Abstract
Aims
Of patients with atrial fibrillation (AF), approximately 10% undergo percutaneous coronary intervention (PCI). We studied the safety and efficacy of dual vs. triple antithrombotic therapy (DAT vs. TAT) in this population.
Methods and results
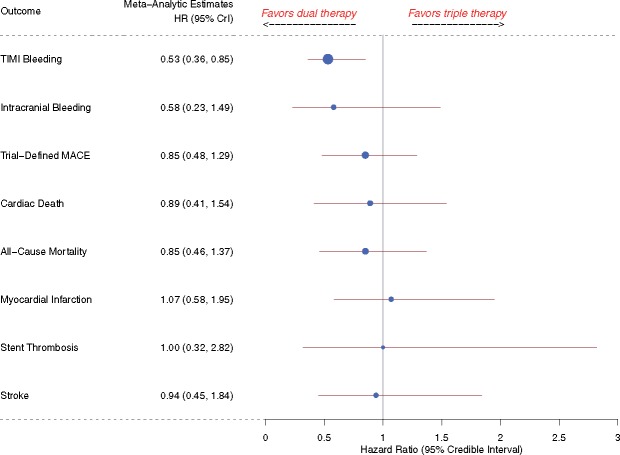
A systematic review and meta-analysis was conducted using PubMed, Embase, EBSCO, Cochrane database of systematic reviews, Web of Science, and relevant meeting abstracts for Phase 3, randomized trials that compared DAT vs. TAT in patients with AF following PCI. Four trials including 5317 patients were included, of whom 3039 (57%) received DAT. Compared with the TAT arm, Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding showed a reduction by 47% in the DAT arm [4.3% vs. 9.0%; hazard ratio (HR) 0.53, 95% credible interval (CrI) 0.36–0.85, I2 = 42.9%]. In addition, there was no difference in the trial-defined major adverse cardiac events (MACE) (10.4% vs. 10.0%, HR 0.85, 95% CrI 0.48–1.29, I2 = 58.4%), or in individual outcomes of all-cause mortality, cardiac death, myocardial infarction, stent thrombosis, or stroke between the two arms.
Conclusion
Compared with TAT, DAT shows a reduction in TIMI major or minor bleeding by 47% with comparable outcomes of MACE. Our findings support the concept that DAT may be a better option than TAT in many patients with AF following PCI.
Keywords: Atrial fibrillation, Antithrombotic therapy, Percutaneous coronary intervention, Dual therapy, Triple therapy
Introduction
Atrial fibrillation (AF) is a major global health problem affecting 33.5 million individuals worldwide.1 Oral anticoagulation (OAC) with either vitamin K antagonists (VKAs) or non-VKA oral anticoagulants (NOACs) is the mainstay for prevention of thrombo-embolic events in this population.2 Approximately 5–10% of these patients also undergo percutaneous coronary intervention (PCI) for concomitant coronary artery disease (CAD).3 This overlap poses significant challenges since prior data from randomized trials have shown superiority of dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor over aspirin and VKA for prevention of stent thrombosis and major adverse cardiac events (MACE) in patients post-PCI.4,5 Consequently, most patients with AF following PCI are subjected to treatment with a combination of both these therapies (DAPT plus OAC), the so-called ‘triple antithrombotic therapy (TAT)’ approach.
While this approach is reasonable and endorsed by American College of Cardiology/American Heart Association/European Society of Cardiology guidelines6,7 as well as expert consensus8–10 for varying durations, the evidence supporting the same is sparse. A major limitation of TAT is bleeding.11 One proposed approach is to curtail the TAT to a minimum duration with a goal to reduce bleeding events. However, this strategy has been challenged by data from the What is the Optimal AntiplatElet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary StenTing (WOEST) as well as Intracoronary Stenting and Antithrombotic Regimen: Testing of a Six-Week vs. a Six-Month Clopidogrel Treatment Regimen In Patients With Concomitant Aspirin and OraL Anticoagulant Therapy Following Drug-Eluting Stenting (ISAR-TRIPLE) trials which demonstrated that many of the bleeding events occur in the first few weeks after the initiation of TAT.12,13
In the last several years, a number of randomized trials14,15 have attempted to evaluate the strategy of dual antithrombotic therapy (DAT) vs. TAT in this patient population with DAT defined as a combination of one antiplatelet agent and an anticoagulant.16,17 In aggregate, studies have suggested reduction in bleeding by almost half in patients on DAT compared with TAT.18–21 These results are complemented by similar incidence of thrombo-embolic and MACE between the two groups. A major criticism for all the randomized trials is that none of them is sufficiently powered to assess thrombo-embolic (efficacy) outcomes. To reduce the selection bias introduced by observational data and to enhance the power for assessment of efficacy outcomes, we conducted a systematic review and meta-analysis of Phase 3, randomized trials examining DAT vs. TAT in patients with AF, following PCI with inclusion of the most recently published Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI) trial, which is the largest of all the trials on this topic to date.
