DOI] [PubMed] [Google Scholar]
- 7. Buls N, Pages J, Mana F et al. Patient and staff exposure during endoscopic retrograde cholangiopancreatography. Br J Radiol 2002;75:435–43. [DOI] [PubMed] [Google Scholar]
- 8. Oonsiri S, Jumpangern C, Sanghangthum T et al. Radiation dose to medical staff in interventional radiology. J Med Assoc Thai 2007;90:823–8. [PubMed] [Google Scholar]
- 9. Zagorska A, Romanova K, Hristova-Popova J et al. Eye lens exposure to medical staff during endoscopic retrograde cholangiopancreatography. Phys Med 2015;31:781–4. [DOI] [PubMed] [Google Scholar]
- 10. Vano E, Rosenstein M, Liniecki J et al. ICRP Publication 113. Education and training in radiological protection for diagnostic and interventional procedures. Ann ICRP 2009;39:7–68. [DOI] [PubMed] [Google Scholar]
- 11. Politi L, Biondi-Zoccai G, Nocetti L et al. Reduction of scatter radiation during transradial percutaneous coronary angiography: a randomized trial using a lead-free radiation shield. Catheter Cardiovasc Interv 2012;79:97–102. [DOI] [PubMed] [Google Scholar]
- 12. Shorrock D, Christopoulos G, Wosik J et al. Impact of a disposable sterile radiation shield on operator radiation exposure during percutaneous coronary intervention of chronic total occlusions. J Invasive Cardiol 2015;27:313–6. [PubMed] [Google Scholar]
- 13. Abdelaal E, Plourde G, MacHaalany J et al. Effectiveness of low rate fluoroscopy at reducing operator and patient radiation dose during transradial coronary angiography and interventions. JACC Cardiovasc Interv 2014;7:567–74. [DOI] [PubMed] [Google Scholar]
- 14. Domienik J, Bissinger A, Grabowicz W et al. The impact of various protective tools on the dose reduction in the eye lens in an interventional cardiology–clinical study. J Radiol Prot 2016;36:309–18. [DOI] [PubMed] [Google Scholar]
- 15. Minami T, Sasaki T, Serikawa M et al. Occupational radiation exposure during endoscopic retrograde cholangiopancreatography and usefulness of radiation protective curtains. Gastroenterol Res Pract 2014;2014:926876 10.1155/2014/926876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chida K, Morishima Y, Masuyama H et al. Effect of radiation monitoring method and formula differences on estimated physician dose during percutaneous coronary intervention. Acta Radiol 2009;50:170–3. [DOI] [PubMed] [Google Scholar]
- 17. Muniraj T, Aslanian HR, Laine L et al. A double-blind, randomized, sham-controlled trial of the effect of a radiation-attenuating drape on radiation exposure to endoscopy staff during ERCP. Am J Gastroenterol 2015;110:690–6. [DOI] [PubMed] [Google Scholar]
- 18. Alzimami K, Sulieman A, Paroutoglou G et al. Optimisation of radiation exposure to gastroenterologists and patients during therapeutic ERCP. Gastroenterol Res Pract 2013;2013:587574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- 20. Dumonceau JM, Garcia-Fernandez FJ, Verdun FR et al. Radiation protection in digestive endoscopy: European Society of Digestive Endoscopy (ESGE) guideline. Endoscopy 2012;44:408–21. [DOI] [PubMed] [Google Scholar]
- 21. Morishima Y, Chida K, Katahira Y et al. Need for radiation safety education for interventional cardiology staff, especially nurses. Acta Cardiol 2016;71:151–5. [DOI] [PubMed] [Google Scholar]
- 22. O’Connor U, Gallagher A, Malone L et al. Occupational radiation dose to eyes from endoscopic retrograde cholangiopancreatography procedures in light of the revised eye lens dose limit from the International Commission on Radiological Protection. Br J Radiol 2013;86:20120289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sulieman A, Elzaki M, Khalil M. Occupational exposure to staff during endoscopic retrograde cholangiopancreatography in Sudan. Radiat Prot Dosimetry 2011;144:530–3. [DOI] [PubMed] [Google Scholar]
- 24. Wright RE, Boyd CS, Workman A. Radiation doses to patients during pharyngeal videofluoroscopy. Dysphagia 1998;13:113–5. [DOI] [PubMed] [Google Scholar]
- 25. Morishima Y, Chida K, Watanabe H. Estimation of the dose of radiation received by patient and physician during a videofluoroscopic swallowing study. Dysphagia 2016;31:574–8. [DOI] [PubMed] [Google Scholar]
- 26. Chida K, Kaga Y, Haga Y et al. Occupational dose in interventional radiology procedures. AJR Am J Roentgenol 2013;200:138–41. [DOI] [PubMed] [Google Scholar]
- 27. Oztas E, Parlak E, Kucukay F et al. The impact of endoscopic retrograde cholangiopancreatography education on radiation exposure to experienced endoscopist: ‘trainee effect’. Dig Dis Sci 2012;57:1134–43. [DOI] [PubMed] [Google Scholar]
- 28. Naidu LS, Singhal S, Preece DE et al. Radiation exposure to personnel performing endoscopic retrograde cholangiopancreatography. Postgrad Med J 2005;81:660–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin JM, Lee TH, Park SH et al. A survey of the radiation exposure protection of health care providers during endoscopic retrograde cholangiopancreatography in Korea. Gut Liver 2013;7:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chida K, Inaba Y, Morishima Y et al. Comparison of dose at an interventional reference point between the displayed estimated value and measured value. Radiol Phys Technol 2011;4:189–93. [DOI] [PubMed] [Google Scholar]
- 31. Chida K, Saito H, Otani H et al. Relationship between fluoroscopic time, dose–area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. AJR Am J Roentgenol 2006;186:774–8. [DOI] [PubMed] [Google Scholar]
- 32. Inaba Y, Chida K, Shirotori K et al. Comparison of the radiation dose in a cardiac IVR X-ray system. Radiat Prot Dosimetry 2011;143:74–80. [DOI] [PubMed] [Google Scholar]
- 33. Nakamura M, Chida K, Zuguchi M. Red emission phosphor for real-time skin dosimeter for fluoroscopy and interventional radiology. Med Phys 2014;41:101913 10.1118/1.4893534. [DOI] [PubMed] [Google Scholar]
- 34. Kato M, Chida K, Sato T et al. The necessity of follow-up for radiation skin injuries in patients after percutaneous coronary interventions: radiation skin injuries will often be overlooked clinically. Acta Radiol 2012;53:1040–4. [DOI] [PubMed] [Google Scholar]
- 35. Chida K, Kato M, Kagaya Y et al. Radiation dose and radiation protection for patients and physicians during interventional procedure. J Radiat Res 2010;51:97–105. [DOI] [PubMed] [Google Scholar]
- 36. Inaba Y, Chida K, Kobayashi R et al. Fundamental study of a real-time occupational dosimetry system for interventional radiology staff. J Radiol Prot 2014;34:N65–71. [DOI] [PubMed] [Google Scholar]
- 37. Chida K, Morishima Y, Inaba Y et al. Physician-received scatter radiation with angiography systems used for interventional radiology: comparison among many X-ray systems. Radiat Prot Dosimetry 2012;149:410–6. [DOI] [PubMed] [Google Scholar]
- 38. Haga Y, Chida K, Kaga Y et al. Occupational eye dose in interventional cardiology procedures. Sci Rep 2017;7:569. [DOI] [PMC free article] [PubMed] [Google Scholar]