Methods
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the systematic review and meta-analysis. All relevant Phase 3 randomized clinical trials comparing TAT (defined as DAPT plus an OAC) vs. DAT (defined as single antiplatelet agent plus an OAC) in patients with AF following PCI were eligible for inclusion. The primary exclusion criteria were observational non-randomized studies, registry data, ongoing trials without results, editorials, case series, and duplicate studies. A digital computerized search was conducted through PubMed, Embase, EBSCO, Cochrane database of systematic reviews, and Web of Science from its inception up to 25 July 2017 using the following search terms in various combinations: ‘percutaneous coronary intervention’, ‘coronary stenting’, ‘coronary angioplasty’, ‘stents, angioplasty’, ‘PCI’, ‘triple antiplatelet therapy’, ‘dual antiplatelet therapy’, ‘triple antithrombotic therapy’, ‘triple therapy’, ‘dual therapy’, ‘double therapy’, ‘anticoagulants’, ‘antiplatelets’, ‘platelet aggregation inhibitors’, 'vitamin K antagonists’, ‘warfarin’, ‘dabigatran’, ‘rivaroxaban’, ‘apixaban’, ‘edoxaban’, ‘aspirin’, ‘thienopyridine’, ‘clopidogrel’, ‘atrial fibrillation’, and ‘randomized clinical trial’. In addition, references of prior systematic reviews/meta-analysis, as well as abstracts from major cardiology meetings were screened for related studies. There were no restrictions on language, publication date, or publication status. Two investigators (H.G., A.Q.) independently reviewed the titles/abstracts and studies to determine their eligibility to meet the inclusion criteria. The same authors (H.G., A.Q.) independently extracted all the relevant outcomes of interest into a structured data set. The entire tabulated data set was reviewed, and disagreements were resolved via consensus and by a third author (D.L.B.).
Data analysis
We extracted information about the following outcomes from individual trials as well as their Supplementary material online12–15,22: Thrombolysis in Myocardial Infarction (TIMI) major and minor bleeding, intracranial bleeding, all-cause mortality, cardiac death, myocardial infarction (MI), stent thrombosis, and stroke. It is important to note that ISAR-TRIPLE had a slightly different trial design compared with other trials included in the analysis. In ISAR-TRIPLE trial, patients in both the arms were treated with the same triple therapy for the first 6 weeks, after which the DAT group received aspirin and warfarin whereas the TAT group received aspirin, clopidogrel, and warfarin. Henceforth, to have a valid comparison, our analysis incorporated the event data from landmark analysis of the ISAR-TRIPLE trial.
The primary safety outcome was a composite of TIMI major or minor bleeding. The secondary safety outcome was intracranial bleeding. The primary efficacy outcome was ‘trial defined MACE’ which followed the definition of MACE in the respective trials (Supplementary material online, Table S3). Secondary efficacy outcomes were the individual components of the primary efficacy outcome as well as cardiac death and stent thrombosis.
Effect size calculation
It was anticipated that the length of follow-up will differ between trials. Consequently, hazard ratios (HRs) with 95% confidence intervals for each endpoint were extracted for each of the trials when reported. For studies in which HR was not reported, the log (HR) and its variance were estimated using a previously validated method, as follows23,24:
Log-HR = 2 * [(# observed events Group 1) − (# observed events Group 2)]/[(# observed events Group 1) + (# observed events Group 2)]
Variance (log-HR) = 4/[(# observed events Group 1) + (# observed events Group 2)].
Statistical analysis
To accommodate the anticipated heterogeneity across studies, we used random effects meta-analysis to synthesize results. In particular, we used a Bayesian hierarchical model to estimate the random effects model. We assumed a Gaussian distribution for random effects. The freely available, open-source programme OpenBUGS (Bayesian inference using Gibbs Sampling) was used to fit the model.25 Non-informative priors (normal distribution with mean = 0, standard deviation = 1000) for the overall mean HR and inverse-gamma (0.001, 0.001) for the between study variance was used. Convergence of the Markov Chain Monte Carlo sampler was assessed using the Brooks–Gelman–Rubin method.26 In particular, we employed four chains; convergence of the sampler was established if the ratio of within-chain and between-chain variability for the four chains starting at different initial values is close to 1.
Heterogeneity across studies was assessed using the Cochran Q test. Higgins I2 statistics was used to determine the degree of in between study heterogeneity (I2 < 25%—low, 25–50%—moderate, and >50%—high degree of heterogeneity). Sensitivity analyses were conducted to investigate the robustness of our results to assess whether any of the included studies had a large influence on the results.
The methodological quality of the randomized trials was assessed by Cochrane’s Collaboration tool for assessing risk of bias. For each trial, bias was assessed qualitatively as low risk, intermediate risk, or high risk of bias by independent investigators. Publication bias was not assessed as there were small number of studies (<10) included in the analysis.
Results
A total of 232 studies were screened for eligibility, out of which four Phase 3, randomized trials including 5317 patients assessing the strategy of DAT vs. TAT in AF patients following PCI were included in the final analysis (Figure 1). Characteristics of the individual trials included in the analysis with its primary and secondary outcomes are depicted in the Supplementary material online, Table S1. One TAT arm of PIONEER AF-PCI14 (aspirin, P2Y12 inhibitor, and low dose rivaroxaban 2.5 mg twice daily, 709 patients) was not included in the analysis as rivaroxaban 2.5 mg is not an approved dose for thrombo-embolic protection in patients with AF. Baseline characteristics of patients enrolled in the four trials included in this meta-analysis are shown in Table 1. Mean follow-up ranged from 9 to 14 months. Mean age of patients across the trials was 70.9 years in the DAT arm and 71.1 years in the TAT arm. About 47% patients in the DAT arm and 45% patients in the TAT arm underwent PCI for acute coronary syndrome (ACS), whereas the remaining 53% and 55% patients in the DAT and the TAT arm, respectively underwent PCI for non-ACS indications. Approximately, 75% of patients in the DAT arm and 82% of patients in the TAT arm had a CHA2DS2VASc score of >2. Furthermore, 66% of patients in the DAT arm and 71% of patients in the TAT arm had a HAS-BLED score of ≥3. Atrial fibrillation was present in 100% patients in the PIONEER AF-PCI and RE-DUAL PCI trials, whereas AF was present in 84% and 69% of patients enrolled in ISAR-TRIPLE and WOEST, respectively. All the trials were judged to be at low risk of bias via the Cochran’s Collaboration tool for risk assessment (Supplementary material online, Table S2).
Figure 1.
Study selection.
Table 1.
Baseline characteristics of patients in intention-to-treat analysis of randomized trials included in the analysis
| WOEST12 |
ISAR-TRIPLE13 |
PIONEER AF-PCI14 |
RE-DUAL PCI15 |
Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DAT (n = 279) | TAT (n = 284) | DAT (n = 307) | TAT (n = 307) | DAT (n = 709) | TAT (n = 706) | DAT (n = 981) | DAT (n = 763) | TAT (n = 981) | DAT (n= 3039) | TAT (n= 2278) | |
| Dabigatran 110 mg | Dabigatran 150 mg | ||||||||||
| Age (years) | 70.3 (7.0) | 69.5 (8.0) | 73.9 (7.7) | 73.3 (8.7) | 70.4 (9.1) | 69.9 (8.7) | 71.5 (8.9) | 68.6 (7.6) | 71.7 (8.9) | 70.9 | 71.1 |
| Female (%) | 23 | 18 | 25 | 21 | 26 | 27 | 26 | 22 | 24 | 25 | 23 |
| BMI (kg/m2) | 27.5 (4.3) | 27.9 (4.2) | 27.5 (4.2) | 27.9 (4.6) | 28.6 (25.7–32.4) | 29.0 (25.8–32.8) | NR | NR | NR | 27.9 | 28.2 |
| Diabetes (%) | 24 | 25 | 28 | 24 | 29 | 31 | 37 | 34 | 39 | 32 | 32 |
| Hypertension (%) | 69 | 68 | 77 | 76 | 73 | 75 | NR | NR | NR | 73 | 74 |
| Dyslipidaemia (%) | 68 | 72 | 74 | 75 | 43 | 45 | NR | NR | NR | 56 | 58% |
| Current smoker (%) | 22 | 15 | 9 | 10 | 5 | 7 | NR | NR | NR | 10 | 9 |
| History of MI (%) | 34 | 35 | 29 | 25 | 20 | 22 | 24 | 24 | 27 | 25 | 26 |
| History of CABG (%) | 20 | 26 | 24 | 17 | NR | NR | 10 | 10 | 11 | 13 | 15 |
| History of PCI (%) | 31 | 36 | NR | NR | NR | NR | 33 | 31 | 35 | 32 | 35 |
| PPI use (%) | 34 | 39 | NR | NR | 39 | 37 | NR | NR | NR | 37 | 37 |
| Type of index event (%) | |||||||||||
| ACS | 25 | 30 | 33 | 31 | 51 | 52 | 52 | 51 | 48 | 47 | 45 |
| Non-ACS | 75 | 70 | 67 | 69 | 49 | 48 | 48 | 49 | 52 | 53 | 55 |
| Type of stent (%) | |||||||||||
| Drug-eluting stent | 65 | 64 | 99 | 99 | 65 | 66 | 82 | 81 | 84 | 79 | 79 |
| Bare-metal stent | 32 | 30 | 1 | 0 | 33 | 32 | 15 | 16 | 13 | 19 | 19 |
| Drug-eluting and bare-metal stents | 1 | 4 | 0 | 0 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| PTCA/no stent | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Indication for oral anti coagulation (%) | |||||||||||
| Atrial fibrillation | 69 | 69 | 83 | 85 | 100 | 100 | 100 | 100 | 100 | 96 | 95 |
| Mechanical valve | 10 | 11 | 6 | 9 | 0 | 0 | 0 | 0 | 0% | 1 | 2 |
| Other | 20 | 20 | 12 | 6 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| CHA2DS2-VASc score (%) | |||||||||||
| ≤2 | NR | NR | 5 | 7 | 27 | 21 | 23 | 32 | 20 | 25 | 18 |
| >2 | NR | NR | 95 | 93 | 73 | 79 | 77 | 68 | 80 | 75 | 82 |
| HAS-BLED score (%) | |||||||||||
| <3 | NR | NR | NR | NR | 28 | 29 | 33 | 41 | 29 | 34 | 29 |
| ≥3 | NR | NR | NR | NR | 72 | 71 | 67 | 56 | 71 | 66 | 71 |
Data are mean (SD), median (IQR), or percentage unless otherwise indicated.
BMI, body mass index; CABG, coronary artery bypass grafting; CHA2DS2-VASc, stroke risk factor scoring system in which one point is given for history of congestive heart failure, hypertension, age 65-74 years, diabetes, vascular disease, female gender and two points are given for history of age ≥75 years, and stroke or transient ischaemic attack; DAT, dual antithrombotic therapy; HAS-BLED, major bleeding risk factor scoring system in which one point is given to hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drug/alcohol use; MI, myocardial infarction; NR, not reported; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PTCA, percutaneous transluminal coronary angioplasty; TAT, triple antithrombotic therapy.
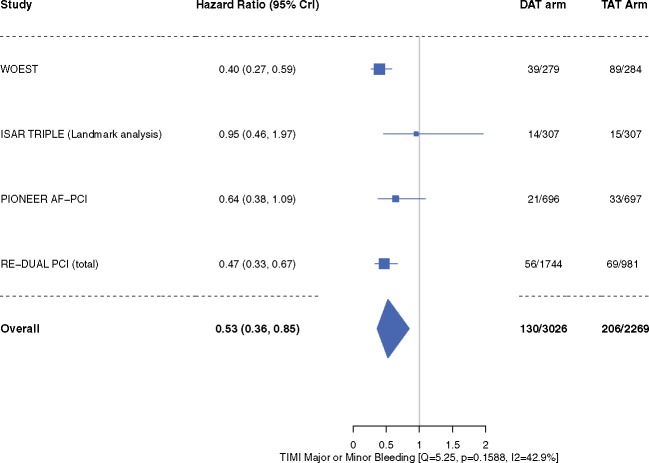
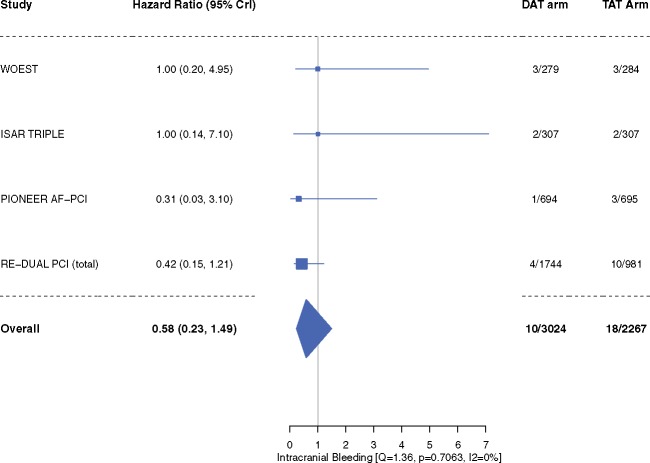
Compared with patients in the TAT arm, patients in the DAT arm demonstrated a 47% relative reduction in the risk of TIMI major or minor bleeding [4.3% vs. 9.0%; HR 0.53, 95% CrI 0.36–0.85, I2 = 42.9%] (Figure 2). The risk of intracranial bleeding was 42% lower, although not achieving statistical significance (HR 0.58, 95% CrI 0.23–1.49, I2 = 0%) (Figure 3). These outcomes did not differ when dabigatran 110 mg or 150 mg doses were analysed separately for the meta-analysis (Supplementary material online, Figures S2A, B and S3A, B).
Figure 2.
Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. CrI, credible interval; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy.
Figure 3.
Intracranial bleeding. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. Note: Primary haemorrhagic stroke reported in PIONEER AF-PCI trial was included as intracranial bleeding. Landmark analysis of ISAR-TRIPLE did not separately report intracranial bleeding rates and henceforth, the total reported event rates of intracranial bleeding reported in the trial are used. CrI, credible interval; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy.
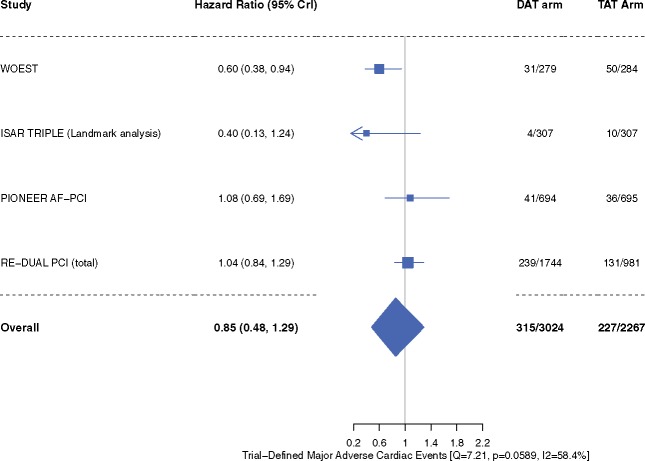
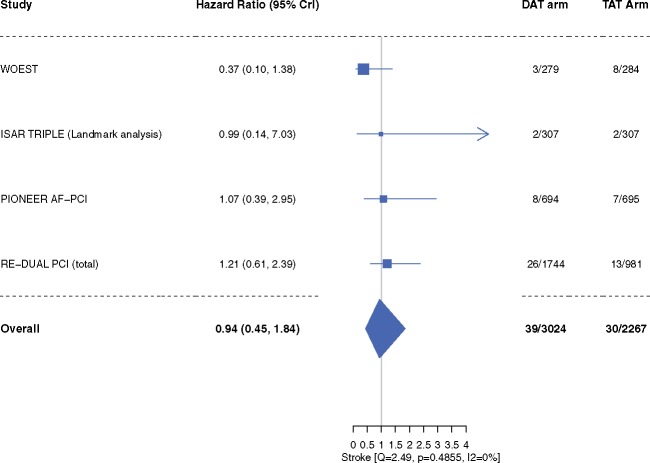
‘Trial-defined MACE’ occurred in 10.4% patients in the DAT arm compared with 10.0% patients in the TAT arm (HR 0.85, 95% CrI 0.48–1.29, I2 = 58.4%) (Figure 4). Inclusion of original results of the ISAR-TRIPLE trial did not alter our efficacy outcome results of trial-defined MACE (Supplementary material online, Figure S1). The individual end-points of all-cause mortality (HR 0.85, 95% CrI 0.46–1.37, I2 = 39.3%), cardiac death (HR 0.89, 95% CrI 0.41–1.54, I2 = 28.7%), MI (HR 1.07, 95% CrI 0.58–1.95, I2 = 15.8%), stent thrombosis (HR 1.00, 95% CrI 0.32–2.82, I2 = 32.1%), and stroke (HR 0.94, 95% CrI 0.45–1.84, I2 = 0%) did not differ between the two groups (Figures 5–7). These outcomes did not differ when dabigatran 110 mg or 150 mg doses were analysed separately for the meta-analysis (Supplementary material online, Figures S4–S9). One-study-omitted sensitivity analysis showed that individual study data did not influence our results (Supplementary material online, Figures S10 and S11).
Figure 4.
Trial-defined major adverse cardiac events. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. CrI, credible interval; DAT, dual antithrombotic therapy; MI, myocardial infarction; TAT, triple antithrombotic therapy.
Figure 5.
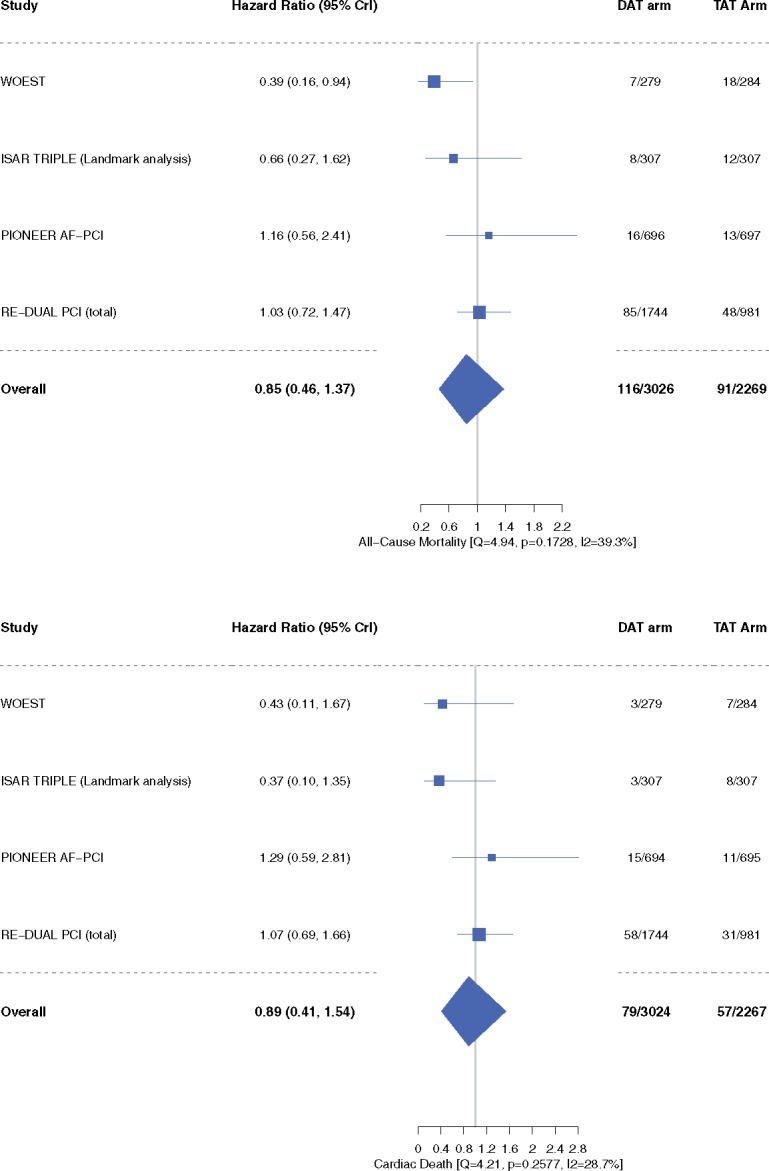
All-cause mortality and cardiac death. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. CrI, credible interval; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy.
Figure 7.
Stroke. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. CrI, credible interval; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy.
Figure 6.

Myocardial infarction and stent thrombosis. Data are n/N unless otherwise indicated. Hazard ratio <1 favours dual antithrombotic therapy and hazard ratio >1 favours triple antithrombotic therapy. Note: There were no events of stent thrombosis in either group in the Landmark Analysis of the ISAR-TRIPLE trial and hence not included in this analysis. CrI, credible interval; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy.
Discussion
Our systematic review and meta-analysis demonstrates several findings that may impact clinical care. First, in patients with AF following PCI, DAT reduces the composite of TIMI major or minor bleeding by 47% compared with TAT. Second, DAT seems comparable to TAT in reducing the trial-defined MACE. Finally, there is no statistically significant difference in individual outcomes of all-cause mortality, cardiac death, MI, stent thrombosis, or stroke between the two arms.
Up to 30% of patients with AF are found to have concomitant CAD, of whom 5–10% undergo PCI.3 It is evident that treating these patients with OAC for thrombo-embolic protection is essential; however, treatment of CAD post-PCI with antiplatelet agents is also equally important. Prior studies have demonstrated that TAT after PCI in these patients is associated with a two-fold increase in bleeding compared with DAT.11,19,20,27 It is also known that bleeding events post-PCI are associated with worse outcomes.28 In our analysis, we found that DAT was associated with a 47% reduction in the composite of TIMI major or minor bleeding compared with TAT. These findings have significant clinical implications, as bleeding is associated with interruption of antithrombotic therapy which in turn is associated with MACE.29 Furthermore, intracranial bleeding, one of the most dreadful and feared complications of TAT, showed a strong numerical trend towards reduction with DAT, particularly driven by patients enrolled in trials evaluating NOACs. This finding is crucial for management of patients at high risk of bleeding in which TAT may carry an even higher risk.
While all the four trials12–15 have demonstrated a reduction in bleeding with DAT compared with TAT, a criticism of all these trials is that they were underpowered to assess efficacy outcomes. In our pooled analysis, we found that DAT may not only reduce bleeding events but is comparable to TAT for the reduction of MACE (Take home figure). These results support the data from prior observational studies.18,21 The precise reasons for similar efficacy of DAT vs. TAT cannot be elucidated from our analysis; however, several mechanisms are possible. Newer generation drug-eluting stents with extremely low (<1%) incidence of stent thrombosis may have played a role.30 In addition, clopidogrel on a biological basis has more platelet inhibition compared with aspirin with less gastrointestinal bleeding which could in combination with OAC avoid the need for aspirin.31,32 This hypothesis has been supported by two randomized trials which demonstrated that OAC is equivalent (or even better) than aspirin for protection against thrombotic events.5,33 Finally, increased bleeding related to TAT has been shown to interrupt DAPT which in turn could increase MACE in the TAT arm.29,34,35
Take home figure.
Summary of bleeding and ischaemic risks for dual versus triple antithrombotic therapy.
Our results may have several clinical implications. In an era where the balance of ischaemic vs. bleeding benefit after PCI is gaining importance, it is prudent that we understand the best approach to antithrombotic therapy in patients with AF following PCI. In this context, several factors that may affect therapeutic decision-making as listed by the recent 2017 ESC focused update on DAPT in CAD (i.e. risk stratification via assessment of ischaemic and bleeding risks using predictor tools such as the CHA2DS2-VASc and HAS-BLED scoring systems) should be emphasized.36 Our study demonstrates that DAT is better than TAT for bleeding outcomes and comparable to TAT for efficacy outcomes. Taking this a step further, the major question that yet remains unanswered is the most appropriate combination for DAT (aspirin, clopidogrel, prasugrel, or ticagrelor with VKAs or any specific NOAC) which provides us with the right balance for minimizing thrombo-embolic vs. bleeding risks in an individual patient. With several such combinations possible, future trials are needed to answer these critical questions.
This meta-analysis has limitations. First, we used trial level data for assessment of outcomes, and hence we could not evaluate if baseline characteristics across various trials were different. Furthermore, several patient level characteristics (e.g. older age, diabetes, renal failure, or prior history of bleeding) as well as procedure related factors (e.g. coronary anatomy complexity, stent length, left main stenting) which could affect the intensity/duration of antithrombotic therapy use were not analysed in our study. Second, we pooled results of all the patients included in WOEST and ISAR-TRIPLE trials, although 69% of patients in the WOEST trial and 84% patients in ISAR-TRIPLE trial had AF. However, subgroup analysis of these trials did not demonstrate any statistical differences in their primary outcomes based on indication of OAC (AF, mechanical valves, or others), and hence we feel that inclusion of all patients enrolled in these trials would be unlikely to affect the final summary estimates of the meta-analysis. Third, in the studies included in our analysis, apart from ISAR-TRIPLE which had aspirin as the antiplatelet agent, the other three trials used a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) combined with either warfarin (WOEST) or NOACs (PIONEER AF, RE-DUAL PCI). However, our one-study-omitted sensitivity analysis does affirm that removal of the ISAR-TRIPLE trial from the analysis does not affect our primary analysis estimate for either safety or efficacy outcomes, indicating robustness of our results. Fourth, due to limited number of studies reporting the data, we could not analyse interaction between several key subgroups such as type of index event (ACS vs. non-ACS), type of stent (drug-eluting vs. bare-metal), CHA2DS2/HAS-BLED risk score etc. and MACE. Finally, substantial heterogeneity exists in between trials in terms of trial design as well as type and duration of antiplatelet/antithrombotic therapy used, which could affect interpretation of our results.
In summary, our systematic review and meta-analysis supports that DAT may be a better option than TAT in many patients with AF following PCI.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We acknowledge the help rendered by Jacqueline Cellini, Reference and Education Librarian at the Countway Library of Medicine at Harvard Medical School, in formulation and execution of the search strategy and systematic review. G.D. performed the statistical analyses, and his time was paid for by Baim Clinical Research Institute. Open access for this article was paid for by Boehringer Ingelheim.
Funding
The systematic review and meta-analysis analysis was funded by Baim Clinical Research Institute. H.B.G. and D.L.B. had full access to all the data in the study and take the responsibility for the decision to submit the manuscript for publication.
Conflict of interest: C.C. reports research Grants from Amgen, Arisaph, Boehringer-Ingelheim (BI), Bristol-Myers Squibb (BMS), Daiichi Sankyo, Janssen, Merck, and Takeda; consulting fees from Alnylam, Amarin, Amgen, Arisaph, Astra Zeneca, BI, BMS, Eisai, GlaxoSmithKline, Kowa, Lipimedix, Merck, Pfizer, Regeneron, Sanofi, and Takeda; P.G.S. receiving fees for serving on a steering committee from Amarin, Janssen, and CSL Behring, fees for serving on a steering committee and lecture fees from AstraZeneca, lecture fees and consulting fees from Bayer and Bristol-Myers Squibb, fees for preparation of educational material from Boehringer Ingelheim, consulting fees and fees for serving on a data and safety monitoring board from Lilly and Merck Sharpe & Dohme, consulting fees from Novartis and Regeneron, fees for serving on a critical event committee from Pfizer, grant support, fees for serving on a steering committee, and consulting fees from Sanofi, and grant support, fees for serving on a steering committee, consulting fees, and fees for serving on a data and safety monitoring board from Servier; G.D. receiving fees from Baim Clinical Research Institute during the conduct of the study; A.Q. is supported by a postdoctoral training grant from the National Heart, Lung, and Blood Institute of the National Institute of Health (T32 HL 007604); S.G.E. has served as a consultant for Abbott Vascular, Boston Scientific, and Medtronic; J.O. receiving lecture fees and consulting fees, paid to his institution, from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Sanofi; J.M.T. receiving grant support, advisory board fees, consulting fees, and lecture fees from AstraZeneca, advisory board fees, consulting fees, and lectures fees from Eli Lilly, Daiichi Sankyo, The Medicines Company, Accumetrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Bayer, and grant support from ZonMw and AstraZeneca; T.K. reports grants from Boerhringer Ingelheim, during the conduct of the study; S.H.H. receiving consulting fees and fees for serving on a speaker bureau from Boehringer Ingelheim, Bayer Healthcare, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, Medtronic, and Zoll; G.Y.L. receiving lecture fees from Bayer and Roche, consulting fees from Bayer Janssen and Biotronik, and lecture fees and consulting fees from Bristol-Myers Squibb Pfizer, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi Sankyo; D.L.B. discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committees, including for his role in RE-DUAL PCI), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. All other authors report no conflict of interest.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ.. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S.. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 3. Kralev S, Schneider K, Lang S, Suselbeck T, Borggrefe M.. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One 2011;6:e24964.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schomig A, Neumann FJ, Kastrati A, Schuhlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann-Roth EM, Richardt G, Alt E, Schmitt C, Ulm K.. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996;334:1084–1089. [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE.. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998;339:1665–1671. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW.. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:2246; e1–76. [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van GIC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 8. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP.. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a North American Perspective—2016 update. Circ Cardiovasc Interv 2016;9:e004395.. [DOI] [PubMed] [Google Scholar]
- 9. Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL.. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014;35:3155–3179. [DOI] [PubMed] [Google Scholar]
- 10. Capodanno D, Angiolillo DJ.. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ Cardiovasc Interv 2014;7:113–124. [DOI] [PubMed] [Google Scholar]
- 11. Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrøm SZ, Poulsen HE, Køber L, Torp-Pedersen C.. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010;170:1433–1441. [DOI] [PubMed] [Google Scholar]
- 12. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van’t Hof AW, ten Berg JM.. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 13. Fiedler KA, Maeng M, Mehilli J, Schulz-Schupke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N.. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE Trial. J Am Coll Cardiol 2015;65:1619–1629. [DOI] [PubMed] [Google Scholar]
- 14. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA.. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 15. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH.. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 16. Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sorensen R, Kober L, Torp-Pedersen C, Hansen ML.. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation 2014;129:1577–1585. [DOI] [PubMed] [Google Scholar]
- 17. Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, Mikkelsen A, Christensen CB, Lip GY, Kober L, Torp-Pedersen C, Hansen ML.. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981–989. [DOI] [PubMed] [Google Scholar]
- 18. Agarwal N, Jain A, Mahmoud AN, Bishnoi R, Golwala H, Karimi A, Mojadidi MK, Garg J, Gupta T, Patel NK, Wayangankar S, Anderson RD.. Safety and efficacy of dual versus triple antithrombotic therapy in patients undergoing percutaneous coronary intervention. Am J Med 2017;130:1280–1289. [DOI] [PubMed] [Google Scholar]
- 19. Bavishi C, Koulova A, Bangalore S, Sawant A, Chatterjee S, Ather S, Valencia J, Sarafoff N, Rubboli A, Airaksinen JK, Lip GY, Tamis-Holland JE.. Evaluation of the efficacy and safety of dual antiplatelet therapy with or without warfarin in patients with a clinical indication for DAPT and chronic anticoagulation: a meta-analysis of observational studies. Catheter Cardiovasc Interv 2016;88:E12–E22. [DOI] [PubMed] [Google Scholar]
- 20. D’Ascenzo F, Taha S, Moretti C, Omede P, Grossomarra W, Persson J, Lamberts M, Dewilde W, Rubboli A, Fernandez S, Cerrato E, Meynet I, Ballocca F, Barbero U, Quadri G, Giordana F, Conrotto F, Capodanno D, DiNicolantonio J, Bangalore S, Reed M, Meier P, Zoccai G, Gaita F.. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol 2015;115:1185–1193. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Fan M, Zhao J, Zhao B, Zhang C, Liu C, Dong Y.. Efficacy and safety of antithrombotic regimens after coronary intervention in patients on oral anticoagulation: traditional and Bayesian meta-analysis of clinical trials. Int J Cardiol 2016;205:89–96. [DOI] [PubMed] [Google Scholar]
- 22. Gibson CM, Pinto DS, Chi G, Arbetter D, Yee M, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Burton P, van Eickels M, Korjian S, Daaboul Y, Jain P, Lip GY, Cohen M, Peterson ED, Fox KA.. Recurrent hospitalization among patients with atrial fibrillation undergoing intracoronary stenting treated with 2 treatment strategies of rivaroxaban or a dose-adjusted oral vitamin K antagonist treatment strategy. Circulation 2017;135:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parmar MK, Torri V, Stewart L.. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 24. Tsiatis A. The asymptomatic joint distribution of the efficient scores test for the proportional hazards model calculated over time. Biometrik 1981;68:311–315. [Google Scholar]
- 25. Lunn D, Spiegelhalter D, Thomas A, Best N.. The BUGS project: evolution, critique and future directions. Stat Med 2009;28:3049–3067. [DOI] [PubMed] [Google Scholar]
- 26. Brooks SP GA. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434–325. [Google Scholar]
- 27. Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, Kober L, Torp-Pedersen C, Gislason GH, Hansen ML.. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126:1185–1193. [DOI] [PubMed] [Google Scholar]
- 28. Chhatriwalla AK, Amin AP, Kennedy KF, House JA, Cohen DJ, Rao SV, Messenger JC, Marso SP.. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. Jama 2013;309:1022–1029. [DOI] [PubMed] [Google Scholar]
- 29. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H.. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 30. Bavry AA, Bhatt DL.. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet 2008;371:2134–2143. [DOI] [PubMed] [Google Scholar]
- 31. Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ.. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000;140:67–73. [DOI] [PubMed] [Google Scholar]
- 32. CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 33. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H.. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002;347:969–974. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL. When is a double better than a TRIPLE? Stenting in patients with atrial fibrillation. J Am Coll Cardiol 2015;65:1630–1632. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt DL. O PIONEERs! The beginning of the end of full-dose triple therapy with warfarin? Circulation 2017;135:334–337. [DOI] [PubMed] [Google Scholar]
- 36. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN.. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.